Abstract
The proportions and activation status of T cells may influence responses to hepatitis C virus (HCV) and treatment outcome in patients receiving pegylated interferon (IFN)-α/ribavirin therapy. We confirmed that IFN-γ enzyme-linked immunospot (ELISPOT) responses to HCV are poor in HCV patients and showed that responses to HCV and cytomegalovirus (CMV) antigens decrease during therapy. This was most apparent in patients with sustained virological response (SVR). Baseline frequencies of CD4+ effector memory (TEM) T cells were lower in SVR than non-SVR. Proportions of CD4+ and CD8+ TEM and terminally differentiated effector memory (TEMRA) T cells declined on therapy in SVR, as did proportions of Fas+ CD8+ TEMRA T cells. Baseline frequencies of programmed death (PD)-1-expressing CD4+ TEM and TEMRA T-cells were higher in SVR. Therapy increased percentages of PD-1+ CD4+ central memory (TCM) T cells and PD-1+ CD8+ TEM and TEMRA T cells in SVR. We conclude that successful therapy depletes circulating antigen-specific CD4+ T cell responses. This paralleled decreases in proportions of effector memory T cells and higher percentages of CD4+ TCM T cells expressing PD-1.
Keywords: HCV, interferon-based therapy, PD-1, T cells
Introduction
Current therapy for hepatitis C virus (HCV) infection comprises pegylated interferon-α (pegIFN-α) and a guanosine analogue, ribavirin. Treatment suppresses viral replication in 70–80% of patients infected with HCV genotypes 2 and 3, and 40–50% of patients infected with HCV genotype 1 [1]. In addition to their anti-viral activities, IFN-α and ribavirin can modulate innate and adaptive immune responses [2]. The mechanisms invoked are not understood clearly.
The importance of cellular immune responses has been established in patients acutely infected with HCV, where viral clearance parallels vigorous and multi-specific HCV-specific T cell responses [3–5]. Effector memory (TEM) T cells are able to eliminate viruses such as HCV through the production of cytolytic molecules (e.g. perforin) and/or cytokines [e.g. interferon (IFN)-γ][6]. Human and chimpanzee studies have shown that the loss of these cells or impairment of their function promotes persistent HCV infection [7–9]. Failure of immune control in chronic HCV infection may result from persistent immune activation and subsequent apoptosis of HCV-specific T cells [10,11], increased expression of inhibitory co-stimulatory receptors such as programmed death (PD)-1 [12], the presence of regulatory T cells (Treg) [13] and/or dysfunction of antigen-presenting cells [14].
Several groups have examined HCV-specific CD4 and CD8 T cell responses in patients receiving anti-viral therapy with contradictory results. Barnes et al. [15] demonstrated diminished IFN-γ and interleukin (IL)-2 HCV- and cytomegalovirus (CMV)-specific T cells during high-dose IFN-α therapy, most notably in patients with a sustained virological response (SVR). This may be attributed to a reduction in the proportions of CD4 and CD8 T cells in the peripheral circulation, particularly memory T cell subsets capable of producing cytokines.
To date, the effects of therapy on naive and memory T cell populations have not been characterized adequately in treatment-naive patients. This study investigates the proportion and phenotype of T cell subsets in untreated and treated chronically HCV-infected patients, with a particular focus on T cell activation status and expression of Fas and PD-1. The proportions of Treg were also evaluated. These parameters are assessed in the context of changes in HCV-specific T cell immune responses during therapy.
