Abstract
Proteinase 3 (PR3) is a major autoantigen in anti-neutrophil cytoplasmic antibodies (ANCA)-associated systemic vasculitis (AASV), and the proportion of neutrophils expressing PR3 on their membrane (mPR3+) is increased in AASV. We have shown recently that mPR3 and CD177 are expressed on the same cells in healthy individuals. In this study we try to elucidate mechanisms behind the increased mPR3 expression in AASV and its relationship to CD177. All neutrophils in all individuals were either double-positive or double-negative for mPR3 and CD177. The proportion of double-positive neutrophils was increased significantly in AASV and systemic lupus erythematosus patients. The proportion of mPR3+/CD177+ cells was not correlated to general inflammation, renal function, age, sex, drug treatment and levels of circulating PR3. AASV patients had normal levels of granulocyte colony-stimulating factor and granulocyte–macrophage colony-stimulating factor. Pro-PR3 was found to constitute 10% of circulating PR3 but none of the mPR3. We found increased mRNA levels of both PR3 and CD177 in AASV, but they did not correlate with the proportion of double-positive cells. In cells sorted based on membrane expression, CD177–mRNA was several-fold higher in mPR3+ cells. When exogenous PR3 was added to CD177-transfected U937 cells, only CD177+ cells bound PR3 to their membrane. In conclusion, the increased membrane expression of PR3 found in AASV is not linked directly to circulating PR3 or PR3 gene transcription, but is dependent upon CD177 expression and correlated with the transcription of the CD177 gene.
Keywords: anti-neutrophil cytoplasmic antibody (ANCA)/anti-PR3/MPO, flow cytometry/FACS, glomerulonephritis, vasculitis, Wegener's granulomatosis
Introduction
PR3 was first described in 1973 as an intracellular protein [1], but it is also found in the circulation in complex with its natural inhibitor, α1-anti-trypsin [2]. PR3 belongs to the microbicidal serine proteases, and is stored in azurophilic granules of neutrophils in a mature form. Early during synthesis, some PR3 molecules escape targeting into granules and become secreted as pro-PR3, which has a negative regulatory effect on haematopoiesis [3]. PR3 is also found in secondary granules, secretory vesicles [4] and on the plasma membrane of neutrophils (mPR3), suggesting additional functions for this protein [5]. The mPR3 is accessible for interaction with the immune system and is not inhibited by α1-anti-trypsin [6]. Moreover, mPR3 has a peculiar feature of being expressed on the plasma membrane of only a subset of neutrophils. The existence of two distinct neutrophil subpopulations within one individual is called bimodal membrane expression [7]. For both membrane-bound and circulating PR3, its functional significance as well as its origin remains unexplained. It is unclear to what extent circulating PR3 emanates from pro-PR3, secreted by proliferating neutrophils in the bone marrow, from the granule-stored mature PR3 released by circulating mature neutrophils or if it is released during apoptosis.
CD177 is the only other molecule known to have a bimodal membrane expression on neutrophils. It is a glycosyl-phosphatidylinositol (GPI)-anchored glycoprotein, first described in 1971 as the NB1 antigen and a member of the leucocyte antigen 6 superfamily [8]. The membrane expression of CD177 is increased during pregnancy [9] and in situations of increased granulopoiesis, such as bacterial infections and burns [10,11]. Several studies have shown that 95–100% of patients with polycythaemia vera (PV) have elevated levels of CD177mRNA [12–14] due to a dominant gain-of-function mutation in the JAK2 gene [15]. CD177mRNA expression in neutrophils is up-regulated in response to administration of granulocyte colony-stimulating factor (G-CSF) to healthy subjects or by stimulation by G-CSF or granulocyte–macrophage colony-stimulating factor (GM-CSF) in vitro[12]. It has also been shown that GM-CSF could increase significantly the PR3 membrane expression on neutrophils in vitro [16].
Anti-neutrophil cytoplasmic antibodies (ANCA)-associated systemic vasculitis (AASV) is a group of diseases characterized histologically by necrotizing vasculitis affecting small blood vessels and which is often associated with pauci-immune necrotizing crescentic glomerulonephritis [17,18]. Serologically, they are characterized by autoantibodies directed against constituents of neutrophil granules (ANCA) [19–21]. The Chapel Hill international consensus conference defined three major categories of AASV: Wegener's granulomatosis (WG), microscopic polyangiitis (MPA) and Churg–Strauss syndrome (CSS) [22]. WG is differentiated from MPA by the presence of necrotizing granulomatous inflammation of the respiratory tract. CSS is differentiated from WG and MPA by the presence of asthma and eosinophilia [23]. In WG, most patients have ANCA with specificity against proteinase 3 (PR3–ANCA), while in MPA and CSS, ANCA is directed most often against myeloperoxidase (MPO–ANCA). Several observations suggest a pathophysiological role of ANCA in AASV [24,25]. However, the mechanisms leading to the production of ANCA are still unknown.
Previous studies have shown higher membrane expression of PR3 and higher plasma PR3 levels in patients with AASV compared to healthy controls [26,27]. In addition, mPR3 has been shown to be co-expressed with CD177 on neutrophils in healthy individuals [28].
In this study, we investigate the mechanisms underlying the elevated plasma PR3 levels, elevated membrane expression of PR3 and the co-expression of mPR3 and CD177 in AASV.
