Abstract
Strong genetic contribution has been demonstrated to influence the development of autoimmune thyroid disease (AITD) as well as thyroid autoantibody production. In order to assess the relation between CT60 cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphism and thyroid autoantibody production, we investigated 180 consecutive newly diagnosed patients with two forms of AITD, 105 with Hashimoto's thyroiditis (HT) and 75 with postpartum thyroiditis (PPT). We evaluated thyroid function, measured antibodies against thyroid peroxidase (TPO) and thyroglobulin (Tg), and determined CT60 CTLA-4 gene polymorphism. In HT, TPO antibody median value was significantly lower in the AA compared to the AG and GG genotypes (65, 122 and 319 U/ml, P < 0·005), while the Tg antibody median value was lower in the AA compared to the AG genotype (91 and 189 U/ml, P < 0·02). In PPT, the frequency of thyroid autoantibody-positive patients was higher among G-allele-carrying genotypes (P < 0·04). Similar to HT, the TPO antibody median value was lower in the AA compared to the AG and GG genotypes (12, 130 and 423 U/ml, P < 0·006). Hypothyroid PPT patients were more often thyroid autoantibody-positive (P < 0·005) and the TPO antibody median value was higher compared to hyperthyroid PPT patients (500 and 32 U/ml, P < 0·0001). The frequency of the G-allele was significantly higher among hypothyroid patients (P < 0·05). Our data suggest that in both HT and PPT, the CT60 CTLA-4 gene polymorphism contributes importantly to thyroid autoantibody production. In PPT, the genotype also seems to influence thyroid function, as patients with the polymorphous allele are more prone to develop hypothyroid form of PPT.
Keywords: CT60, CTLA-4, Hashimoto's thyroiditis, postpartum thyroiditis, thyroid autoantibodies
Introduction
The presence of circulating autoantibodies against major thyroid antigens is the hallmark of thyroid autoimmunity, which comprises several different clinical forms, including Hashimoto's thyroiditis (HT) and postpartum thyroiditis (PPT). In HT, the antibodies against thyroid peroxidase or thyroglobulin (Tg) appear characteristically in the patients' sera, while tissue damage due to T cell-mediated cytotoxicity usually contributes to gradual development of hypothyroidism [1]. In PPT, where the re-establishment of immune responsiveness after delivery leads to thyroid dysfunction in the first year postpartum, two-thirds of females present with positive thyroid peroxidase antibodies, putting them at risk for developing a hypothyroid form of PPT and permanent hypothyroidism. Thyroid peroxidase antibody-negative PPT patients are more likely to experience only a phase of transient hyperthyroidism and 1 year postpartum the euthyroid state is usually restored [2].
Similar to autoimmune thyroid disease (AITD), strong genetic susceptibility is required for the production of thyroid autoantibodies [3]. According to an estimation based on Danish twin pairs, the genetic background contributes 73% to the predisposition to thyroid autoantibody production [4]. Moreover, an earlier performed whole genome linkage study demonstrated the cytotoxic T lymphocyte antigen-4 (CTLA-4) gene to be most probably the putative thyroid autoantibody susceptibility gene [5]. Also, our recent investigation of patients with HT provided evidence that both -318C/T promoter and 49A/G exon 1 CTLA-4 gene single nucleotide polymorphisms (SNPs) were associated with higher thyroid autoantibody concentrations, confirming its important role in thyroid autoantibody production [6]. In the CTLA-4 gene additional polymorphisms were described, among which the CT60 SNP in the 3′-untranslated region was found to affect the efficiency of splicing with reduced production of soluble CTLA-4 [7]. In spite of being associated strongly with AITD [8], the influence of CT60 SNP on thyroid autoantibody production has not been determined until now. Therefore, the objective of the present study was to evaluate the association of CT60 CTLA-4 SNP with thyroid autoantibody production in patients with two different forms of autoimmune thyroid disease, HT and PPT.
Materials and methods
Patients
A total of 180 Caucasian patients from Slovenia were recruited consecutively, including 105 patients with HT and 75 patients with PPT. All patients were newly diagnosed and had been evaluated prior to initiation of treatment. Among HT patients, 96 females and nine males, aged between 17 and 83 (mean 51·1 ± 16·8) years, were investigated. The inclusion criteria were subclinical or clinical and biochemical hypothyroidism, the presence of thyroid peroxidase antibodies and/or thyroglobulin antibodies and characteristic hypoechoic thyroid ultrasound (US) pattern. In females with PPT, aged between 21 and 42 (mean, 30·4 ± 4·7) years, thyroid dysfunction occurred in the first year postpartum. Hyperthyroidism was diagnosed in patients with suppressed thyroid stimulating hormone (TSH) and normal or elevated free thyroid hormones; the mean time from the delivery to diagnosis was 5·5 ± 2·2 months. Hypothyroidism was confirmed in patients with elevated TSH and normal or decreased free thyroid hormones; the mean time from the delivery to diagnosis was 7·1 ± 2·6 months. The patients presented with normal or hypoechoic US pattern, most of them were positive for thyroid peroxidase antibodies or thyroglobulin antibodies. Patients with positive TSH receptor stimulating antibodies, which are distinctive of Graves' disease, were excluded from the study. In all patients, the data on family history of AITD and cigarette smoking were obtained.