Patients and methods
Patients
The cohort comprised Caucasian chronically HCV-infected patients (n = 34) recruited from Royal Perth Hospital (Western Australia) between 2003 and 2005. HCV infection was diagnosed by third-generation enzyme linked immunosorbent assay (ELISA) for antibodies to HCV. Patients were selected for pegIFN-α and ribavirin combination therapy (Pegasys; Roche, Dee Why, NSW, Australia or Pegatron; Schering-Plough, North Ryde, NSW, Australia) on the basis of clinical and laboratory indicators and a liver biopsy. Blood samples were collected at this time and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll (Amersham Biosciences, Buckinghamshire, UK) density gradient centrifugation and cryopreserved in 10% dimethylsulphoxide (DMSO) and 90% fetal calf serum (FCS) for subsequent analyses. PBMC samples were also collected for 19 of the 34 patients during [median time on therapy: 5 (3–10) months]. Patients with HCV genotypes 1 or 4 were treated for 48 weeks and those with genotypes 2 or 3 for 24 weeks. Patients were classified as sustained virological responders (SVR) if their serum HCV RNA was undetectable 24 weeks after end-of-treatment. SVR and non-SVR were sampled after similar intervals on treatment (P > 0·33). Healthy individuals with no evidence of exposure to human immunodeficiency virus (HIV) or HCV were included as controls. They were age- and sex-matched with patients [42 (21–73 years), seven males, nine females]. The study was approved by the Royal Perth Hospital Research Ethics committee and all patients and controls gave informed consent.
Detection of HCV RNA and HCV genotyping
Sera were stored at −80°C. Viral RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA, USA). HCV genotype was determined using the Line Probe Assay (Inno-LiPA; Innogenetics, Gent, Belgium) or by real-time polymerase chain reaction (PCR) and melting curve analysis using fluorescence resonance energy transfer (FRET) probes [16]. HCV viral loads prior to treatment and during therapy were quantitated using specific primers and Taqman probes for the conserved 5′ untranslated region (5′UTR) [17]. The lower limit of detection was 2·54 log10 copies/ml.
Liver biopsy
All patients underwent a liver biopsy prior to treatment. Fibrosis was evaluated according to the Scheuer [18] scoring system. Fibrosis was staged as: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, portal fibrosis with many septa; F4, cirrhosis.
Flow cytometric analyses
Cell surface antigens were detected with CD4-peridinin chlorophyll (PerCP)-cyanine (Cy)5·5, CD8-allophycocyanin (APC)-Cy7, CD45RA-phycoerythrin (PE)-Cy7, CD31-PE, CD95/Fas-APC, PD1-PE (BD Biosciences, San Jose, CA, USA), human leucocyte antigen D-related-fluorescein isothiocyanate (HLA-DR-FITC) (Coulter Immunotech, Marseille, France) and CD57-FITC (eBiosciences, San Diego, CA, USA) applied for 15 min at 22–24°C. Intracellular staining for forkhead box P3 (FoxP3) was performed using PE-conjugated anti-human FoxP3 and proprietary buffers from BD Biosciences. Stained cells were washed twice and analysed on a FACSCanto™ (Becton Dickinson, San Jose, CA, USA). At least 200 000 events were acquired and analysed using FlowJo (Treestar, San Carlos, CA, USA). Lymphocytes were identified by forward- and side-scatter (FSC and SSC). Absolute lymphocyte and CD4 and CD8 T lymphocyte counts from whole blood were performed on the day of sample collection in a routine laboratory at Royal Perth Hospital.
Expression of CD57 and CD45RA was used to define naive (CD45RA+CD57−), central memory (TCM) (CD45RA−CD57−), effector memory (TEM) (CD45RA−CD57+) and terminally differentiated effector memory (TEMRA) (CD45RA+CD57+) T cells. Many studies have used CCR7 to distinguish T cell differentiation status. CCR7 expression could not be detected on cryopreserved PBMC with the monoclonal antibody available commercially when this study was performed, but an alternative antibody is now available (clone 3D12; BD Biosciences). A pilot study of seven healthy controls and five HCV-infected patients confirmed that CCR7 and CD57 define similar percentages of naive and CD4+ TEMRA T cells and all CD8+ T cell subsets (see Supplementary Table S1). However, percentages of CD4+ TCM T cells were lower and CD4+ TEM T cells were higher using CCR7 compared to CD57.