Patients and methods
Patients and controls
Fifty-five AASV were recruited from Department of Nephrology, Lund University Hospital in the period 2006–08. As controls, we recruited 93 healthy blood donors (HBD) from the local blood bank, 20 renal transplant (TP) recipients from the Department of Nephrology, 17 PV patients and one paroxysmal nocturnal haemoglobinuria (PNH) patient from the Department of Haematology, 21 systemic lupus erythematosus (SLE) and 21 rheumatoid arthritis (RA) patients as disease controls from the Department of Rheumatology (Table 1).
Table 1.
Demographic data and membrane expression of PR3 and CD177.
| AASV |
||||||||
|---|---|---|---|---|---|---|---|---|
| HBD | PR3-ANCA | MPO-ANCA | ANCA-neg | PV | TP | SLE | RA | |
| Total no. | 93 | 33 | 18 | 4 | 17 | 20 | 21 | 21 |
| Age years | 41 ± 13 | 61 ± 18 | 64 ± 13 | 57 ± 17 | 61 ± 12 | 51 ± 11 | 44 ± 13 | 63 ± 13 |
| F/M ratio (n) | 33/57 | 15/18 | 11/7 | 2/2 | 7/10 | 7/13 | 20/1 | 13/8 |
| Double-membrane expression | 58·4% | 71·2%** | 66·5% | 63·5% | 60·6% | 60·4% | 69·9%* | 53·2% |
P-value < 0·05
P-value < 0·01, compared to HBD.
All results are expressed as mean ± standard deviation of the mean. AASV, anti-neutrophil cytoplasmic antibodies (ANCA)-associated systemic vasculitis; ANCA-neg, ANCA-negative patients; F, female; HBD, healthy blood donors; M, male; MPO-ANCA, myeloperoxidase-ANCA-positive patients; PR3-ANCA, proteinase 3-ANCA-positive patients; PV, polycythaemia vera; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TP, renal transplant recipients.
Patients were diagnosed as vasculitis and classified into WG or MPA according to the European Medicines Agency (EMEA) algorithm [23]. The Birmingham Vasculitis Activity Score (BVAS) was used to determine the activity of vasculitis [29].
The study was approved by the Regional Ethical Review Board and informed signed consent was obtained from all individuals participated in the study.
Blood sampling and separation
Leucocytes were isolated by centrifugation on Polymorphprep (Axis-Shield, Oslo, Norway). Plasma band was used to measure PR3, pro-PR3, G-CSF and GM-CSF levels. Neutrophil band was used to study membrane and RNA expression.
Membrane expression and flow cytometry
The neutrophil-containing samples were blocked using human immunoglobulin (Ig)G (0·5 mg/ml; European Institute of Science AB, Lund, Sweden) for 20 min on ice. Neutrophils (1 × 106) were single-labelled with a primary antibody, affinity-purified rabbit anti-PR3 (3·3 µg/ml) purified at our nephrology laboratory as described previously [27], mouse-anti-PR3 (4A5, 5 µg/ml; Wieslab, Lund, Sweden), mouse anti-CD177 (1:200; Serotec, Oxford, UK). In addition, 1 × 106 cells were double-labelled with two primary antibodies: rabbit anti-PR3 and mouse anti-CD177. For detection of pro-PR3 on the neutrophil membrane, a new antibody was made by immunization of rabbits with a keyhole limpet haemocyanin (KLH)-conjugated peptide from the C-terminal end of the pro-PR3 (CRRVEAKGRP). Peptide synthesis, conjugation, immunization and antibody purification using immobilized peptides was performed by Innovagen AB (Lund, Sweden). After washing, the samples incubated with secondary antibodies goat-anti-rabbit Alexa 488 (1 : 600; Molecular Probes, Eugene, OR, USA) and goat-anti-mouse Alexa647-RPE (1:600; Molecular Probes) for 15 min on ice. These samples were fixed using 2% paraformaldehyde. Fluorescence was measured by fluorescence activated cell sorter (FACS) and neutrophil population was selected by gating for appropriate forward- and side-scatter.
Quantitative polymerase chain reaction (PCR) assay
Total RNA was isolated by RNeasy Mini kit (Qiagen, Gaithersburg, MD, USA), and reverse transcription was performed using the TaqMan Reverse Transcription Reagents kit (Applied Biosystems, Foster City, CA, USA). The gene expression of PR3, CD177, MPO and interleukin (IL)-8 was determined using quantitative PCR assays on an ABI PRISM 7000 Sequence Detector (Applied Biosystems) with TaqMan Universal Master Mix UNG, as described previously [30]. Relative expression was determined by the difference in the Ct values for the target genes after normalization to RNA input level, using Cyclophilin A Ct values. Relative quantification was determined by standard 2(–ΔΔ Ct) calculations [31].
Separation of mPR3-positive and mPR3-negative cells
Neutrophils were isolated from three donors and three patients, labelled with anti-PR3 (monoclonal mouse anti-PR3, 4A5, Wieslab; conjugated with Alexa647, Molecular Probes). For separation of the two subpopulations, mPR3-positive and mPR3-negative, we used FACS Aria flow cytometer equipped with automatic cell deposition unit (BD Biosciences, Immunocytometry Systems, San Jose, CA, USA). After sorting, RNA was isolated and CD177- as well as the PR3-specific cDNA was measured in each subpopulation by real time-PCR.