TSH was measured by commercially available chemiluminescent immunoassay kit (TSH-3; Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA; reference range, 0·35–5·5 mU/l). Thyroid peroxidase antibodies and thyroglobulin antibodies were determined using commercially available enzyme-linked immunosorbent assay kit (ETI-AB-TPOK and ETI-AB-HTGK; Dia Sorin, Saluggia, Vercelli, Italy; positive value, above 15 U/ml and above 100 U/ml, respectively). The study was approved by the local ethical committee, while the subjects signed a written informed consent form.
Genotyping
DNA was prepared from 2 ml of whole blood using the commercially available DNA Isolation kit (FlexiGene DNA kit; Qiagen, Hilden, Germany) following the manufacturer's instructions. Each patient was genotyped for CT60 CTLA-4 polymorphism.
CTLA-4 CT60 polymorphism analysis
CT60 polymorphism was detected using technology Taqman Assay By Design (Applied Biosystems, Carlsbad, CA, USA). A 200 base pairs-long sequence containing A6230G (CT60) polymorphism was amplified in real-time polymerase chain reaction (RT–PCR) using specific primers, forward 5′-CCATCCTCTTTCCTTTTGATTTCTT-3′ and reverse 5′-GTTAAACAGCATGCCAATTGATTT-3′, and the Taqman MGB probes, Fam-AACCCATGTTATATCC and Vic-ACCCACGTTATATCC for the recognition of A and G allele, respectively. The reaction was performed in a final volume of 25 µl containing 200 ng of genomic DNA, 0·9 µM of each primer, 0·25 µM of each probe and TaqMan universal PCR master mix (Thermo Fisher Scientific, Abgene, Epsom, UK). After incubation at 95°C for 10 min, 40 cycles of 15 s at 95°C and 1 min at 60°C, individual genotypes were established using ABI Prism 7000 Sequence Detection System (Applied Biosystems, Carlsbad, CA, USA) and sds version 1·1 software.
Statistical analysis
We compared various parameters in HT and PPT patients carrying different CT60 CTLA-4 genotypes, and in PPT patients with different thyroid function. Hardy–Weinberg equilibrium (HWE) for genotype distribution was calculated using the χ2 test. The clinical characteristics and median values of thyroid peroxidase antibodies and thyroglobulin antibodies were analysed using the non-parametric Kruskal–Wallis analysis of variance (anova) test. We used the χ2 test to compare the distribution of patients being either positive or negative for thyroid autoantibodies. Multiple logistic regression analysis was applied in order to analyse the independent effect of genetic and non-genetic factors on the development of thyroid autoantibodies, and on thyroid function in PPT patients. Statistical analysis was performed using statistica software (StatSoft, Tulsa, OK, USA). P-values of <0·05 were considered significant.
Results
The level of thyroid autoantibodies in HT patients regarding CT60 genotypes
With genotyping of 105 HT patients we established the AA genotype in 22 (20·9%) patients, the AG genotype in 47 patients (44·8%) and the GG genotype in 36 patients (34·3%), indicating that the distribution was in HWE (χ2 0·823, P = 0·364). The groups of patients carrying different genotypes did not differ significantly with regard to their age, TSH concentration, family history of AITD, smoking status or the proportion of thyroid peroxidase antibody positivity, while the proportion of thyroglobulin antibody-positive patients was significantly higher in AG genotype (Table 1). However, compared to the AA genotype, groups with the AG and GG genotypes presented with significantly higher median values of thyroid peroxidase antibodies (median, 65, 122 and 319 U/ml, respectively; P < 0·005) (Fig. 1a). Comparing the median values of thyroglobulin antibodies, we found a significantly lower concentration in the group with the AA genotype compared to the AG genotype (median, 91 and 189, respectively; P < 0·02), while the thyroglobulin antibody median value in the GG genotype was not significantly higher (median, 79·5 U/ml) (Fig. 1b).
Table 1.
