Abstract
Gut inflammation is characterized by mucosal recruitment of activated cells from both the innate and adaptive immune systems. In addition to immune cells, inflammation in the gut is associated with an alteration in enteric endocrine cells and various biologically active compounds produced by these cells. Although the change in enteric endocrine cells or their products is considered to be important in regulating gut physiology (motility and secretion), it is not clear whether the change plays any role in immune activation and in the regulation of gut inflammation. Due to the strategic location of enteric endocrine cells in gut mucosa, these gut hormones may play an important role in immune activation and promotion of inflammation in the gut. This review addresses the research on the interface between immune and endocrine systems in gastrointestinal (GI) pathophysiology, specifically in the context of two major products of enteric endocrine systems, namely serotonin (5-hydroxytryptamine: 5-HT) and chromogranins (Cgs), in relation to immune activation and generation of inflammation. The studies reviewed in this paper demonstrate that 5-HT activates the immune cells to produce proinflammatory mediators and by manipulating the 5-HT system it is possible to modulate gut inflammation. In the case of Cgs the scenario is more complex, as this hormone has been shown to play both proinflammatory and anti-inflammatory functions. It is also possible that interaction between 5-HT and Cgs may play a role in the modulation of immune and inflammatory responses. In addition to enhancing our understanding of immunoendocrine interaction in the gut, the data generated from the these studies may have implications in understanding the role of gut hormone in the pathogenesis of both GI and non-GI inflammatory diseases which may lead ultimately to improved therapeutic strategies in inflammatory disorders.
Keywords: 5-HT, chromogranins, gut, immune response, inflammation
Introduction
The gastrointestinal (GI) tract contains the largest endocrine organ in the body. A large numbers of endocrine cells are dispersed among the epithelial cells of gut mucosa and react to changes in gut contents by releasing hormones that are, in general, targeted to other parts of the digestive system [1]. There are at least 14 different populations of enteric endocrine cells scattered throughout GI epithelia [2]. Enteric endocrine cells release various biologically active compounds such as gastrin, secretin, stomatostatin, cholecystokinin, chromogranins (Cgs) and serotonin (5-hydroxytryptamine: 5-HT) [3–5]. The hormones released from the enteric endocrine cells are important enteric mucosal signalling molecules influencing gut physiology (motor and secretory function). Alteration of endocrine cell function, particularly in the context of 5-HT, has been shown to be associated in a number of GI diseases including inflammatory bowel disease (IBD), coeliac disease, enteric infections, colon carcinoma and functional disorders such as irritable bowel syndrome (IBS) [6–14]. The association between alteration in the production of gut hormones from enteric endocrine cells and various GI diseases emphasizes highly the significance of these hormones in intestinal homeostasis.
Due to the strategic location of enteric endocrine cells in gut mucosa, interaction between immune and endocrine systems is very likely to play an important role in immune activation in relation to gut pathology and pathophysiology in various GI disorders, including IBD. This paper reviews information on the role of two major hormones of the GI tract, namely 5-HT and Cgs, in immune activation in the context of gut inflammation and highlights its implications in understanding the pathology and pathophysiology of inflammatory disorders of the gut.
5-HT in GI tract
Enterochromaffin (EC) cells are the best-characterized GI endocrine cells, which are dispersed throughout the GI mucosa and are the main source of biogenic amine 5-HT in gut [5,15]. EC cells have specialized microvilli that project into the lumen, and contain enzymes and transporters known to be present in the apical parts of the enterocytes [16]. EC cells function as sensors for the gut contents and respond to luminal stimuli directly via these transporters and/or indirectly by mediators from the surrounding cells [16]. The GI tract contains about 95% of the body's 5-HT, and EC cells are its main source [15,17]. 5-HT is also found in enteric neurones, but the 5-HT amount present in enteric neurones appears very small in comparison to that present in EC cells (approximately 90% of 5-HT in EC cells and 10% in enteric neurones) [17]. EC cells release 5-HT in a regulated and calcium-dependent manner in response to various mechanical and chemical stimuli, including bacterial toxins [3–5]. EC cells synthesize 5-HT from its precursor l-tryptophan. Tryptophan hydroxylase (TPH) catalyzes the rate-limiting step in the synthesis of 5-HT from tryptophan and has been detected prominently in EC cells [18]. Recent studies show that there are two isoforms of TPH enzymes regulating 5-HT system. TPH1 is present mainly in peripheral organs such as the intestine and spleen, while TPH2 predominates in the brain stem [19,20]. Thus 5-HT seems to be synthesized independently in peripheral tissues and neurones by two different rate-limiting TPH isoenzymes.
The synthesis of 5-HT by EC cells begins by conversion of dietary tryptophan to 5-hyroxytryptophan (5-HTP) by the rate-limiting TPH1. 5-HTP is then converted to 5-HT by the enzyme l-amino acid decarboxylase. Newly produced 5-HT is packaged into granules/vesicles by the vesicular monoamine transporter 1. 5-HT is released mainly from the granules stored near the basal border of the EC cell, but studies have also identified granules near the apical membrane where release may also take place [21]. Once released, 5-HT is transported into surrounding epithelial cells by the serotonin reuptake transporter (SERT) and degraded to 5-hydroxyindoleacetic acid by monoamine oxidase A.