Cell culture, transfection and addition of exogenous PR3
Human histiocytic lymphoma cells (U937) were stably transfected with CD177–cDNA in a pcDNAvector3·1 or with a negative control vector by electroporation [Gene Pulser II (Bio-Rad); 0·4 cm cuvette; 0·2 kV, 950 µF]. Two clones transfected with CD177–cDNA were selected, one positive for CD177 expression and one negative for CD177 expression. They were cultured for 1 week in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS). Both clones were then incubated with exogenous PR3 for 2 h on ice (1 µg/ml in each tube). The cells were labelled with anti-PR3 and anti-CD177 antibodies and the fluorescence was measured by FACS.
Measurement of plasma G-CSF by enzyme-linked immunosorbent assay (ELISA)
Plasma G-CSF and GM-CSF were measured using Quantikine® (R&D systems, Abingdon, UK) ELISA Development kits from R&D Systems.
PR3 and pro-PR3
Plasma PR3 level was detected by sandwich ELISA, as described previously [26,27]. For detection of plasma Pro-PR3, a new sandwich ELISA was developed. Briefly, a microtitre plate was coated overnight with new affinity-purified anti-pro-PR3 antibody (2 µg/ml). Plasma samples were added and the plates were incubated for 2 h. After washing, bound pro-PR3 was detected by incubation for 2 h with monoclonal murine anti-PR3 (4A3, 0·5 µg/ml) in sample buffer. After washing, a conjugated anti-mouse antibody (1:2000, alkaline phosphatase-labelled rabbit-anti-mouse IgG; Dako, Glostrup, Denmark) was added and incubated for 1 h. P-nitrophenyl-phosphate disodium (Sigma, St Louis, MO, USA) 1 mg/ml in substrate buffer was used as substrate and incubated with the samples for 30 min. Optical densities were read at 405 nm. A standard curve was produced by incubation of a twofold dilution series of recombinant PR3 containing the C-terminal pro-peptide, starting with 0·25 ng/ml and using the sample buffer as a blank.
Statistical analyses
Differences in continuous variables between two groups were analysed using the unpaired t-test and results are given as mean ± standard deviation (s.d.). For data sets that did not follow Gaussian distribution, the Mann–Whitney U-test was used and results were given as median and range.
Correlations were analysed using Pearson's rank test and for non-parametric data Spearman's rank test was used. A two-sided P < 0·05 was considered to be statistically significant.
Results
Demographic data
Fifty-five patients with AASV were included in this study (Table 1). At the time of sampling, 40 patients were in stable remission (BVAS 0-1), 13 moderately active in their disease (BVAS 2-5) and two patients highly active in their disease (BVAS > 5). Twenty-three patients were treated with cytotoxic drugs together with steroids, 13 with cytotoxic drugs only, seven with steroids only and 12 patients did not have any form of immunosuppressive treatment.
Membrane expression results
Neutrophils from 223 individuals were analysed for membrane expression of PR3 and CD177. A strong correlation between the percentage of mPR3+ subpopulation and the percentage of CD177+ subpopulation (r = 0·93, P < 0·0001, n = 223) was observed (Fig. 1). In the patient with PNH, which is characterized by blood cells lacking GPI-anchors, there were fewer than 1% cells positive for CD177 and fewer than 1% cells positive for mPR3. Neither in any specific disease condition nor in any single individual did we find a substantial number of single-positive cells. We concluded that mPR3+ cells are identical to CD177+ cells and defined this subpopulation as double-positive for PR3 and CD177. The mPR3+/CD177+ subpopulation was used as the standard tool for subsequent comparisons and correlations.
Fig. 1.
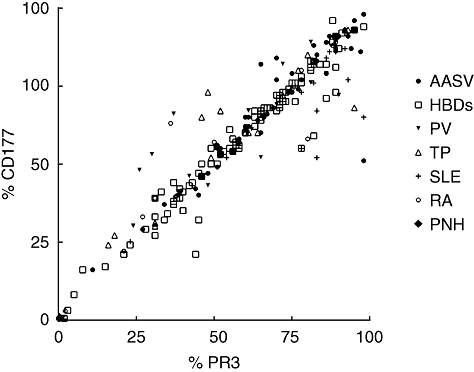
Correlation between mPR3 and CD177 among all the groups. Shows the results of 91 HBDs, 52 AASV patients, 17 PV patients, 20 TP, 21 SLE patients and 17 RA patients and one patient with PNH. There was a strong correlation between % of mPR3-positive neutrophils and % of CD177-positive neutrophils among all the groups, i.e. they define the same population of neutrophils (mPR3- and CD177-positive population). AASV, anti-neutrophil cytoplasmic antibodies (ANCA)-associated systemic vasculitis; HBD, healthy blood donors; PNH, paroxysmal nocturnal haemoglobinuria; PV, polycythaemia vera; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TP, renal transplant recipients.
We found that the percentage of mPR3+/CD177+ neutrophils was significantly higher in AASV patients (69%, P = 0·0042) and SLE patients (70%, P = 0·022) compared to healthy blood donors (HBD, 58%). Meanwhile, PV patients, renal transplant (TP) recipients and rheumatoid arthritis (RA) patients did not show any significant difference in the percentage of mPR3+/CD177+ neutrophils compared to healthy controls (61%, 60% and 53% versus 58%, respectively), Fig. 2.