Enzyme-linked immunospot assay (ELISPOT)
Nitrocellulose plates (Millipore, Danvers, MA, USA) were coated with anti-human IFN-γ antibody (15 µg/ml; Mabtech, Stockholm, Sweden) overnight at 4°C. Viable PBMC in RPMI-1640 with 10% fetal calf serum were plated in duplicate at 1·0 or 2·0 × 105 cells per well and stimulated for 24 h at 37°C with the following antigens: recombinant HCV core and NS3 (final concentration, 2·5 µg/ml; Virogen, Watertown, MA, USA), CMV lysate [19], medium alone as a negative control or anti-human CD3 (final concentration, 10 ng/ml; BD Pharmingen, San Diego, CA, USA) as a positive control. Co-stimulatory antibodies anti-CD28 and anti-CD49d (final concentration 1 µg/ml; BD Pharmingen) were added to all wells except those stimulated with anti-human CD3. Spots were detected with biotinylated anti-human IFN-γ antibody (Mabtech), streptavidin–horseradish peroxidase conjugate (BD Pharmingen) and tetramethylbenzidine substrate (Mabtech) and counted using AID ELISPOT reader version 2·9 software (Autoimmun Diagnostika GmbH, Strasberg, Germany). Frequencies of reactive T cells were determined by subtracting average numbers of spot-forming cells in negative control wells from numbers in stimulated wells, and are expressed per 2 × 105 PBMC. To determine the cellular source of IFN-γ production following stimulation with HCV core and NS3 antigens, PBMC samples from two HCV-infected patients were depleted of CD4+ or CD8+ cells using magnetic beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). This established that secretion of IFN-γ was limited to CD4+ T cells (data not shown).
Statistics
Statistical analyses were performed using Graphpad Prism version 5·01 (Graphpad Software, San Diego, CA, USA). Differences between study groups were evaluated using Mann–Whitney U-tests. Correlation analyses were carried out using Spearman's rank correlation coefficients. P-values < 0·05 were considered statistically significant and 0·05 < P < 0·10 is noted. Data in figures are presented as box-and whisker-plots showing median, 25th and 75th percentiles and range.
Results
Neither T cell responses to HCV antigens nor the severity of HCV disease at baseline predict treatment outcome
Sustained virological responses (SVR) after pegIFN-α/ribavirin therapy were achieved in 20 of 34 patients (59%). Baseline characteristics for patients who achieved an SVR and those who failed to clear the virus (non-SVR) were similar (Table 1), as were baseline IFN-γ responses to HCV and CMV antigens. T cell responses to HCV did not correlate with HCV genotype, viral loads, serum alanine aminotransferase (ALT) levels or liver histology before or during treatment (data not shown).
Table 1.
Baseline characteristics in hepatitis C virus (HCV)-infected patients.
| SVR (n = 20) | Non-SVR (n = 14) | |
|---|---|---|
| Age (years) | 48 (21–56) | 48 (21–66) |
| Sex(male/female) | 11/9 | 9/5 |
| HCV genotype 1/2/3 | 11/0/9 | 9/2/3 |
| Serum HCV RNA (log10 copies/ml) | 5·44 (3·97–6·55) | 5·32 (4·20–6·11) |
| Serum ALT(U/ml) | 123 (30–449) | 107 (25–224) |
| Liver histology(Scheuer) | ||
| Fibrosis 1/2/3/4 | 8/7/1/4 | 3/5/3/3 |
| IFN-γ responses* | ||
| HCV core | 44 (0–104) | 43 (0–146) |
| HCV NS3 | 43 (0–91) | 29 (0–180) |
| CMV | 53 (0–376) | 66 (14–410) |
Interferon (IFN)-γ responses are expressed as spots per 2 × 105 peripheral blood mononuclear cells. ALT, serum alanine aminotransferase; CMV, cytomegalovirus; SVR, sustained virological response.