5-HT is released from EC cells into the blood, into the surrounding tissue and into the gut lumen and participates in various gut functions [22]. Secretion of 5-HT by EC cells can be enhanced or attenuated by the action of signalling molecules released from surrounding cells, and alteration of 5-HT release may contribute to intestinal pathophysiology. Our recent work has shown an important immunoendocrine axis in the gut, where secretory products from CD4+ T cells interact with EC cells or their precursors to enhance 5-HT production in the gut via T helper type (Th2)-based mechanisms [23]. Recently we have observed that EC cell and 5-HT responses to the same enteric infectious agent are influenced by Th1 or Th2 cytokine predominance, suggesting the importance of the immunological profile of the inflammatory response in the regulation of EC cell biology [24]. The role of the host's immune response underlying changes in EC cells and 5-HT has also been demonstrated in a number of GI infection-induced gut inflammations, which include infections with Salmonella typhimurium, rotavirus, Citrobacter rodentium, Trichuris muris, Nippostrongylus brasiliensis and Trichinella spiralis[10–12,23–26]. Thus the close proximity between EC cells and immune cells in the gut mucosa, and the recent knowledge showing that cytokines from immune cells can activate EC cell secretion, suggest that interaction between gut endocrine and immune systems may be responsible for aspects of pathophysiology in GI inflammation.
5-HT exerts a confounding range of effects in the gut, due largely to the presence of multiple receptor subtypes which are present on smooth muscle, enteric neurones and enterocytes [27,28]. Seven types of 5-HT receptors are now identified and among these, 5-HT3 and 5-HT4 receptors are shown to play important roles in GI physiology, including motor and secretory function. The increased availability of selective 5-HT receptor agonists and antagonists has prompted numerous studies aimed at developing therapeutic agents for functional bowel disorders such as IBS. Alosetron (5-HT3 receptor antagonist) became the first agent approved by the United States Food and Drug Administration for the treatment of diarrhoea-predominant IBS. However, the drug was associated unexpectedly with ischaemic colitis and, rarely, with severe constipation-induced complications [29]. The patients diagnosed with ischaemic colitis were not at ischaemic risk, and there is no evidence of 5-HT receptor on vascular smooth muscle. The case of alosetron prompts a rethinking of our approaches to the pharmacological modulation of the 5-HT pathway and warrants more studies on 5-HT in the context of intestinal pathology and pathophysiology.
5-HT in immune activation
There is now abundant evidence to suggest that mucosal 5-HT modulates the immune response and, thus, is able potentially to influence intestinal inflammation [30]. Several serotonergic receptors have been characterized in lymphocytes, monocytes, macrophages and dendritic cells, which suggests a role of 5-HT in immune cell function [31]. The presence of EC cells in contact with, or very close proximity to, CD3+ and CD20+ lymphocytes [32] indicates clearly the existence of interaction between EC and immune cells. 5-HT influences in vitro proliferation of lymphocytes [33], protects natural killer (NK) cells from oxidative damage [34] and promotes the recruitment of T cells [35]. It has also been shown that 5-HT inhibits apoptosis of immune cells and contributes to chronic atopic dermatitis [36]. Exogenous 5-HT induces rapid phosphorylation of extracellular signal-regulated kinase-1 and -2 (ERK1/2) and nuclear factor of kappa light polypeptide gene enhancer in B cell inhibitor, alpha (IκBα) in naive T cells. We have demonstrated recently that macrophages isolated from the peritoneal cavity of mice produced interleukin (IL)-1β via the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway in response to treatment with 5-HT, implying a role of 5-HT in activation of innate immune cells and production of proinflammatory cytokines [37]. Inhibition of 5-HT-mediated activation of T cells has also been shown by preincubation with a specific 5-HT receptor antagonist, suggesting that 5-HT can also play important role in the generation of adaptive immunity [38].
5-HT in gut inflammation
EC cells and 5-HT have been evaluated in IBD and in animal models of intestinal inflammation and data indicate that inflammation results in changes in various aspects of 5-HT signalling in the GI tract. It has become increasingly evident that interactions between the gut hormones and the immune system play an important role in the pathophysiology of IBD. Changes in the EC cell population and in 5-HT content have been reported in association with both Crohn's disease (CD) and ulcerative colitis (UC) [6,9,39,40]. A study on the quality of life in IBD in remission shows that approximately 50% of patients with IBD in long-standing remission have IBS-like symptoms [41]. Psychological wellbeing and levels of anxiety and depression of these patients having IBS-like symptoms are comparable to the general population, supporting the hypothesis that transient or chronic inflammation may lead to persistent gut dysfunction. In addition, it has been shown that TPH1 mRNA levels are up-regulated in CD patients in remission who experience IBS-like symptoms [42]. As 5-HT signalling is altered in IBS, and 5-HT has been shown to possess a proinflammatory role, these observations may be related to inflammation-induced alterations in EC cells and 5-HT signalling. In addition, SERT transcription is decreased in patients with UC as well as in patients with a recent history of diverticulitis [9,43]. These data support the notion that inflammation alters the normal 5-HT signalling cascade producing chronic IBS-like symptoms in addition to the direct effects of the inflammatory response. In addition, it has been shown recently that reduced expression of phospho-MEK, a downstream target of c-Raf, in neuroendocrine cells in the human colonic biopsies correlates with clinical responses in CD due to treatment with the anti-inflammatory small molecule semapimod, suggesting that neuroendocrine cells, which are important regulators of gut physiology, may be involved in the pathogenesis of human colonic inflammation [44]. Recently it has been shown that IL-1β and bacterial products [Escherichia coli lipopolysaccharide (LPS)] stimulated 5HT secretion from EC cells via Toll-like receptor (TLR) receptor activation (TLR-4 and IL-1β) of patients suffering from CD, implying that immune-mediated alterations in 5HT production may represent a component of the pathogenesis of abnormal bowel function in CD [45].