Fig. 2.
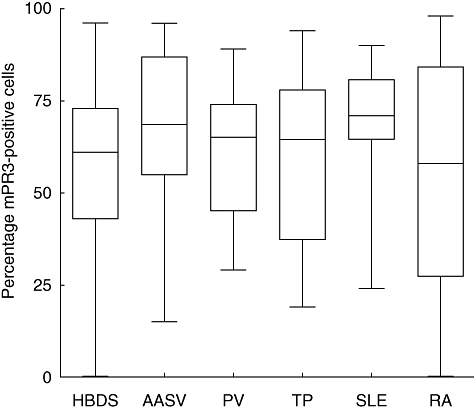
Double membrane expression. Compares the percentage of the double-positive population of neutrophils (mPR3- and CD177-positive population) among all groups of patients and healthy controls. The percentage of double-positive neutrophils was significantly higher in AASV patients and SLE patients compared to HBD. AASV, anti-neutrophil cytoplasmic antibodies (ANCA)-associated systemic vasculitis; HBD, healthy blood donors; PV, polycythaemia vera; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TP, renal transplant recipients.
Correlation between membrane expression and clinical data
In AASV patients, PR3–ANCA-positive patients had a significantly higher percentage of mPR3+/CD177+ neutrophils compared to HBD (71·2% versus 58·4%, P = 0·0044), while MPO–ANCA-positive patients had non-significantly higher mPR3+/CD177+ cells compared to HBD (66·5% versus 58·4%, P = 0·142). Otherwise, no correlation was found between the percentage of mPR3+/CD177+ neutrophils and clinical data.
Similarly, no difference in percentage of mPR3+/CD177+ neutrophils and current disease status or treatment was observed. There was no correlation with CRP, estimated GFR, cytotoxic drug treatment, steroid dose or BVAS.
Correlation between membrane expression and gene expression
In 115 samples, we measured mRNA levels of PR3, CD177, MPO and IL-8 (Table 2). Data are expressed as calibrated fold change of mRNA; setting mRNA expression of the healthy controls equal to 1.
Table 2.
Gene expression of PR3 and CD177.
| HBD | AASV | PV | TP | SLE | RA | |
|---|---|---|---|---|---|---|
| n (RNA data) | 32 | 26 | 13 | 16 | 17 | 21 |
| PR3-mRNA | 1 | 2·5* | 1·5 | 2·0 | 5·4** | 1·3 |
| CD177-mRNA | 1 | 4·5** | 26·7*** | 1·8 | 6·0*** | 5·0** |
| MPO-mRNA | 1 | 2·1** | 1·2 | 1·2 | 3·2** | 0·5* |
| IL-8-mRNA | 1 | 0·6 | 0·1** | 0·6 | 1·2 | 0·8 |
P-value < 0·05
P-value < 0·01
P-value < 0·001.
All results are expressed as mean. AASV, anti-neutrophil cytoplasmic antibodies (ANCA)-associated systemic vasculitis; HBD, healthy blood donors; PV, polycythaemia vera; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TP, renal transplant recipients.
PR3-mRNA expression was significantly higher in AASV and SLE patients (×2·5 and ×5·4, respectively) compared to healthy controls. The CD177-mRNA expression was significantly higher among AASV, SLE and RA patients (4·5, 6·0 and 5·0, respectively) compared to healthy controls and even more elevated among PV patients (×26·7) compared to healthy controls (Table 2).
There was a weak positive correlation between the CD177–mRNA expression and the percentage of mPR3+/CD177+ neutrophils, which was statistically significant only when all samples were pooled together (Spearman's r = 0·37, P < 0·0001, n = 115).
Gene expression of sorted cells
To explore further the relationship between gene transcription in mature neutrophils and membrane PR3 expression, neutrophils were sorted based on their mPR3 expression and their mRNA levels of PR3 and CD177 were measured. We found that PR3-mRNA expression did not differ between mPR3-positive and -negative cells. On the other hand, the median mRNA levels of CD177 was 13 times higher in the mPR3+ cells compared to the negative ones, as shown in Table 3.
Table 3.
Gene expression of sorted cells.
| PR3-RNA levels |
CD177-RNA levels |
|||||
|---|---|---|---|---|---|---|
| mPR3+ cells | mPR3– cells | PR3-index | mPR3+ cells | mPR3– cells | CD177-index | |
| Patient no 1 | 0·22 | 0·23 | 0·96 | 57·81 | 0·8 | 72·3 |
| Patient no 2 | 0·01 | 1·34 | 0·007 | 24·2 | 9·19 | 2·63 |
| Patient no 3 | 0·03 | 0·39 | 0·077 | 308 | 15·42 | 19·9 |
| HC no 1 | 0·11 | 0·01 | 11 | 37·44 | 0·26 | 141 |
| HC no 2 | 0·01 | 0·01 | 1 | 0·2 | 0·08 | 2·5 |
| HC no 3 | 0·02 | 0·04 | 0·5 | 6·08 | 0·92 | 6·6 |
| Median | 0·025 | 0·13 | 0·73 | 30·8 | 0·86 | 13·3 |
All results are expressed as calibrated fold change compared to standard RNA; HC, healthy control; PR3-index, the index between PR3-RNA levels in mPR3-positive cells and PR3-RNA levels in mPR3-negative cells; CD177-index, the index between CD177-RNA levels in mPR3-positive cells and CD177-RNA levels in mPR3-negative cells.