Characteristics of patients with Hashimoto's thyroiditis and the presence of thyroglobulin antibodies and thyroid peroxidase antibodies in relation to the CT60 cytotoxic T lymphocyte antigen-4 (CTLA-4) genotype.
| Characteristics | AA (n = 22) | AG (n = 47) | GG (n = 36) | P-value |
|---|---|---|---|---|
| Sex (male/female) | 0/22 | 5/42 | 4/32 | |
| Age, mean ± s.d. (years) | 54·5 ± 16·5 | 51·0 ± 17·0 | 49·2 ± 16·8 | 0·448 |
| Family history of AITD (yes/no) | 9/13 | 19/28 | 16/20 | 0·930 |
| Cigarette smoking (yes/no) | 5/17 | 12/35 | 10/26 | 0·913 |
| TSH, mean ± s.d. (mU/l) | 10·1 ± 7·9 | 22·1 ± 28·1 | 24·2 ± 37·1 | 0·255 |
| Thyroid peroxidase antibody-positive | 21 (95·5%) | 42 (89·4%) | 35 (97·2%) | 0·328 |
| Thyroid peroxidase antibody-negative | 1 (4·5%) | 5 (10·6%) | 1 (2·8%) | |
| Thyroglobulin antibody-positive | 10 (45·5%) | 36 (76·6%) | 14 (38·9%) | <0·05 |
| Thyroglobulin antibody-negative | 12 (54·5%) | 11 (23·4%) | 22 (61·1%) |
Kruskal–Wallis analysis of variance test for clinical characteristics analysis, χ2 test for comparison of the distribution of patients being positive or negative for thyroid peroxidase antibodies or thyroglobulin antibodies. AITD, autoimmune thyroid disease; s.d., standard deviation.
Fig. 1.
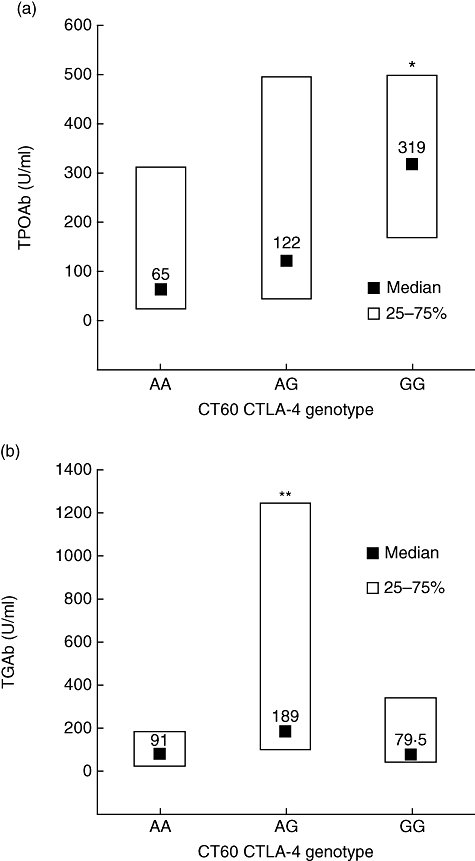
The relation of CT60 cytotoxic T lymphocyte antigen-4 (CTLA-4) polymorphism with the concentration of thyroid peroxidase antibodies (TPOAb) (a) and thyroglobulin antibodies (TGAb) (b) in patients with newly diagnosed Hashimoto's thyroiditis. Data are median values and quartiles. *P < 0·002 compared with the AA genotype and P < 0·04 compared with the AG genotype, **P < 0·02 compared with the AA genotype (Kruskal–Wallis analysis of variance test).
Using multiple regression analysis, we evaluated independent effects of genetic and non-genetic factors on the development of thyroid autoantibodies. The reference categories for the analysis were CT60 CTLA-4 genotype, age, family history of AITD and cigarette smoking. In the case of thyroid peroxidase antibodies, we confirmed a significant contribution of CT60 CTLA-4 genotype (P < 0·007) and younger age (P < 0·05), while family history and cigarette smoking did not prove to have any effect. In thyroglobulin antibodies, no contribution of either genotype or non-genetic factors was confirmed.
The level of thyroid autoantibodies in PPT patients regarding CT60 genotypes
The genotyping in the group of 75 PPT patients revealed the AA genotype in 17 (22·7%) patients, the AG genotype in 36 (48%) and the GG genotype in 22 (29·3%) patients, showing no deviation from HWE (χ2 0·096, P = 0·757). As presented in Table 2, the patients with different genotypes did not differ in age, number of pregnancies, family history of AITD and smoking status. However, females with the G-allele carrying genotypes presented significantly more often with positive values of thyroid peroxidase antibodies (P < 0·04), while the proportion of thyroglobulin antibody-positive patients did not differ significantly between the three genotypes. Similarly, more patients with the G-allele carrying genotypes had at least one type of thyroid autoantibody elevated compared to the AA genotype (P < 0·04) (Table 2). Furthermore, the median value of thyroid peroxidase antibodies was significantly lower in the AA genotype compared to the AG and GG genotypes (median, 12, 130 and 423 U/ml, respectively, P < 0·006) (Fig. 2a). In contrast to thyroid peroxidase antibodies, the median values of thyroglobulin antibodies did not differ significantly between the three genotypes (Fig. 2b).