In the experimental models of colitis induced by trinitrobenzene sulphonic acid (TNBS), dinitrobenzenesulphonic acid (DNBS) and dextran sodium sulphate (DSS), an increase in 5-HT content has been observed [46–48]. By using the DNBS model of experimental colitis, we have shown an amelioration of colonic inflammation in monocyte chemoattractant protein-1-deficient mice in association with a reduction of EC cells [46]. Very recently it has been shown that the 5-HT3 antagonist tropisetron decreased colonic damage that was associated with decreased neutrophil infiltration, lipid peroxidation and colonic inflammatory cytokines in an acetic acid model of experimental colitis [49]. Experimental inflammation in animals induced by TNBS or infection with either T. spiralis or C. rodentium leads to down-regulation of SERT with a concomitant increase in EC cell number and/or 5-HT release, further supporting a role for 5-HT in inflammatory states [25,26,50]. Although these observations clearly show changes in EC cells and 5-HT during mucosal inflammation, it is unknown whether the change plays any role in regulating gut inflammation. It is very likely that the activated mucosal immune system in enteric inflammation influences 5-HT production, which subsequently plays an important role in the regulation of gut inflammation. 5-HT can regulate inflammation by acting on signalling pathways in inflammation, production of inflammatory mediators from immune cells and promoting interaction between innate and adaptive immune response.
Recently we have investigated the role of 5-HT in colonic inflammation in two different models of colitis (DSS and DNBS) using tryptophan hydroxylase1-deficient (TPH1−/−), mice, which have significantly reduced amounts of 5-HT in gut, and in mice treated with 5-HT synthesis inhibitor parachlorophenylalanine (PCPA) [37]. Delayed onset and decreased severity of colitis were observed in TPH1−/− mice compared to wild-type mice and in PCPA-treated mice after induction of colitis by DSS. This was associated with down-regulation of macrophage infiltration and production of proinflammatory cytokines. Restoration of 5-HT amounts in TPH1−/− mice by administration of 5-HT precursor 5-HTP enhanced the severity of DSS-induced colitis. We also observed a significant reduction in severity of colitis in TPH1−/− mice after induction of DNBS-colitis. Our data complement the recent study published by Bischoff et al., which demonstrated that TNBS-induced colitis is increased in severity when coupled with the 5-HT-enhancing effects by knock-out of SERT gene [51]. Recent studies from our laboratory also demonstrate that dendritic cells from TPH1−/− mice in DSS-colitis produced reduced IL-12 compared to TPH1+/+ mice and stimulation with 5-HT restored IL-12 production from the dendritic cells from naive TPH1−/− mice [52]. Taken together, these studies show a critical role of 5-HT in the pathogenesis of inflammation in gut by influencing proinflammatory cytokine production in experimental colitis and provide new insights into the mechanisms of gut inflammation. In a wider context, a beneficial effect with treatment with 5-HT receptor antagonist has been shown in both clinical and experimental arthritis [53], implicating a role of 5-HT in the pathogenesis of non-GI-inflammation in addition to GI inflammation.
The chromogranin/secretogranin family
As presented above, 5-HT is present throughout the GI tract and plays an important role in the regulation of the development of gut inflammation and various physiological activities in the gut. In addition to 5-HT, enteric endocrine cells produce the granins family [40] of biologically active products, which include Cgs A/B [54] and secretogranin, which can also contribute to various GI functions including immune modulation and inflammation.
The granin family consists of single-polypeptide chains of 185–657 amino acid residues. The numerous pairs of basic amino acids indicate a potential site for cleavage by prohormone convertases PC1/3 and PC2 in the secretory granules [55]. More than 10 different proteolytic sites have been identified in the CgA. For example, proteolytic processing of CgC by PCs reveals that both PC1 and PC2 can cleave the CgC precursor at sites of pairs of dibasic amino acid to yield intermediate-sized fragments, but only PC1 was capable of producing active neuropeptide secretoneurin (CgC 154–186). Moreover, other proteases have been indentified in chromaffines granules, including the neuroendocrine-specific carboxypeptidase E/H and the Lys/Arg-amino peptidases [55]. These data suggest that Cgs might serve as a prohormone for a shorter fragment having regulatory properties [56]. In the rat and human GI tract, the presence of cell- and tissue-specific processing of CgA has been shown [57–59], but very little is known about the functional role of Cgs in GI pathophysiology. Herein we will discuss the several data related to the role of Cgs in immune function and inflammation.
Cgs in immune activation
Due to the similarity of sequence with the cell-penetrating peptides family [60], Cgs-derived peptides such as chromofungin (CHR, bCgA 47–66) and vasostatin-I (VS-I, bCgA 1–76) are able to penetrate into polymorphonuclear neutrophils (PMNs), inducing an extracellular calcium entry by a CaM-regulated iPLA2 pathway. This study highlights the role of CgA-derived peptides in active communication between the neuroendocrine and immune systems [61]. Keeping within the endocrine–immune context, not only can the PMN be regulated by Cgs-derived peptides, but catestatin (CAT; bCgA 344–364) stimulates chemotaxis of human peripheral blood monocytes dose-dependently, exhibiting its maximal effect at a concentration of 1 nM comparable to the established chemoattractant-formulated peptide Met-Leu-Phe (fMLP) [62], suggesting a role of this peptide as an inflammatory mediator. In the same inflammatory context, secretoneurin reduces IL-16 release from eosinophils; this effect is in addition to that observed with granulocyte–macrophage colony-stimulating factors or IL-5. Results suggest that distinct neuropeptides are able to reduce the number of lymphocytes at inflammatory sites during existing eosinophilia by inhibiting the relaease of IL-16, thus attenuating the proinflammatory action of lymphocytes and monocytes. It has also been demonstrated that secretoneurin stimulates migration and cytokine release from human peripheral blood NK cells, implying that activation of this cell type by secretoneurin could affect the accumulation of these cells at loci of neurogenic inflammation [63]. A role for the neuropeptide on neutrophil adhesion and transmigration through a lung fibroblast barrier in vitro has also been shown [64].