PegIFN-α/ribavirin therapy depresses IFN-γ responses to viral antigens
From the 34 patients studied, 19 had donated blood during therapy [median (range) time on therapy; 6 (3–10) months]. SVR was achieved in 13 of 19 patients (68%). IFN-γ responses to HCV antigens and CMV were similar in patients and controls (Fig. 1) and declined during treatment. Classification of patients based on virological response demonstrated significantly lower HCV core-specific responses during therapy in SVR [44 (0–104) versus 3·5 (0·0–106), P = 0·03], with marginal significances observed for NS3 [43 (0–91) versus 3·0 (0·0–294), P = 0·11] and CMV [53 (0–376) versus 34 (0·0–99), P = 0·06]. Median values were not significantly lower during therapy in non-SVR [core: 43 (0–146) versus 37 (2·5–106), P = 0·63, NS3: 29 (0–180) versus 12 (0–93), P = 0·56, CMV: 66 (14–410) versus 16 (5·5–186), P = 0·32].
Fig. 1.
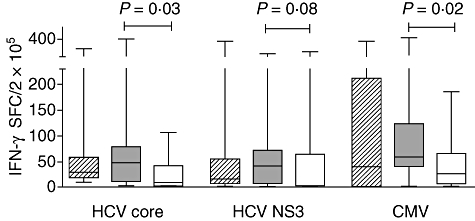
Interferon (IFN)-γ responses to hepatitis C virus (HCV) and cytomegalovirus (CMV) antigens in healthy controls (diagonal) and patients assessed at baseline (closed) and during therapy (open). Results are presented as box-and-whisker plots showing median values and the 25th and 75th percentiles.
We considered whether the reduced IFN-γ ELISPOT counts on therapy reflected absolute numbers or proportions of T cells. Absolute numbers of CD4+ and CD8+ T cells declined in SVR (P = 0·03 and P = 0·0009, respectively) (Table 2). However, the percentage of CD4+ T cells increased while CD8+ T cells decreased during treatment (P = 0·01), so a lack of CD4+ T cells does not explain the reduced ELISPOT counts. It may be important that the decline occurred in the context of small reductions in immune activation. HLA-DR expression declined slightly during therapy on CD4+[15 (4·6–37)% versus 12 (5·0–22)%, P = 0·09] and CD8+ T cells [46 (11 77)% versus 34 (11–61)%, P = 0·03] in SVR but not in non-SVR [CD4+: 17 (7·7–22)% versus 16 (5·0–18)%, P = 0·52 and CD8+: 46 (17–61)% versus 50 (15–61)%, P = 0·39]. Phenotypic changes were therefore explored in more detail.
Table 2.
Proportions and phenotype of naive T cells in hepatitis C virus (HCV)-infected patients in relation to treatment outcome.
| Healthy controls |
SVR |
Non-SVR |
|||
|---|---|---|---|---|---|
| (n = 16) | Baseline (n = 20) | During therapy (n = 13) | Baseline (n = 14) | During therapy (n = 6) | |
| Lymphocyte count (cells/µl) | 2900 (1700–4200) | 1800 (900–3200)* | 3200 (2000–4500) | 2000 (1200–4800)* | |
| Lymphocyte (%) | 70 (52–77) | 69 (59–84) | 71 (65–84) | 79 (65–82) | 74 (63–78) |
| CD4+ T cell count (cells/µl) | 1289 (798–2016) | 895 (530–1888)* | 1504 (920–2262) | 943 (680–2544)* | |
| CD4+ (%) | 46 (31–65) | 44 (35–69) | 51 (33–76)* | 45 (35–53) | 50 (40–58) |
| CD8+ T cell count (cells/µl) | 800 (352–1287) | 400 (110–768)* | 925 (352–1440) | 414 (264–1632)* | |
| CD8+ (%) | 17 (8·9–29) | 26 (16–40) | 23 (10–30)* | 27 (14–43) | 22 (16–36) |
| CD31+CD45RA+ (% CD4) | 28 (3·8–38) | 25 (4·4–47) | 26 (6·3–37) | 21 (11–44) | 26 (16–34) |
| CD31−CD45RA+ (% CD4) | 15 (7·4–30) | 15 (4·4–35) | 16 (7·8–33) | 14 (7·8–35) | 14 (7·8–46) |
| CD45RA+CD57− (% CD8) | 41 (5·5–70) | 34 (9·7–62) | 51 (19–68)* | 33 (12–63) | 51 (19–64) |
| HLA-DR expression | |||||
| CD31+CD45RA+ (% CD4) | 2·5 (0·9–11) | 2·6 (0·9–10) | 2·7 (1·3–58) | 2·4 (1·3–5·9) | 2·2 (1·1–7·7) |
| CD31−CD45RA+ (% CD4) | 1·6 (0·4–21) | 2·5 (1·0–13) | 2·1 (1·1–6·7) | 3·9 (1·1–42) | 3·5 (1·4–6·4) |
| Fas expression | |||||
| CD31+CD45RA+ (% CD4) | 4·1 (1·4–14) | 4·1 (1·6–12) | 3·4 (2·3–20) | 4·2 (2·8–11) | 4·7 (2·3–7·7) |
| CD31−CD45RA+ (% CD4) | 15 (7·6–32) | 15 (6·6–39) | 14 (6·9–41) | 18 (8·7–58) | 22 (9·2–34) |
Results are presented as median (range). P-values determined by Mann–Whitney U-test.