U937 cells and exogenous PR3 binding
To correlate CD177 to mPR3-membrane binding, we used U937 cells that normally express low mPR3 but high PR3–mRNA levels. These cells were stably transfected with CD177-cDNA. Of 20 clones, eight clones became stably positive for CD177 surface expression. Surprisingly, all the 20 clones shut down their PR3-mRNA expression and neither the protein (measured by immunoblotting and FACS) nor the mRNA expression (measured by real-time PCR) could be detected. Sixteen of the 20 mock-transfected clones continued to express PR3.
Two clones transfected with CD177–cDNA were selected, one positive for CD177-membrane expression and one negative. These two clones were incubated with exogenous PR3. As shown in Fig. 3, only cells expressing CD177 on their membrane bound PR3.
Fig. 3.
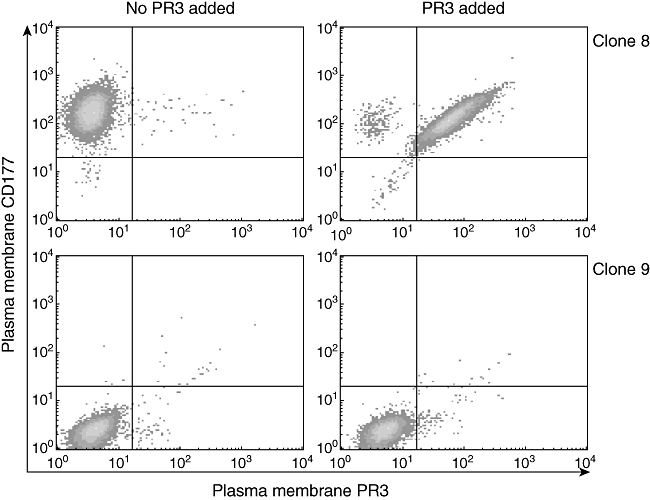
U937 and exogenous PR3 binding. The left panel shows the membrane expression of U937 clone 8 cells (express CD177 but not proteinase 3 (PR3) on their plasma membrane), and U937 clone 9 cells clone (do not express PR3 or CD177 on their plasma membrane), measured by fluorescence activated cell sorter analysis. In the right panel, membrane expression of PR3 and CD177 was measured again on the same cells after incubation with mature PR3 for 2 h. Clone 8 cells expressed the PR3 on their plasma membrane (upper right), while clone 9 cells did not express any PR3 or CD177 on their membranes (lower right).
G-CSF and GM-CSF in the plasma
We measured the plasma levels G-CSF and GM-CSF because they have been shown to increase the membrane expression of mPR3 and the mRNA expression of CD177 [12,16].
There was no significant difference between the groups regarding their plasma levels of G-CSF, and most of the samples were within normal or slightly higher than the normal plasma levels (normal range 2·2–30·9 pg/ml).
GM-CSF levels were within the normal range (< 2 pg/ml) for the majority of the samples. Elevated levels were found in four AASV patients (median 484·2, range 7·7–3135 pg/ml), eight RA patients (54·7, 11–178 pg/ml), one PV patient (23 pg/ml) and one SLE patient (42·7 pg/ml). However, there was no correlation between plasma levels of G-CSF or GM-CSF and size of the mPR3+/CD177+ subpopulation.
Pro-PR3 and PR3
Looking for the source of the circulating PR3, we measured the mature form of PR3 as well as the pro-PR3 in plasma. Total plasma PR3 was elevated significantly in AASV patients (median 148, range 30–2553 µg/l, n = 49) compared to healthy controls (84, 38–246 µg/l, n = 63) (Mann–Whitney U-test, P < 0·0001). Using a pro-PR3 specific anti-serum we found that plasma pro-PR3 constitute only a small fraction of the total plasma PR3, in both AASV patients and in healthy controls (10% each). Accordingly, pro-PR3 levels were higher in AASV patients (12, 6–184 µg/l) compared to healthy controls (7, 4–28 µg/l) (P < 0·0001). When correlating levels of total PR3, mature PR3 and pro-PR3 to the percentage of mPR3+/CD177+ neutrophils, no significant correlation was found. The new pro-PR3 specific anti-serum did not bind to mPR3, indicating that the mPR3 is mature PR3 (data not shown).
Discussion
In this study, we show that mPR3 and CD177 are also co-expressed on the plasma membrane of neutrophils in all individuals under pathological conditions. In no subgroup including AASV, PV, SLE and RA patients, as well as renal transplant recipients, did we find evidence for any significant amounts of single-positive cells (Fig. 1). Moreover, the mPR3+/CD177+ neutrophil subpopulation was larger in AASV and SLE patients compared to other diseases and healthy controls, suggesting a specific distinct cause of this phenomenon and raising several questions regarding the pathophysiological significance and origin of this subpopulation. Is this a result of increased plasma levels of specific cytokines, or could it be due to increased production of one of the two proteins on mRNA level in mature neutrophils?