Cgs-derived peptides can not only regulate the immune system during inflammation, but can also modulate the endothelial permeability during the inflammatory process, but the actual role of Cgs and derived peptide are not really clear. CgA prevents the vascular leakage induced by tumour necrosis factor (TNF)-α in a mouse model [65]. Studies of the mechanism of action show that CgA and its NH(2)-terminal fragments inhibit TNF-α-induced vascular permeability by preventing endothelial cytoskeleton rearrangements. It has been proposed that neuronal/endocrine secretion of CgA could contribute to the regulation of endothelial barrier function and the protection of vessels against plasma leakage in inflammatory diseases [65]. In keeping with the effects on angiogenesis induced by contact hypersensitivity reactions in mouse ears, VS-I-treated mice revealed significantly reduced oedema formation, resulting from lower plasma leakage and inhibition of inflammation-associated vascular remodelling [66]. Intravital microscopy studies of inflamed ears showed a decrease in the fraction of rolling leucocytes in VS-I-treated mice [66].
Cgs in gut inflammation
In addition to anti-microbial activity [67] Cgs may play important role in the neuroimmune interaction in relation to inflammatory function. This review will remain focused upon the function of Cgs in inflammatory responses in the gut. Circulating CgA levels, a marker for neuroendocrine tumours including carcinoids, have recently been found elevated in some patients with IBD [68]. In this context the disease activity and TNF-α levels influence the CgA pattern, which could reflect the neuroendocrine system activation in response to inflammation [69]. In a recent letter addressed to the aforementioned study, Sidhu and collaborators [70,71] confirmed the observation of Sciolia et al.[69] of an elevated level of CgA serum in both IBD and diarrhoea-predominant IBS patients. The unifying hypothesis proposed could be the EC cell hyperplasia producing an elevated serum CgA levels, as reported previously [72]. The differential replication of EC cells in IBS patients could also explain why elevated levels are found only in a proportion of patients, and levels decline with time. Further studies of serial serum CgA measurements in both these conditions would strengthen our understanding of the plausible mechanisms behind these observations.
In the context of experimental colitis, intrarectal injection of CAT can decrease the inflammatory markers [73]. Disease activity index, macroscopic and histological scores, as well as myeloperoxidase (MPO) activity, were decreased significantly in mice treated with CAT compared to mice that received DSS only. Treatment decreased the onset of clinical disease as assessed by loose stools, weight loss and rectal bleeding. In addition, colonic tissue levels of IL-1β, IL-6 and TNF-α were decreased significantly in mice treated with CAT. Conversely, the biochemically modified fragment had no effect on the severity of colitis. These results support the hypothesis that Cgs-derived peptides modulate intestinal inflammation in a murine model of colitis by acting directly or indirectly on the microbiota and the immune system. Identification of the molecular and cellular mechanisms underlying the protective role of this peptide may lead to a novel therapeutic option in IBD.
In addition to gut inflammation, the relation between TNF-α and CgA has been demonstrated in rheumatoid arthritis (RA), a disease well known to share some common features with IBD. Correlation between CgA and TNF receptor-I (TNFR-I) and TNFR-II has been evaluated in patients before the initiation of treatment with Infliximab® and compared it with the value calculated during treatment [74]. The authors observed a high correlation between CgA and both receptors. Moreover, they found that treatment with anti-TNF-α monoclonal antibodies (mAbs) abrogated the correlation between CgA and TNFR-I and TNFR-II, but it should be mentioned that in this study anti-TNF-α mAbs treatment did not modify the mean levels of CgA and TNFRs but led only to the abrogation of the correlation between CgA and TNFRs, implying that perhaps other indirect factors are associated in this effect. Three years later, the same group described that patients with RA have significantly higher serum levels of CgA and TNFRs compared with controls and that the highest levels of CgA identify the population of patients with extra-articular manifestations [74]. Taken together, these results suggest that CgA might be involved in the pathogenesis of inflammatory autoimmune disease through a complex interaction with TNF-α, mediated by as yet-undefined factors. In a series of papers Metz-Boutigue's group, who have published extensively on granins, showed a link between serum concentration of CgA and outcome in patients admitted with or without systemic inflammatory response syndrome. CgA concentrations were correlated positively with inflammation markers such as procalcitonin and C-reactive protein, but also with simplified acute physiological score (SAPS). A Cox model confirmed that CgA and SAPS were independent predictors of outcome [75,76]. In addition, a significant association has been reported between CgA level and periodontitis, again showing a close relationship between the level of CgA and the inflammatory process [77].
The hypothesis that Cgs-derived peptides are involved in mechanisms modulating altered colonic motility and visceral pain induced by gut inflammation was tested for the first time in 2004 using an application of acetic acid (AA) in vitro and in vivo. Using the writhing test, a model of somato-visceral pain, we have demonstrated that depending upon the Cgs-derived peptides used (bCgA 4–16, 47–66), they could display pro- and anti-nocicpetive effects [78,79]. In the context of smooth muscle contraction, Cgs-derived peptides modulate the effect of AA on human and rat smooth muscle contraction via a direct action on the calcium L-type channel or towards an indirect action through the enteric nervous system (motorneurone and type-C sensitive fibre) [80,81].
All these data provide proof of concept that Cgs and Cgs-derived peptides seem to play an important role in the development of inflammatory pathologies, and different groups have now focused their attention upon characterizing a mechanistic explanation.