P < 0·05 baseline versus during therapy. SVR: sustained virological response.
Proportions of naive CD8 T cells increased during therapy while proportions and phenotype of naive CD4+ T cells do not predict treatment outcome or change on therapy
The naive T cell compartment is replenished from the thymus and by homeostatic proliferation in the periphery. Naive CD4+ T cells can be classified as thymus-naive (CD31+CD45RA+) or central-naive (CD31−CD45RA+) based on expression of CD45RA with or without CD31 [20]. CD31 is expressed on CD45RA+ CD8+ T cells but ∼50% of CD45RA− CD8+ T cells also express CD31 [21], so expression of CD31 on CD8+ T cells cannot be interpreted meaningfully and is not reported here. Instead we defined naive CD8+ T cells as CD45RA+CD57−.
Before the start of therapy, percentages of naive CD8+ T cells and both subsets of naive CD4+ T cells were similar in controls and all patients. Baseline percentages of thymus-naive and central-naive CD4+ T cells did not differ between SVR and non-SVR and these values did not change on therapy in both groups (Table 2). Treatment increased percentages of naive CD8+ T cells compared to baseline in SVR (P = 0·02) and marginally in non-SVR (P = 0·06).
The combined patient cohort at baseline had higher HLA-DR and Fas expression on central-naive CD4+ T cells than controls (P = 0·02 and P = 0·09, respectively), but percentages of thymus-naive CD4+ expressing HLA-DR or Fas did not vary (data not shown). Classification of patients based on treatment outcome revealed similar percentages of naive T cells expressing HLA-DR and Fas in SVR and non-SVR at baseline (Table 2). Proportions remained unchanged during therapy.
Effector memory T cells change on therapy and baseline levels may predict treatment outcome
Cell surface markers used to distinguish effector memory T cells was explained earlier (see also Supplementary Table S1; Fig. 2 top panels). Percentages of CD4+ and CD8+ memory T cell subsets were similar in controls and patients, analysed as a combined patient cohort or classified by virological response. The frequency of CD4+ TEM (CD45RA−CD57+) was lower in SVR than non-SVR at baseline (P = 0·003) (Fig. 2). Frequencies of TEM and TEMRA (CD45RA+CD57+) declined on therapy in SVR within CD4+ T cells (P = 0·006 and P = 0·03, respectively) and CD8+ T cells (P < 0·0001 and P = 0·02, respectively).
Fig. 2.