First, there could have been several relatively trivial reasons for the increase of mPR3+/CD177+ cells, such as general inflammatory activity, reduced renal function or specific drug therapy. To address these possibilities, we correlated the mPR3+/CD177+ cells with clinical data from AASV patients. According to our results, the elevated percentage of mPR3+/CD177+ cells found in AASV patients does not seem to be due to treatment, general inflammation or renal failure.
Even though there was no correlation with either CRP or BVAS, ruling out that the elevation of mPR3+/CD177+ cells could be caused by general inflammation, it could still be mediated by some specific set of cytokines. Human blood plasma comprises a very effector-enriched environment for the neutrophils [32]. The search for external factors that are responsible for the elevated mPR3+/CD177+ cells in vasculitis patients is not easy. We focused upon two cytokines in the plasma, G-CSF and GM-CSF. We found that only GM-CSF levels were elevated in four AASV patients. Although this may explain the increased percentage of mPR3+/CD177+ neutrophils in these four patients, it does not explain it in the remaining 51 AASV patients. Thus, these experiments do not explain if the elevated mPR3+/CD177+ cells are due to external stimuli, a genetic predisposition to develop the disease, or if it is a reflection of a disease-specific defect in the neutrophils.
One explanation that could account for an increased number of mPR3+/CD177+ neutrophils is a continued production of PR3 and/or CD177 on a gene transcription level in mature neutrophils. To determine if this was the case, we measured the mRNA expression by TaqMan real-time PCR in neutrophils. The up-regulated PR3-mRNA levels described previously in AASV patients [33] was reconfirmed. Furthermore, we found significantly higher mRNA expression of CD177 among AASV, PV, SLE and RA patients compared to HBD, as shown in Table 2.
From the finding that CD177–mRNA expression, but not PR3–mRNA expression, correlates with the percentage of mPR3+/CD177+ cells we concluded that the underlying mechanism behind the shift of the neutrophil subpopulation towards an mPR3+/CD177+ phenotype could be linked to an over-production of CD177 by mature human neutrophils. However, increases only in CD177 gene transcription is not sufficient to achieve an increase in the percentage of double-positive cells, as the PV patients who had a very high CD177–mRNA expression (× 26·7) did not exhibit a significantly increased proportion of mPR3+/CD177+ cells compared to healthy controls.
When we sorted the human neutrophils into two groups according to their mPR3 expression, the PR3 mRNA level did not differ between the mPR3-positive and -negative cells. However, the mRNA level of CD177 was significantly higher (× 13) in the positive group compared to the negative one. Similar results for CD177 have been shown previously [34]. In order to show the importance of CD177 on protein level for the mPR3 expression we transfected U937 cells with CD177 cDNA. The results showed that only cells expressing CD177 on their membranes were able to bind exogenously added PR3. These two experiments, together with the correlations discussed above, suggest clearly that CD177 expression on the plasma membrane is responsible for the bimodal expression pattern of mPR3. In other words, CD177 seems to be a prerequisite for PR3 to be expressed on the plasma membrane of neutrophils.
von Vietinghoff et al. has shown a direct physical binding between PR3 and CD177 (NB-1) [35]. Despite several attempts, we have not been able to reproduce their results at our laboratory, and hence cannot share their conclusion that CD177 is a neutrophil membrane receptor for PR3. Saying that CD177 is a prerequisite for mPR3 expression with no evidence for direct physical binding is contradictory, but could be explained if CD177 assist PR3 to bind directly to the membrane or to another membrane protein, but it does not stay attached afterwards. A similar theory has been postulated previously for the relation between the soluble endothelial protein C receptor (sEPCR) and PR3, where sEPCR attaches more effectively to the neutrophil membrane in the presence of mPR3 [36]. Another example is the relationship between klotho and FGF23 in renal cells, where klotho is essential for the binding of FGF23 to its receptor in a specific and high-affinity manner [37].
Recently, it has been shown by Hajjar et al., via computational simulation, that PR3 binding to the membrane depends partly upon electrostatic and hydrophobic interactions that keep the PR3 stably inserted into the lipid bilayer structure of the membrane, without the need of another molecule [38]. This theory does not explain why PR3 is expressed on only a subset of neutrophils, even though they have similar membrane structure and contain equal amounts of intracellular PR3 protein [39].
A recent study by Hu et al. has shown similar results to ours. However, they show that ANCA-induced activation of neutrophils is independent of CD177/mPR3 positivity. Cells that are CD177-negative become mPR3-positive after tumour necrosis factor (TNF)-α priming and are equally stimulated with ANCA as CD177-positive cells [40].
The origin of the mPR3 and the PR3 found in plasma is not known; nor is it known if the mPR3 or the plasma PR3 is in a pro-form or mature protein. We have measured the amounts of circulating plasma PR3 previously and found it to be elevated in AASV patients compared to healthy controls [27]. This finding was verified in this study. To be able to measure the pro-PR3 we had to develop a new antibody recognizing the pro-form only. The new ELISA based on this new anti-pro-PR3 antibody showed that the pro-PR3 was also elevated in our AASV patients compared to healthy blood donors. However, the proportion between the pro-PR3 and total PR3 was, on average, 10% and did not differ significantly between AASV patients and HBD. There could be several different explanations for these findings: the elevated levels of the pro-PR3 could indicate an increased production of neutrophils in the bone marrow or possibly reflect an increased synthesis of PR3 in mature cells; or the elevated levels of mature PR3 could reflect an increased degranulation upon activation or release of granular content as a consequence of necrosis. Arguing against the degranulation theory is the fact that there is no correlation between plasma PR3 and neutrophil gelatinase-associated lipocalin (NGAL), a marker of secondary granules [27]. None the less, the anti-pro-PR3 antibody did not recognize the plasma membrane-bound PR3, indicating that mPR3 is not the pro-form of PR3 but mature PR3.