Conclusion
The studies discussed in this review provide evidence in favour of a key role of gut hormones in intestinal inflammation. In addition to the contribution in GI physiology, such as motility and secretion, gut hormones can also play an important role in immune activation and in the generation of inflammation in gut. The precise mechanisms by which gut hormones regulate the inflammation remain to be determined. The data generated from the studies on 5-HT in gut inflammation suggest strongly that increased 5-HT released by luminal inflammatory stimuli can activate immune cells such as macrophages, dendritic cells, lymphocytes and enteric nerves via specific 5-HT receptors, which can enhance the production of proinflammatory mediators via triggering activation of the NF-κB pathway and/or other possible proinflammatory signalling systems, and which subsequently can up-regulate the inflammatory response (Fig. 1). It will be interesting to see roles of specific 5-HT receptor subtype(s) in immune activation and generation of intestinal inflammation.
Fig. 1.
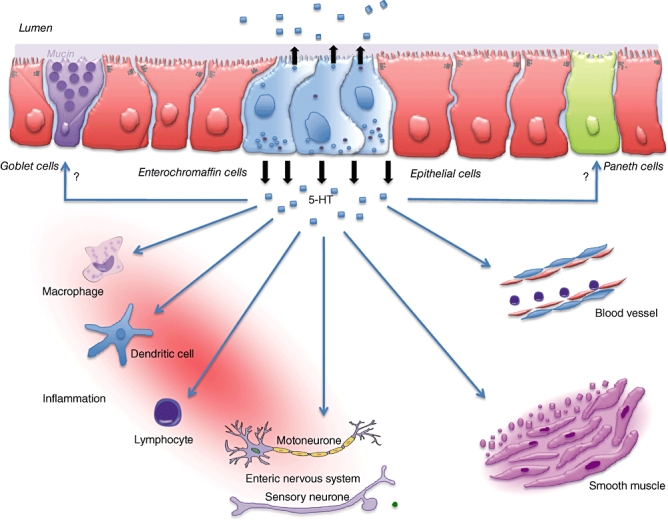
Putative role of 5-hyroxytryptophan (5-HT) in immune activation and inflammation. 5-HT released from enterochromaffin cells in response to luminal stimuli (chemical, mechanical or microbial) can act on innate immune cells such as macrophages and dendritic cells to activate proinflammatory cytokine production and can also influence interaction between innate immune and adaptive immune cells to promote inflammation. 5-HT can also act directly on goblet cells to induce mucin production, and on smooth muscle and nerves to alter gut motility.
The role of Cgs in inflammation is not as clear at present, as it is with 5-HT; however, the available data suggest that it is an important and interesting area for further exploration. Cgs can interact with immune cells to increase or decrease in proinflammatory mediators such as TNF-α, IL-1β and IL-6 (Fig. 2), depending upon the signals that initiate the inflammation, the site of inflammation and the type of peptide. It will be interesting to determine whether experimental modulation in the amount of Cgs has any effect on immune activation and the generation of inflammation in gut and in other parts of the body. In addition, it seems possible that 5-HT and Cgs systems can interact with each other in the context of inflammation. Neuroendocrine secretory protein of Mr 55 000 (NESP55), a novel member of Cgs, has been identified recently as an endogenous antagonist of the serotonergic 5-HT1B receptor subtype [82]. As alteration in the serotonergic system is considered to play an important role in inflammatory response, it is alluring to speculate that Cgs may contribute to the inflammatory mechanism by modulating the 5-HT response.
Fig. 2.
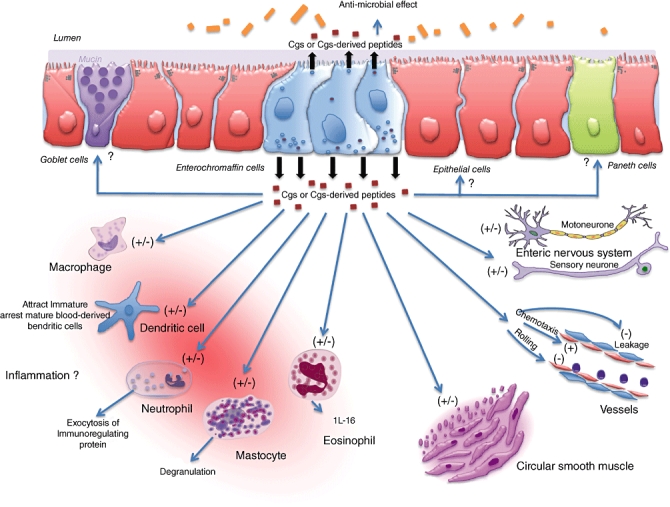
Putative role of chromogranins (Cgs) in immune activation and inflammation. Luminal or internal inflammatory stimuli causes alteration in Cgs or Cgs-derived peptides release. They may act locally on paneth, globet and epithelial cells as well as on immune cells, such as macrophages, dendritic cells, neutrophil, mastocytes and eosinophils. Endothelial permeability, chemotaxis, rolling, smooth muscle contractility and the enteric nervous system can also be modulated. IL: interleukin; (−) inhibition; (+) activation.
These studies provide novel information on the role of gut hormones in immune signalling and regulation of gut inflammation. Despite being a challenging and complicated area to explore, recent studies on immunoendocrine interaction has generated new interest to elucidate the role of gut hormones in the inflammatory process and immune function. In addition to enhancing our understanding on the pathogenesis of inflammatory changes, these studies give new information on 5-HT and Cgs in the context of immunoendocrine interactions in gut and intestinal homeostasis. This is very important, due not only to the alteration in enteric endocrine cells functions observed in various GI inflammatory conditions but also in non-GI inflammatory disorders and functional GI disorders such as IBS. These data may have implications in understanding the role of gut hormone in the pathogenesis of both GI and non-GI inflammation, which may lead ultimately to improved therapeutic strategies in inflammatory disorders.