Table 2.
Characteristics of patients with postpartum thyroiditis and the presence of thyroglobulin antibodies and thyroid peroxidase antibodies in relation to the CT60 cytotoxic T lymphocyte antigen-4 (CTLA-4) genotype.
| Characteristics | AA (n = 17) | AG (n = 36) | GG (n = 22) | P-value |
|---|---|---|---|---|
| Age, mean ± s.d. (years) | 30·3 ± 5·2 | 31·1 ± 4·8 | 29·0 ± 3·3 | 0·238 |
| Number of pregnancies, mean ± s.d. | 1·94 ± 0·97 | 1·86 ± 1·07 | 1·77 ± 0·75 | 0·998 |
| Family history of AITD (yes/no) | 7/13 | 15/21 | 9/10 | 0·981 |
| Cigarette smoking (yes/no) | 6/11 | 6/30 | 3/19 | 0·485 |
| Thyroid peroxidase antibody-positive | 8 (47·1%) | 28 (77·8%) | 18 (81·8%) | <0·04 |
| Thyroid peroxidase antibody-negative | 9 (52·9%) | 8 (22·2%) | 4 (18·2%) | |
| Thyroglobulin antibody-positive | 7 (41·2%) | 26 (72·2%) | 15 (68·2%) | 0·079 |
| Thyroglobulin antibody-negative | 10 (58·8%) | 10 (27·8%) | 7 (31·8%) | |
| Thyroid peroxidase antibody and thyroglobulin antibody-positive | 3 (17·7%) | 22 (61·1%) | 13 (59·1%) | <0·04 |
| Thyroid peroxidase antibody or thyroglobulin antibody-positive | 9 (52·9%) | 10 (27·8%) | 7 (31·8%) | |
| Thyroid peroxidase antibody and thyroglobulin antibody-negative | 5 (29·4%) | 4 (11·1%) | 2 (9·1%) |
Kruskal–Wallis analysis of variance test for clinical characteristics analysis, χ2 test for comparison of the distribution of patients being positive or negative for thyroid peroxidase antibodies or thyroglobulin antibodies. AITD, autoimmune thyroid disease; s.d., standard deviation.
Fig. 2.
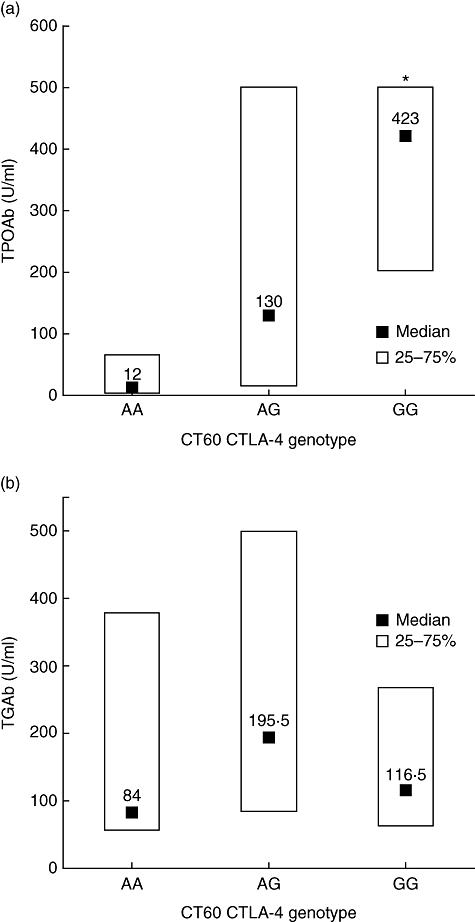
The relation of CT60 cytotoxic T lymphocyte antigen-4 (CTLA-4) polymorphism with the concentration of thyroid peroxidase antibodies (TPOAb) (a) and thyroglobulin antibodies (TGAb) (b) in patients with newly diagnosed postpartum thyroiditis. Data are median values and quartiles. *P < 0·005 compared with the AA genotype (Kruskal–Wallis analysis of variance test).
For the evaluation of thyroid autoantibody development with multiple regression analysis, the reference categories were CT60 CTLA-4 genotype, age, number of pregnancies, family history of AITD and cigarette smoking. For thyroid peroxidase antibodies, we established a significant contribution of CT60 CTLA-4 genotype (P < 0·04), while the effect of other factors was not confirmed. In thyroglobulin antibodies, no significant contribution of genetic or non-genetic factors was found.