To conclude, in this study we show that mature PR3 and CD177 are co-expressed on the plasma membrane of neutrophils in all individuals including AASV patients. The mPR3+/CD177+ phenotype as well as mRNA expression of PR3 and CD177 are increased in neutrophils from patients with AASV and SLE, while in PV patients only the mRNA expression of CD177 is increased. The increased levels of PR3 and CD177 are not related to plasma levels of G-CSF or GM-CSF. We also show that PR3 depends upon CD177 for its membrane expression. Thus, further studies are needed to reveal the potential factors leading to this over-expression of PR3 and CD177 in AASV patients. Understanding the PR3–CD177 interaction may improve our knowledge of the pathophysiology of AASV and thereby facilitate the search for better treatment modalities for this serious and devastating illness.
Acknowledgments
This study was supported by Swedish Research Council (grant 71X-15152) and the Crafoord Foundation. The authors would like to thank Wieslab AB for providing monoclonal anti-PR3 antibodies (4A5) and Ellinor Johnsson for technical assistance.
Disclosure
None.
References
- 1.Ohlsson K, Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of two granulocyte collagenases. Eur J Biochem. 1973;36:473–81. doi: 10.1111/j.1432-1033.1973.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 2.van der Wiel BA, Dolman KM, Goldschmeding R, von dem Borne AEG, Hack CE. Alpha-1 anti-trypsin is the major inhibitor of the 29 kD cANCA antigen. Am J Kidney Dis. 1991;18:206A. [Google Scholar]
- 3.Skold S, Rosberg B, Gullberg U, Olofsson T. A secreted proform of neutrophil proteinase 3 regulates the proliferation of granulopoietic progenitor cells. Blood. 1999;93:849–56. [PubMed] [Google Scholar]
- 4.Witko-Sarsat V, Cramer EM, Hieblot C, et al. Presence of proteinase 3 in secretory vesicles: evidence of a novel, highly mobilizable intracellular pool distinct from azurophil granules. Blood. 1999;94:2487–96. [PubMed] [Google Scholar]
- 5.Csernok E, Ernst M, Schmitt W, Bainton DF, Gross WL. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994;95:244–50. doi: 10.1111/j.1365-2249.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell EJ, Campbell MA, Owen CA. Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity, and susceptibility to inhibition. J Immunol. 2000;165:3366–74. doi: 10.4049/jimmunol.165.6.3366. [DOI] [PubMed] [Google Scholar]
- 7.Halbwachs-Mecarelli L, Bessou G, Lesavre P, Lopez S, Witko-Sarsat V. Bimodal distribution of proteinase 3 (PR3) surface expression reflects a constitutive heterogeneity in the polymorphonuclear neutrophil pool. FEBS Lett. 1995;374:29–33. doi: 10.1016/0014-5793(95)01073-n. [DOI] [PubMed] [Google Scholar]
- 8.Lalezari P, Murphy GB, Allen FH., Jr NB1, a new neutrophil-specific antigen involved in the pathogenesis of neonatal neutropenia. J Clin Invest. 1971;50:1108–15. doi: 10.1172/JCI106582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caruccio L, Bettinotti M, Matsuo K, Sharon V, Stroncek D. Expression of human neutrophil antigen-2a (NB1) is increased in pregnancy. Transfusion. 2003;43:357–63. doi: 10.1046/j.1537-2995.2003.00320.x. [DOI] [PubMed] [Google Scholar]
- 10.Bux J, Goehring K, Wolff J, et al. Expression of NB1 glycoprotein (HNA-2a, CD177) on neutrophils is upregulated in inflammatory diseases and during G-CSF expression. Blood. 2002;100:462a. [Google Scholar]
- 11.Gohring K, Wolff J, Doppl W, et al. Neutrophil CD177 (NB1 gp, HNA-2a) expression is increased in severe bacterial infections and polycythaemia vera. Br J Haematol. 2004;126:252–4. doi: 10.1111/j.1365-2141.2004.05027.x. [DOI] [PubMed] [Google Scholar]
- 12.Temerinac S, Klippel S, Strunck E, et al. Cloning of PRV-1, a novel member of the uPAR receptor superfamily, which is overexpressed in polycythemia rubra vera. Blood. 2000;95:2569–76. [PubMed] [Google Scholar]
- 13.Kralovics R, Buser AS, Teo SS, et al. Comparison of molecular markers in a cohort of patients with chronic myeloproliferative disorders. Blood. 2003;102:1869–71. doi: 10.1182/blood-2003-03-0744. [DOI] [PubMed] [Google Scholar]
- 14.Teofili L, Martini M, Luongo M, et al. Overexpression of the polycythemia rubra vera-1 gene in essential thrombocythemia. J Clin Oncol. 2002;20:4249–54. doi: 10.1200/JCO.2002.11.507. [DOI] [PubMed] [Google Scholar]
- 15.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 16.Hellmich B, Csernok E, Trabandt A, Gross WL, Ernst M. Granulocyte–macrophage colony-stimulating factor (GM-CSF) but not granulocyte colony-stimulating factor (G-CSF) induces plasma membrane expression of proteinase 3 (PR3) on neutrophils in vitro. Clin Exp Immunol. 2000;120:392–8. doi: 10.1046/j.1365-2249.2000.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135:921–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Jennette JC. Antineutrophil cytoplasmic autoantibody-associated diseases: a pathologist's perspective. Am J Kidney Dis. 1991;18:164–70. doi: 10.1016/s0272-6386(12)80874-2. [DOI] [PubMed] [Google Scholar]