Acknowledgments
Supported by grants from the Crohn's and Colitis Foundation of Canada (CCFC) and by the Canadian Institutes of Health Research (CIHR) to Dr Waliul I. Khan.
Disclosure
None.
References
- 1.Miller LJ. Gastrointestinal hormones and receptors. In: Yamada T, Alpers DH, Laine L, Owyang C, Powell DW, editors. Textbook of gastroenterology. 3rd edn. Philadelphia, PA: Lippincott-Williams and Wilkins; 1999. pp. 35–66. [Google Scholar]
- 2.Rindi G, Kloppel G. Endocrine tumors of the gut and pancreas tumor biology and classification. Neuroendocrinology. 2004;80(Suppl. 1):12–15. doi: 10.1159/000080733. [DOI] [PubMed] [Google Scholar]
- 3.Gershon MD. Review article. Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13(Suppl. 2):15–30. [PubMed] [Google Scholar]
- 4.Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol. 2002;90:109–20. doi: 10.1034/j.1600-0773.2002.900301.x. [DOI] [PubMed] [Google Scholar]
- 5.Sharkey KA, Mawe GM. Neuroimmune and epithelial interactions in intestinal inflammation. Curr Opin Pharmacol. 2002;2:669–77. doi: 10.1016/s1471-4892(02)00215-1. [DOI] [PubMed] [Google Scholar]
- 6.Ahonen A, Kyosola K, Penttila O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976;8:1–7. [PubMed] [Google Scholar]
- 7.Belai A, Boulos PB, Robson T, Burnstock G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut. 1997;40:767–74. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–40. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 9.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Grondahl ML, Jensen GM, Nielsen CG, Skadhauge E, Olsen JE, Hansen MB. Secretory pathways in Salmonella typhimurium-induced fluid accumulation in the porcine small intestine. J Med Microbiol. 1998;47:151–7. doi: 10.1099/00222615-47-2-151. [DOI] [PubMed] [Google Scholar]
- 11.Kordasti S, Sjovall H, Lundgren O, Svensson L. Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut. 2004;53:952–7. doi: 10.1136/gut.2003.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore BA, Sharkey KA, Mantle M. Role of 5-HT in cholera toxin-induced mucin secretion in the rat small intestine. Am J Physiol. 1996;270:G1001–9. doi: 10.1152/ajpgi.1996.270.6.G1001. [DOI] [PubMed] [Google Scholar]
- 13.Sjolund K, Alumets J, Berg NO, Hakanson R, Sundler F. Enteropathy of coeliac disease in adults: increased number of enterochromaffin cells the duodenal mucosa. Gut. 1982;23:42–8. doi: 10.1136/gut.23.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulich TR, Cheng L, Glover H, Yang K, Lewin KJ. A colonic adenocarcinoma with argentaffin cells. An immunoperoxidase study demonstrating the presence of numerous neuroendocrine products. Cancer. 1983;51:1483–9. doi: 10.1002/1097-0142(19830415)51:8<1483::aid-cncr2820510822>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Cetin Y, Kuhn M, Kulaksiz H, et al. Enterochromaffin cells of the digestive system: cellular source of guanylin, a guanylate cyclase-activating peptide. Proc Natl Acad Sci USA. 1994;91:2935–9. doi: 10.1073/pnas.91.8.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchan AM. Nutrient tasting and signaling mechanisms in the Gut III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999;277:G1103–7. doi: 10.1152/ajpgi.1999.277.6.G1103. [DOI] [PubMed] [Google Scholar]
- 17.Kim DY, Camilleri M. Serotonin: a mediator of the brain–gut connection. Am J Gastroenterol. 2000;95:2698–709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–81. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- 19.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–80. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 20.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 21.Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2009;153:47–57. doi: 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52:1–30. [PubMed] [Google Scholar]
- 23.Wang H, Steeds J, Motomura Y, et al. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–57. doi: 10.1136/gut.2006.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motomura Y, Ghia JE, Wang H, et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008;57:475–81. doi: 10.1136/gut.2007.129296. [DOI] [PubMed] [Google Scholar]
- 25.O'Hara JR, Skinn AC, MacNaughton WK, Sherman PM, Sharkey KA. Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol. 2006;8:646–60. doi: 10.1111/j.1462-5822.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 26.Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–70. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 27.Gershon MD. Serotonin and its implication for the management of irritable bowel syndrome. Rev Gastroenterol Disord. 2003;3(Suppl. 2):S25–34. [PubMed] [Google Scholar]
- 28.Nakajima M, Shiihara Y, Shiba Y, et al. Effect of 5-hydroxytryptamine on gastrointestinal motility in conscious guinea-pigs. Neurogastroenterol Motil. 1997;9:205–14. doi: 10.1046/j.1365-2982.1997.d01-56.x. [DOI] [PubMed] [Google Scholar]
- 29.Ladabaum U. Safety, efficacy and costs of pharmacotherapy for functional gastrointestinal disorders: the case of alosetron and its implications. Aliment Pharmacol Ther. 2003;17:1021–30. doi: 10.1046/j.1365-2036.2003.01545.x. [DOI] [PubMed] [Google Scholar]
- 30.Spiller R. Serotonin, inflammation, and IBS: fitting the jigsaw together? J Pediatr Gastroenterol Nutr. 2007;45(Suppl. 2):S115–19. doi: 10.1097/MPG.0b013e31812e66da. [DOI] [PubMed] [Google Scholar]
- 31.Cloez-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc Biol. 2007;81:599–606. doi: 10.1189/jlb.0906544. [DOI] [PubMed] [Google Scholar]