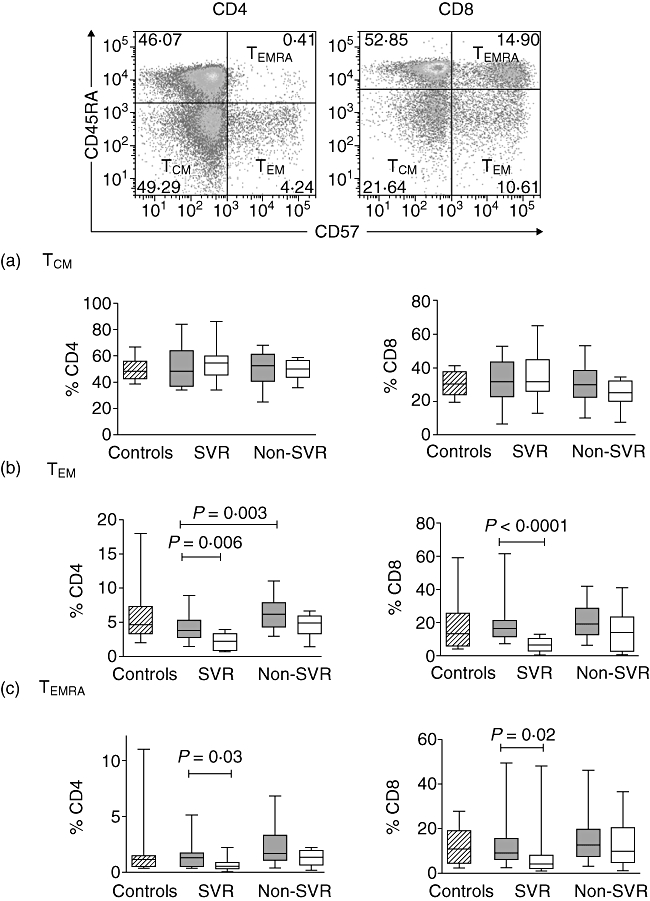
Representative example of CD45RA and CD57 expression on CD4+ and CD8+ T cells. Proportions of central memory (TCM) (a), effector memory (TEM) (b) and terminally differentiated effector memory (TEMRA) (c) T cells in healthy controls (diagonal) and in hepatitis C virus (HCV)-infected patients at baseline (grey) and during therapy (white). Results are presented as box-and-whisker plots showing median values and the 25th and 75th percentiles.
Patients with an SVR showed a decline in Fas expression on CD8+ TEMRA T cells during therapy [79 (20-97) versus 59 (8·0-97); P = 0·01], with no clear changes on other memory T cell subsets. Proportions of Fas expressing CD4+ and CD8+ T cells were not modified during treatment in non-SVR (data not shown).
PD-1 expression on the different T cell subsets was similar in the combined patient cohort and controls. Prior to therapy, PD-1 expression on CD4+ TEMRA T cells was higher in SVR than non-SVR (P = 0·001), whereas no difference was observed for CD8+ T cells (Fig. 3). In SVR, treatment increased expression of PD-1 on CD4+ TCM (CD45RA−CD57−) T cells (P = 0·01) and on CD8+ TEM and TEMRA T cells (P = 0·01 and P = 0·007, respectively). There was also increased PD-1 expression on CD4+ and CD8+ TEMRA T cells from non-SVR (P = 0·05 and P = 0·02).
Fig. 3.
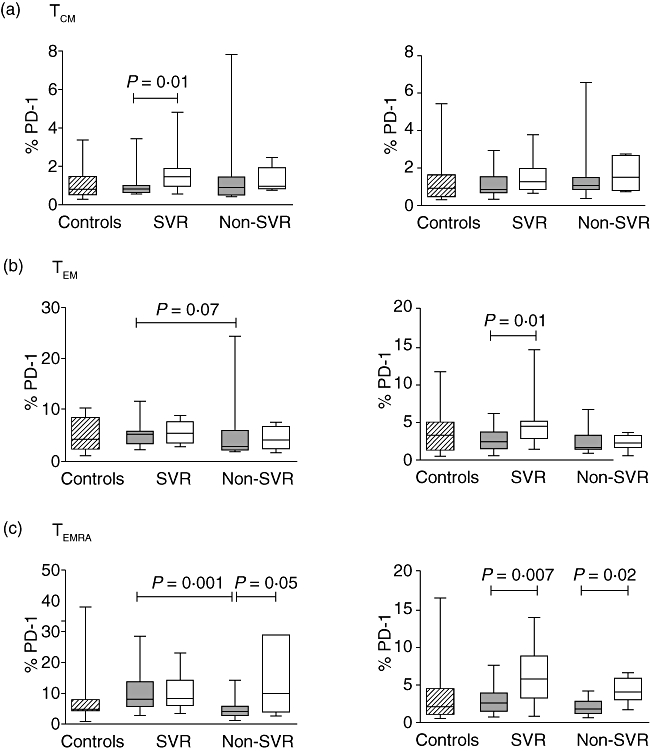
Proportions of central memory (TCM) (a), effector memory (TEM) (b) and terminally differentiated effector memory (TEMRA) (c) T cells expressing programmed death (PD)-1 (CD4+ on left panel and CD8+ on right panel) in healthy controls (diagonal) and in hepatitis C virus (HCV)-infected patients at baseline (grey) and during therapy (white). Results are presented as box-and-whisker plots showing median values and the 25th and 75th percentiles.