Thyroid function in PPT patients with different CT60 genotypes
In PPT patients, 41 (54·7%) were hyperthyroid at presentation, while hypothyroidism was established in 34 (45·3%) patients. As presented in Table 3, the median value of thyroid peroxidase antibodies was significantly higher in the hypothyroid form of disease (P < 0·0001). Similarly, the median value of thyroglobulin antibodies was higher, although the difference was statistically insignificant. Accordingly, hypothyroid patients presented with elevated thyroid autoantibody concentrations far more frequently compared to the hyperthyroid form of PPT (P < 0·005). Further comparison of thyroid function in patients with different genotypes showed that the frequency of the G-allele was significantly higher among hypothyroid patients (P < 0·05). Interestingly, among 25 hypothyroid patients with both elevated thyroid peroxidase antibody and thyroglobulin antibody concentrations, 14 presented with the AG genotype and 11 with the GG genotype, while no AA genotype was found in this group.
Table 3.
Thyroid antibodies and CT60 cytotoxic T lymphocyte antigen-4 (CTLA-4) polymorphism in hyperthyroid or hypothyroid patients with newly diagnosed postpartum thyroiditis.
| Hyperthyroid form (n = 41) | Hypothyroid form (n = 34) | P-value | |
|---|---|---|---|
| Thyroid peroxidase antibodies, median (Q25–Q75) (U/ml) | 32 (5–129) | 500 (375–500) | <0·0001 |
| Thyroglobulin antibodies, median (Q25–Q75) (U/ml) | 108 (58–283) | 201·5 (103–497) | 0·055 |
| Thyroid peroxidase antibody and thyroglobulin antibody-positive | 13 (31·7%) | 25 (73·5%) | <0·005 |
| Thyroid peroxidase antibody or thyroglobulin antibody-positive | 19 (46·3%) | 7 (20·6%) | |
| Thyroid peroxidase antibody and thyroglobulin antibody-negative | 9 (22·0%) | 2 (5·9%) | |
| AA genotype | 12 (29·3%) | 5 (14·7%) | 0·086 |
| AG genotype | 21 (51·2%) | 15 (44·1%) | |
| GG genotype | 8 (19·5%) | 14 (41·2%) | |
| A allele | 45 (54·9%) | 25 (36·8%) | <0·05 |
| G allele | 37 (45·1%) | 43 (63·2%) |
Kruskal–Wallis analysis of variance test for thyroid peroxidase antibody and thyroglobulin antibody median analysis, χ2 test for comparison of the distribution of patients being positive or negative for thyroid peroxidase antibodies or thyroglobulin antibodies and for comparison of genotype and allele distribution.
Evaluating the independent effect of different genetic and non-genetic factors on thyroid function with multiple regression analysis, we established a strong contribution of thyroid peroxidase antibodies (P < 0·0002) and an insignificant contribution of thyroglobulin antibodies, CT60 genotype, age, family history and smoking. After elimination of the thyroid autoantibody effect, the contribution of the CT60 genotype reached the level of significance (P < 0·05).
Discussion
This study of patients with two different forms of thyroid autoimmune disease, HT and PPT, demonstrates a strong contribution of CT60 CTLA-4 SNP to thyroid autoantibody production. The significant increase of thyroid peroxidase antibody concentration and slight increase of thyroglobulin antibody concentration found in patients carrying the polymorphous CT60 CTLA-4 allele is consistent with our previous report on HT patients, where exon 1 and promoter CTLA-4 polymorphisms were studied [6]. Exon 1 SNP has also been shown to influence higher thyroid autoantibody production in Graves' disease [9]. Nevertheless, no data are available in the literature on association of CT60 SNP with thyroid autoantibody production.
Similarly, the data on genetic susceptibility in PPT are scarce in spite of the relatively high prevalence of 8% in the postpartum period [10]. A few earlier reports suggested an association with human leucocyte antigen (HLA) status, which was not confirmed afterwards [11]. The first report referring to the CTLA-4 gene in PPT was published a decade ago, describing no association between PPT and microsatellite CTLA-4 polymorphism [12]. The second report was our recent case–control study, where we were not able to demonstrate a link between CT60 CTLA-4 SNP and PPT [13]. However, the strong influence of thyroid peroxidase antibodies on development, thyroid function and prognosis of PPT was reported, as patients with higher thyroid peroxidase antibodies in the postpartum period developed PPT more often, presented with hypothyroidism more often and developed permanent hypothyroidism more often [2,11,14,15]. The current study also showed that thyroid peroxidase antibody concentrations were significantly higher in the hypothyroid form of PPT and the frequency of patients positive for thyroid autoantibodies was also significantly higher among hypothyroid patients. Nevertheless, the present data have, for the first time, provided evidence that thyroid autoantibody production in PPT patients is influenced strongly by the CTLA-4 gene. This association between polymorphous CT60 allele and higher thyroid autoantibody levels might also be reflected indirectly in the association between the polymorphous CT60 allele and the hypothyroid form of PPT, where patients present with higher thyroid autoantibody levels. Concordantly, in our study only G-allele carrying genotypes were found among hypothyroid PPT patients positive for both thyroid peroxidase antibodies and thyroglobulin antibodies.