- 32.Yang GB, Lackner AA. Proximity between 5 and HT secreting enteroendocrine cells and lymphocytes in the gut mucosa of rhesus macaques (Macaca mulatta) is suggestive of a role for enterochromaffin cell 5-HT in mucosal immunity. J Neuroimmunol. 2004;146:46–9. doi: 10.1016/j.jneuroim.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 33.Stefulj J, Cicin-Sain L, Schauenstein K, Jernej B. Serotonin and immune response: effect of the amine on in vitro proliferation of rat lymphocytes. Neuroimmunomodulation. 2001;9:103–8. doi: 10.1159/000049013. [DOI] [PubMed] [Google Scholar]
- 34.Betten A, Dahlgren C, Hermodsson S, Hellstrand K. Serotonin protects NK cells against oxidatively induced functional inhibition and apoptosis. J Leukoc Biol. 2001;70:65–72. [PubMed] [Google Scholar]
- 35.Laberge S, Cruikshank WW, Beer DJ, Center DM. Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J Immunol. 1996;156:310–15. [PubMed] [Google Scholar]
- 36.Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol. 2007;127:1947–55. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- 37.Ghia JE, Li N, Wang H, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–60. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 38.Leon-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139–46. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bishop AE, Pietroletti R, Taat CW, Brummelkamp WH, Polak JM. Increased populations of endocrine cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol. 1987;410:391–6. doi: 10.1007/BF00712758. [DOI] [PubMed] [Google Scholar]
- 40.El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–19. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 41.Simren M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Bjornsson ES. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389–96. doi: 10.1111/j.1572-0241.2002.05475.x. [DOI] [PubMed] [Google Scholar]
- 42.Minderhoud IM, Oldenburg B, Schipper ME, ter Linde JJ, Samsom M. Serotonin synthesis and uptake in symptomatic patients with Crohn's disease in remission. Clin Gastroenterol Hepatol. 2007;5:714–20. doi: 10.1016/j.cgh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Costedio MM, Coates MD, Danielson AB, et al. Serotonin signaling in diverticular disease. J Gastrointest Surg. 2008;12:1439–45. doi: 10.1007/s11605-008-0536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowenberg M, Verhaar A, van den Blink B, et al. Specific inhibition of c-Raf activity by semapimod induces clinical remission in severe Crohn's disease. J Immunol. 2005;175:2293–300. doi: 10.4049/jimmunol.175.4.2293. [DOI] [PubMed] [Google Scholar]
- 45.Kidd M, Gustafsson BI, Drozdov I, Modlin IM. IL1beta- and LPS-induced serotonin secretion is increased in EC cells derived from Crohn's disease. Neurogastroenterol Motil. 2009;21:439–50. doi: 10.1111/j.1365-2982.2008.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan WI, Motomura Y, Wang H, et al. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G803–11. doi: 10.1152/ajpgi.00069.2006. [DOI] [PubMed] [Google Scholar]
- 47.Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–16. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 48.Oshima S, Fujimura M, Fukimiya M. Changes in number of serotonin-containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextran sodium sulfate. Histochem Cell Biol. 1999;112:257–63. doi: 10.1007/s004180050445. [DOI] [PubMed] [Google Scholar]
- 49.Mousavizadeh K, Rahimian R, Fakhfouri G, Aslani FS, Ghafourifar P. Anti-inflammatory effects of 5-HT receptor antagonist, tropisetron on experimental colitis in rats. Eur J Clin Invest. 2009;39:375–83. doi: 10.1111/j.1365-2362.2009.02102.x. [DOI] [PubMed] [Google Scholar]
- 50.Linden DR, Foley KF, McQuoid C, Simpson J, Sharkey KA, Mawe GM. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–74. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 51.Bischoff SC, Mailer R, Pabst O, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–95. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 52.Ghia JE, Nan L, Wang H, et al. Role of serotonin in immune activation and inflammation in experimental colitis. Gastroenterology. 2009;136:A397. [Google Scholar]
- 53.Hrycaj P. Serotonin type 3 receptor antagonist tropisetron in the treatment of chronic inflammatory rheumatic conditions – preliminary clinical experience. Scand J Rheumatol Suppl. 2004;119:55–8. doi: 10.1080/03009740410007069. [DOI] [PubMed] [Google Scholar]
- 54.Buffa R, Mare P, Gini A, Salvadore M. Chromogranins A and B and secretogranin II in hormonally identified endocrine cells of the gut and the pancreas. Basic Appl Histochem. 1988;32:471–84. [PubMed] [Google Scholar]
- 55.Seidah NG, Chretien M. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848:45–62. doi: 10.1016/s0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- 56.Eiden LE. Is chromogranin a prohormone? Nature. 1987;325:301. doi: 10.1038/325301a0. [DOI] [PubMed] [Google Scholar]
- 57.Curry WJ, Johnston CF, Hutton JC, et al. The tissue distribution of rat chromogranin A-derived peptides: evidence for differential tissue processing from sequence specific antisera. Histochemistry. 1991;96:531–8. doi: 10.1007/BF00267079. [DOI] [PubMed] [Google Scholar]
- 58.Portela-Gomes GM, Stridsberg M. Selective processing of chromogranin A in the different islet cells in human pancreas. J Histochem Cytochem. 2001;49:483–90. doi: 10.1177/002215540104900408. [DOI] [PubMed] [Google Scholar]
- 59.Portela-Gomes GM, Stridsberg M. Chromogranin A in the human gastrointestinal tract: an immunocytochemical study with region-specific antibodies. J Histochem Cytochem. 2002;50:1487–92. doi: 10.1177/002215540205001108. [DOI] [PubMed] [Google Scholar]