Proportions of regulatory T cells do not correlate with treatment outcome and are not affected by therapy
Before the start of therapy, proportions of CD4+ T cells expressing CD25+CD127lo were similar in controls, SVR and non-SVR [4·1 (3·0–6·0)%, 4·6 (2·5–7·9)%, 3·6 (1·8–5·8)%]. Similarly, when FoxP3 was used to identify Treg cells, there was no difference in the proportions between the three groups [4·4 (3·4–6·5)%, 5·4 (2·9–12)%, 4·9 (2·0–8·5)%]. In patients with SVR, the median frequencies of CD25+CD127lo and FoxP3+ expressing CD4+ T cells during therapy were 5·6 (3·4–7·2)% and 6·9 (3·4–9·5)%, respectively, and in non-SVR the median frequencies were 4·2 (3·2–5·8)% and 4·1 (3·2–5·9)%, respectively. For both groups, these frequencies were not statistically different from baseline.
Discussion
Studies examining the effects of IFN-α and ribavirin treatment on T cell subsets and IFN-γ responses to HCV antigens have yielded conflicting results. We report reduced IFN-γ responses to HCV core and NS3 antigens in patients during therapy, consistent with several studies [15,22,23]. However, increased proliferative and IFN-γ responses to HCV antigens have also been reported in patients receiving pegIFN-α and ribavirin [24,25]. Detailed analysis of our data revealed reduced IFN-γ responses to HCV proteins in SVR rather than non-SVR. This is unlikely to reflect the smaller number of non-SVR, because the median value dropped by 90% in SVR and only 14% in non-SVR. The difference may be related to suppression of HCV viral load during therapy with an associated reduction in antigenic stimulation and immune activation. Decreased HLA-DR expression on CD4+ and CD8+ T cells in SVR during therapy supports this finding further. Neau-Cransac et al. [26] described decreased HLA-DR expression on CD4+ and CD8+ T cells during IFN-α and ribavirin therapy, regardless of treatment outcome, while Appasamy et al. [27] reported increased frequencies of HLA-DR-expressing CD4+ T cells during IFN-α monotherapy and did not distinguish SVR and non-SVR. Differences in treatment regimen could explain the discrepancies in the results.
Longitudinal studies demonstrate recovery of HCV-specific responses upon cessation of treatment [15,23]. This was evident in patients who achieved an SVR, suggesting that T cell responses are not related directly to HCV viral loads. Furthermore, suppression of T cell responses was not limited to HCV, as IFN-γ production was also decreased after stimulation with CMV antigen. This is consistent with a previous study [15] and could be explained by a global loss of antigen-specific T cells from the peripheral circulation. Lymphopenia is one of the side effects of pegIFN-α therapy and we showed decreased absolute CD4+ and CD8+ T cell counts in all patients (Table 2). However, the proportions of T lymphocytes did not fall, regardless of treatment outcome, and the percentage of CD4+ T cells increased in SVR. Therefore, suppressed IFN-γ responses cannot be attributed to the scarceness of CD4+ T cells in the PBMC preparation used in the ELISPOT assay. This study was designed to investigate the frequencies and phenotypes of different lymphocyte subsets to explain the decreased T cell responses in SVR.