The present results of an association between the CTLA-4 gene and thyroid autoantibody concentrations support previous findings provided by different genetic and epidemiological studies. With a whole genome linkage study the CTLA-4 gene has been recognized as a major thyroid autoantibody susceptibility gene [5], which has been confirmed subsequently in an expanded data set [16,17]. The studies on twin pairs indicated a higher prevalence of thyroid autoantibodies in healthy twin siblings [18] and provided the estimation that a 73% likelihood of being thyroid autoantibody-positive might be attributed to genetic susceptibility [4]. Furthermore, in monozygotic twins the concordance rates of thyroid autoantibodies were higher than in dizygotic twins [19]. Also, according to several family studies, positive thyroid autoantibodies appeared more frequently in the first-degree relatives of AITD patients [20–22]. Although our data confirm a strong association between genotype and thyroid autoantibody production, limitations of the study based on the sample size should be considered. A larger sample size would decrease the risk of false negative or false positive results, especially in the evaluation of variables with minor effects.
In spite of the strong influence of CT60 SNP on thyroid autoantibody production, the results of our recent study did not confirm the association of CT60 with HT or PPT, as the frequency of the G allele was 56·3% or 57% compared to 51·7% in the control population [13]. Similarly, the association with HT has not been established in the Japanese population [23,24]. However, an earlier study of a large group of Caucasian HT patients indicated CT60 as the HT susceptibility gene [7], and a similar finding has been reported recently in a small group of Slovak children [25]. Furthermore, a large meta-analysis, based on six published and unpublished studies of a total of 839 HT cases, indicated a significant association of CT60 SNP with HT [8]. As suggested by Ueda et al., the underlying mechanism by which CT60 triggers thyroid autoimmunity might be the reduced efficiency of splicing leading to a decrease of soluble CTLA-4 product and impaired CTLA-4 function [7]. This observation has not been supported by subsequent studies [26,27]. Another mechanism might be the linkage disequilibrium of CT60 with one or more nearby-lying polymorphisms, which alter CTLA-4 expression and function at the level of transcription, translation, mRNA stability or splicing [28]. Among them, promoter (−318C/T) and exon 1 (+49A/G) polymorphisms have been the most frequently shown to reduce the CTLA-4 efficiency [28–33].
As in our previous investigations [6,9], the current study demonstrates clearly higher thyroid peroxidase antibody concentrations associated with the polymorphous CTLA-4 gene. Heterozygotic individuals carrying the AG genotype also present with significantly higher thyroid peroxidase antibody levels compared to the protective AA genotype, and this observation is consistent with the previous suggestion of a dominant pattern of thyroid autoantibody inheritance [34]. In comparison to thyroid peroxidase antibodies, the association of genotype with thyroglobulin antibodies is less obvious. We have no feasible explanation for the difference between thyroid peroxidase antibodies and thyroglobulin antibodies. Perhaps in some patients the interference of thyroglobulin antibodies with elevated serum Tg might be involved, or perhaps it is a case of variable immunogenicity of Tg due to variable iodine intake influencing thyroglobulin antibody production [35].
In conclusion, our results provide convincing evidence that the CT60 CTLA-4 gene SNP or nearby-lying polymorphism influences increased thyroid autoantibody production in patients with HT and PPT. Therefore, they strongly support the assumption that CTLA-4 essentially contributes to thyroid autoantibody diathesis. In PPT, CT60 SNP also seems to influence the thyroid function, as patients carrying the polymorphous CT60 CTLA-4 allele present with higher thyroid peroxidase antibodies and are more prone to develop the hypothyroid form of the disease. Further studies are needed to estimate the association of CTLA-4 gene polymorphisms with the clinical presentation of different AITD forms.
Acknowledgments
This work was supported by the Slovenian Research Agency.
Disclosure
The authors declare no interests to disclose.