- 19.Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? BMJ (Clin Res Ed) 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Woude FJ, Rasmussen N, Lobatto S, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 21.Savage CO, Winearls CG, Jones S, Marshall PD, Lockwood CM. Prospective study of radioimmunoassay for antibodies against neutrophil cytoplasm in diagnosis of systemic vasculitis. Lancet. 1987;1:1389–93. doi: 10.1016/s0140-6736(87)90591-5. [DOI] [PubMed] [Google Scholar]
- 22.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 23.Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66:222–7. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–19. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdgawad M, Hellmark T, Gunnarsson L, Westman KW, Segelmark M. Increased neutrophil membrane expression and plasma level of proteinase 3 in systemic vasculitis are not a consequence of the –564 A/G promotor polymorphism. Clin Exp Immunol. 2006;145:63–70. doi: 10.1111/j.1365-2249.2006.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohlsson S, Wieslander J, Segelmark M. Increased circulating levels of proteinase 3 in patients with anti-neutrophilic cytoplasmic autoantibodies-associated systemic vasculitis in remission. Clin Exp Immunol. 2003;131:528–35. doi: 10.1046/j.1365-2249.2003.02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer S, Abdgawad M, Gunnarsson L, Segelmark M, Tapper H, Hellmark T. Proteinase 3 and CD177 are expressed on the plasma membrane of the same subset of neutrophils. J Leukoc Biol. 2007;81:458–64. doi: 10.1189/jlb.0806514. [DOI] [PubMed] [Google Scholar]
- 29.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994;87:671–8. [PubMed] [Google Scholar]
- 30.Ohlsson S, Hellmark T, Pieters K, Sturfelt G, Wieslander J, Segelmark M. Increased monocyte transcription of the proteinase 3 gene in small vessel vasculitis. Clin Exp Immunol. 2005;141:174–82. doi: 10.1111/j.1365-2249.2005.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pendergraft WF, Alcorta DA, Segelmark M, et al. ANCA antigens, proteinase 3 and myeloperoxidase, are not expressed in endothelial cells. Kidney Int. 2000;57:1981–90. doi: 10.1046/j.1523-1755.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- 32.Schenk S, Schoenhals GJ, de Souza G, Mann M. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics. 2008;1:41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang JJ, Pendergraft WF, Alcorta DA, et al. Circumvention of normal constraints on granule protein gene expression in peripheral blood neutrophils and monocytes of patients with antineutrophil cytoplasmic autoantibody-associated glomerulonephritis. J Am Soc Nephrol. 2004;15:2103–14. doi: 10.1097/01.ASN.0000135058.46193.72. [DOI] [PubMed] [Google Scholar]
- 34.Wolff J, Brendel C, Fink L, Bohle RM, Kissel K, Bux J. Lack of NB1 GP (CD177/HNA-2a) gene transcription in NB1 GP- neutrophils from NB1 GP-expressing individuals and association of low expression with NB1 gene polymorphisms. Blood. 2003;102:731–3. doi: 10.1182/blood-2002-09-2831. [DOI] [PubMed] [Google Scholar]
- 35.von Vietinghoff S, Tunnemann G, Eulenberg C, et al. NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood. 2007;109:4487–93. doi: 10.1182/blood-2006-10-055327. [DOI] [PubMed] [Google Scholar]
- 36.Kurosawa S, Esmon CT, Stearns-Kurosawa DJ. The soluble endot helial protein C receptor binds to activated neutrophils: involvement of proteinase-3 and CD11b/CD18. J Immunol. 2000;165:4697–703. doi: 10.4049/jimmunol.165.8.4697. [DOI] [PubMed] [Google Scholar]
- 37.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 38.Hajjar E, Mihajlovic M, Witko-Sarsat V, Lazaridis T, Reuter N. Computational prediction of the binding site of proteinase 3 to the plasma membrane. Proteins. 2008;71:1655–69. doi: 10.1002/prot.21853. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber A, Busjahn A, Luft FC, Kettritz R. Membrane expression of proteinase 3 is genetically determined. J Am Soc Nephrol. 2003;14:68–75. doi: 10.1097/01.asn.0000040751.83734.d1. [DOI] [PubMed] [Google Scholar]
- 40.Hu N, Westra J, Huitema MG, et al. Coexpression of CD177 and membrane proteinase 3 on neutrophils in antineutrophil cytoplasmic autoantibody-associated systemic vasculitis: anti-proteinase 3-mediated neutrophil activation is independent of the role of CD177-expressing neutrophils. Arthritis Rheum. 2009;60:1548–57. doi: 10.1002/art.24442. [DOI] [PubMed] [Google Scholar]