- 60.Henriques ST, Melo MN, Castanho MA. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem J. 2006;399:1–7. doi: 10.1042/BJ20061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang D, Shooshtarizadeh P, Laventie BJ, et al. Two chromogranin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS One. 2009;4:e4501. doi: 10.1371/journal.pone.0004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Egger M, Beer AG, Theurl M, et al. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol. 2008;598:104–11. doi: 10.1016/j.ejphar.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 63.Feistritzer C, Mosheimer BA, Colleselli D, Wiedermann CJ, Kahler CM. Effects of the neuropeptide secretoneurin on natural killer cell migration and cytokine release. Regul Pept. 2005;126:195–201. doi: 10.1016/j.regpep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Kahler CM, Pischel A, Kaufmann G, Wiedermann CJ. Influence of neuropeptides on neutrophil adhesion and transmigration through a lung fibroblast barrier in vitro. Exp Lung Res. 2001;27:25–46. doi: 10.1080/019021401459752. [DOI] [PubMed] [Google Scholar]
- 65.Ferrero E, Magni E, Curnis F, Villa A, Ferrero ME, Corti A. Regulation of endothelial cell shape and barrier function by chromogranin A. Ann NY Acad Sci. 2002;971:355–8. doi: 10.1111/j.1749-6632.2002.tb04495.x. [DOI] [PubMed] [Google Scholar]
- 66.Huegel R, Velasco P, De la Luz Sierra M, et al. Novel anti-inflammatory properties of the angiogenesis inhibitor vasostatin. J Invest Dermatol. 2007;127:65–74. doi: 10.1038/sj.jid.5700484. [DOI] [PubMed] [Google Scholar]
- 67.Shooshtarizadeh P, Zhang D, Chich JF, et al. The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul Pept. 2009 doi: 10.1016/j.regpep.2009.11.014. doi: 10.1016/j.regpep.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 68.Conlon JM. Granin-derived peptides as diagnostic and prognostic markers for endocrine tumors. Regul Pept. 2009 doi: 10.1016/j.regpep.2009.11.013. doi: 10.1016/j.regpep.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 69.Sciola V, Massironi S, Conte D, et al. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:867–71. doi: 10.1002/ibd.20851. [DOI] [PubMed] [Google Scholar]
- 70.Sidhu R, Drew K, McAlindon ME, Lobo AJ, Sanders DS. Elevated serum chromogranin A in irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD): a shared model for pathogenesis? Inflamm Bowel Dis. 2009;16:361. doi: 10.1002/ibd.20982. [DOI] [PubMed] [Google Scholar]
- 71.Sidhu R, McAlindon ME, Leeds JS, Skilling J, Sanders DS. The role of serum chromogranin A in diarrhoea predominant irritable bowel syndrome. J Gastrointest Liver Dis. 2009;18:23–6. [PubMed] [Google Scholar]
- 72.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–9. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 73.Park AJ, Metz-Boutigue MH, Collins SM, Ghia J-E. Inhibitory influence of catestatin a chromogranin A-derived peptide in experimental colitis. Can J Gastroenterol. 2009;23:254. [Google Scholar]
- 74.di Comite G, Marinosci A, Di Matteo P, et al. Neuroendocrine modulation induced by selective blockade of TNF-alpha in rheumatoid arthritis. Ann NY Acad Sci. 2006;1069:428–37. doi: 10.1196/annals.1351.041. [DOI] [PubMed] [Google Scholar]
- 75.Zhang D, Lavaux T, Sapin R, et al. Serum concentration of chromogranin A at admission: an early biomarker of severity in critically ill patients. Ann Med. 2009;41:38–44. doi: 10.1080/07853890802199791. [DOI] [PubMed] [Google Scholar]
- 76.Zhang D, Lavaux T, Voegeli AC, et al. Prognostic value of chromogranin A at admission in critically ill patients: a cohort study in a medical intensive care unit. Clin Chem. 2008;54:1497–503. doi: 10.1373/clinchem.2007.102442. [DOI] [PubMed] [Google Scholar]
- 77.Hironaka M, Ansai T, Soh I, et al. Association between salivary levels of chromogranin A and periodontitis in older Japanese. Biomed Res. 2008;29:125–30. doi: 10.2220/biomedres.29.125. [DOI] [PubMed] [Google Scholar]
- 78.Ghia JE, Crenner F, Metz-Boutigue MH, Aunis D, Angel F. Effects of a chromogranin-derived peptide (CgA 47-66) in the writhing nociceptive response induced by acetic acid in rats. Regul Pept. 2004;119:199–207. doi: 10.1016/j.regpep.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 79.Ghia JE, Crenner F, Metz-Boutigue MH, Aunis D, Angel F. The effect of a chromogranin A-derived peptide (CgA4-16) in the writhing nociceptive response induced by acetic acid in rats. Life Sci. 2004;75:1787–99. doi: 10.1016/j.lfs.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 80.Ghia JE, Crenner F, Rohr S, et al. A role for chromogranin A (4-16), a vasostatin-derived peptide, on human colonic motility. An in vitro study. Regul Pept. 2004;121:31–9. doi: 10.1016/j.regpep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Ghia JE, Pradaud I, Crenner F, Metz-Boutigue MH, Aunis D, Angel F. Effect of acetic acid or trypsin application on rat colonic motility in vitro and modulation by two synthetic fragments of chromogranin A. Regul Pept. 2005;124:27–35. doi: 10.1016/j.regpep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 82.Ischia R, Lovisetti-Scamihorn P, Hogue-Angeletti R, Wolkersdorfer M, Winkler H, Fischer-Colbrie R. Molecular cloning and characterization of NESP55, a novel chromogranin-like precursor of a peptide with 5-HT1B receptor antagonist activity. J Biol Chem. 1997;272:11657–62. doi: 10.1074/jbc.272.17.11657. [DOI] [PubMed] [Google Scholar]