References
- 1.Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003;148:1–9. doi: 10.1530/eje.0.1480001. [DOI] [PubMed] [Google Scholar]
- 2.Kuijpens JL, Pop VJ, Vader HL, Drexhage HA, Wiersinga WM. Prediction of post partum thyroid dysfunction: can it be improved? Eur J Endocrinol. 1998;139:36–43. doi: 10.1530/eje.0.1390036. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: back to the future. J Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen PS, Brix TH, Iachine I, Kyvik KO, Hegedüs L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: a study of healthy Danish twins. Eur J Endocrinol. 2006;154:29–38. doi: 10.1530/eje.1.02060. [DOI] [PubMed] [Google Scholar]
- 5.Tomer Y, Greenberg DA, Barbesino G, Concepcion E, Davies TF. CTLA-4 and not CD28 is a susceptibility gene for thyroid antibody production. J Clin Endocrinol Metab. 2001;86:1687–93. doi: 10.1210/jcem.86.4.7372. [DOI] [PubMed] [Google Scholar]
- 6.Zaletel K, Krhin B, Gaberšček S, Hojker S. Thyroid autoantibody production is influenced by exon 1 and promoter CTLA-4 polymorphisms in patients with Hashimoto's thyroiditis. Int J Immunogenet. 2006;33:87–91. doi: 10.1111/j.1744-313X.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- 7.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 8.Kavvoura FK, Akamizu T, Awata T, et al. Cytotoxic T-lymphocyte associated antigen 4 gene polymorphisms and autoimmune thyroid disease: a meta-analysis. J Clin Endocrinol Metab. 2007;92:3162–70. doi: 10.1210/jc.2007-0147. [DOI] [PubMed] [Google Scholar]
- 9.Zaletel K, Krhin B, Gaberšček S, Pirnat E, Hojker S. The influence of the exon 1 polymorphism of the cytotoxic T lymphocyte antigen 4 gene on thyroid antibody production in patients with newly diagnosed Graves' disease. Thyroid. 2002;2:373–6. doi: 10.1089/105072502760043431. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;6:573–82. doi: 10.1089/thy.2006.16.573. [DOI] [PubMed] [Google Scholar]
- 11.Premawardhana LDKE, Parkes AB, John R, Harris B, Lazarus J. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610–15. doi: 10.1089/1050725041692828. [DOI] [PubMed] [Google Scholar]
- 12.Waterman EA, Watson PF, Lazarus JH, Parkes AB, Darke C, Weetman A. A study of the association between a polymorphism in the CTLA-4 gene and postpartum thyroiditis. Clin Endocrinol. 1998;49:251–5. doi: 10.1046/j.1365-2265.1998.00537.x. [DOI] [PubMed] [Google Scholar]
- 13.Bicek A, Zaletel K, Gaberscek S, et al. 49A/G and CT60 polymorphisms of the cytotoxic T-lymphocyte-associated antigen 4 gene associated with autoimmune thyroid disease. Hum Immunol. 2009;70:820–4. doi: 10.1016/j.humimm.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab. 2000;85:71–5. doi: 10.1210/jcem.85.1.6227. [DOI] [PubMed] [Google Scholar]
- 15.Nøhr SB, Jørgensen A, Pedersen KM, Laurberg P. Postpartum thyroid dysfunction in pregnant thyroid peroxidase antibody-positive women living in an area with mild to moderate iodine deficiency: is iodine supplementation safe? J Clin Endocrinol Metab. 2000;85:3191–8. doi: 10.1210/jcem.85.9.6799. [DOI] [PubMed] [Google Scholar]
- 16.Ban Y, Davies TF, Greenberg DA, et al. Analysis of the CTLA-4, CD28, and inducible costimulator (ICOS) genes in autoimmune thyroid disease. Genes Immun. 2003;4:586–93. doi: 10.1038/sj.gene.6364018. [DOI] [PubMed] [Google Scholar]
- 17.Ban Y, Greenberg DA, Davies TF, Jacobson E, Concepcion E, Tomer Y. Linkage analysis of thyroid antibody production: evidence for shared susceptibility to clinical autoimmune thyroid disease. J Clin Endocrinol Metab. 2008;93:3589–96. doi: 10.1210/jc.2008-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brix TH, Hansen PS, Kyvik KO, Hegedüs L. Aggregation of thyroid antibodies in first-degree relatives of patients with autoimmune thyroid disease is mainly due to genes: a twin study. Clin Endocrinol. 2004;60:329–34. doi: 10.1111/j.1365-2265.2004.01983.x. [DOI] [PubMed] [Google Scholar]
- 19.Phillips DIW, Osmond C, Baird J, Huckle A, Rees-Smith B. Is birthweight associated with thyroid autoimmunity? A study in twins. Thyroid. 2002;12:377–80. doi: 10.1089/105072502760043440. [DOI] [PubMed] [Google Scholar]
- 20.Hall R, Stanbury JB. Familial studies of autoimmune thyroiditis. Clin Exp Immunol. 1967;2:719–25. [PMC free article] [PubMed] [Google Scholar]
- 21.Strieder TGA, Prummel MF, Tijssen JGP, Endert E, Wiersinga WM. Risk factors for and the prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin Endocrinol. 2003;59:396–401. doi: 10.1046/j.1365-2265.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 22.Marwaha RK, Sen S, Tandon N, et al. Familial aggregation of autoimmune thyroiditis in first degree relatives of patients with juvenile autoimmune thyroid disease. Thyroid. 2003;13:297–300. doi: 10.1089/105072503321582114. [DOI] [PubMed] [Google Scholar]
- 23.Ban Y, Tozaki T, Taniyama M, Tomita M, Ban Y. Association of a CTLA-4 3′ untranslated region (CT60) single nucleotide polymorphism with autoimmune thyroid disease in the Japanese population. Autoimmunity. 2005;38:151–3. doi: 10.1080/08916930500050319. [DOI] [PubMed] [Google Scholar]
- 24.Ikegami H, Awata T, Kawasaki E, et al. The association of CTLA4 polymorphism with type 1 diabetes is concentrated in patients complicated with autoimmune thyroid disease: a multicenter collaborative study in Japan. J Clin Endocrinol Metab. 2006;91:1087–92. doi: 10.1210/jc.2005-1407. [DOI] [PubMed] [Google Scholar]
- 25.Dallos T, Avbelj M, Barák L, et al. CTLA-4 gene polymorphisms predispose to autoimmune endocrinopathies but not to celiac disease. Neuro Endocrinol Lett. 2008;29:334–40. [PubMed] [Google Scholar]
- 26.Purohit S, Podolsky R, Collins C, et al. Lack of correlation between the levels of soluble cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and the CT-60 genotypes. J Autoimmune Dis. 2005;2:8. doi: 10.1186/1740-2557-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayans S, Lackovic K, Nyholm C, et al. CT60 genotype does not affect CTLA-4 isoform expression despite association to T1D and AITD in northern Sweden. BMC Med Genet. 2007;8:3. doi: 10.1186/1471-2350-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anjos SM, Tessier MC, Polychronakos C. Association of the cytotoxic T lymphocyte-associated antigen 4 gene with type 1 diabetes: evidence for independent effects of two polymorphisms on the same haplotype block. J Clin Endocrinol Metab. 2004;89:6257–65. doi: 10.1210/jc.2004-0881. [DOI] [PubMed] [Google Scholar]
- 29.Ligers A, Teleshova N, Masterman T, Huang WX, Hilleret J. CTLA-4 gene expression is influenced by promoter and exon 1 polymorphisms. Genes Immun. 2001;2:145–52. doi: 10.1038/sj.gene.6363752. [DOI] [PubMed] [Google Scholar]
- 30.Wang XB, Zhao X, Giscombe R, Lefvert AK. A CTLA-4 gene polymorphism at position -318 in the promoter region affects the expression of protein. Genes Immun. 2002;3:233–4. doi: 10.1038/sj.gene.6363869. [DOI] [PubMed] [Google Scholar]
- 31.Kouki T, Sawai Y, Gardine CA, Fisfalen ME, Alegre ML, DeGroot LJ. CTLA-4 gene polymorphism at position 49 in exon 1 reduces the inhibitory function of CTLA-4 and contributes to the pathogenesis of Graves' disease. J Immunol. 2000;165:6606–11. doi: 10.4049/jimmunol.165.11.6606. [DOI] [PubMed] [Google Scholar]
- 32.Mäurer M, Loserth S, Kolb-Mäurer A, et al. A polymorphism in the human cytotoxic T-lymphocyte antigen (CTLA4) gene (exon1 +49) alters T-cell activation. Immunogenetics. 2002;54:1–8. doi: 10.1007/s00251-002-0429-9. [DOI] [PubMed] [Google Scholar]
- 33.Anjos S, Nguyen A, Ounissi-Benkalha H, Tessier MC, Polychronakos C. A common autoimmunity predisposing signal peptide variant of cytotoxic T-lymphocyte antigen 4 results in inefficient glycosylation of the susceptibility allele. J Biol Chem. 2002;277:46478–86. doi: 10.1074/jbc.M206894200. [DOI] [PubMed] [Google Scholar]
- 34.Phillips D, McLachlan S, Stephenson A, et al. Autosomal dominant transmission of autoantibodies to thyroglobulin and thyroid peroxidase. J Clin Endocrinol Metab. 1990;70:742–6. doi: 10.1210/jcem-70-3-742. [DOI] [PubMed] [Google Scholar]
- 35.Carayanniotis G. Recognition of thyroglobulin by T cells: the role of iodine. Thyroid. 2007;17:963–73. doi: 10.1089/thy.2007.0199. [DOI] [PubMed] [Google Scholar]