We evaluated expression of CD31 and CD45RA to distinguish between thymus and central naive CD4+ T cells. We found no differences in the percentages of thymus-naive and central-naive CD4+ T cells in SVR and non-SVR at baseline and proportions did not change during therapy. The only previous studies evaluating changes in naive T cell frequencies during IFN-α therapy included patients co-infected with HIV. During therapy, absolute numbers of CD4+ T cells expressing CD45RA decreased while their proportions increased [28,29]. However, the use of CD45RA as a single marker to identify naive T cells may also include CD45RO+ T cells that have reverted to CD45RA+ as late effector memory T cells.
Effector T cells are essential for viral clearance and protection from disease progression. Expression of CD57 alone on CD8+ T cells usually identifies cells as terminally differentiated with a reduced ability to proliferate, increased susceptibility to activation-induced apoptosis and the production of IFN-γ but not IL-2 [30]. CD4+ T cells can also express CD57 to share similar characteristics as CD57-expressing CD8+ T cells [31]. Here, CD45RA and CD57 were used to determine the proportions of TCM (CD45RA−CD57−), TEM (CD45RA−CD57+) and TEMRA (CD45RA+CD57+) T cells. Before therapy, percentages of TEM CD4+ T cells were higher in non-SVR than SVR (Fig. 2). Frequencies of TEM and TEMRA CD4+ T cells declined further on therapy in SVR, consistent with the decreased CD4+ T cell IFN-γ ELISPOT responses. As CD4+ T cells are required for the maintenance of CD8+ T cells during chronic infection, the reduced percentages of memory CD4+ T cells may contribute to the decreased proportions of TEM and TEMRA CD8+ T cells observed in SVR. We lacked samples with defined HLA genotypes to evaluate HCV-specific CD8+ T cell responses, but decreased frequencies of tetramer-positive CD8+ T cells recognizing HCV core and NS3 peptides have been reported during pegIFN-α/ribavirin therapy [32].
We speculated that apoptosis of effector T cells in the peripheral circulation may limit T cell responses. However, our results demonstrated no change in Fas expression on effector CD4+ T cells and even decreased expression on effector CD8+ T cells in SVR. PD-1 is expressed predominately on activated T cells, and interaction with its ligands inhibits T cell proliferation and production of cytokines [33]. In acute HCV infection, expression of PD-1 on virus-specific CD8+ T cells in the peripheral blood and livers declined in patients who resolved their infections, whereas high levels were maintained in those who developed chronic infection [12]. This is the first study to evaluate PD-1 expression on central memory and effector memory CD4+ and CD8+ T cells from treatment-naive patients with chronic HCV and during therapy. PD-1 was expressed preferentially on effector T cells, notably TEMRA cells. This is consistent with a report of PD-1high HCV-specific CD8 T cells expressing high levels of CD57 [12]. Prior to therapy, PD-1 expression on TEM and TEMRA CD4+ T cells was higher in SVR compared to non-SVR (Fig. 3). This did not associate with HCV viral load or CD4+ T cell responses at baseline in patients with an SVR (data not shown). During therapy, proportions of PD-1 expressing cells increased in TCM CD4+ T cells and in effector memory CD8+ T cell subsets in SVR. Up-regulation of PD-1 expression on these cells could limit HCV-specific CD8+ T cell responses [32].
Treg can suppress antigen-specific T cells resulting in the establishment of chronicity in many viral infections [34]. Treg cells are identified by the expression of FoxP3, a forkhead transcription factor [35]. Cells sorted with the phenotype CD4+CD25+CD127lo demonstrated increased levels of FoxP3 and were highly suppressive in functional assays [36]. The only study examining Treg in patients receiving pegIFN-α and ribavirin combination therapy found no difference in FoxP3-expressing CD4+CD25hi T cells or suppressive function in patients who were able to control viral replication and those with detectable viral load throughout therapy [23]. As most CD4+CD25hi Treg cells express FoxP3 [37], we also used proportions of CD4+ T cells expressing FoxP3 in addition to CD4+CD25+CD127lo to identify Treg cells. A strong positive correlation was observed between these parameters (data not shown). At baseline, frequencies of CD4+ T cells expressing FoxP3 or CD25hiCD127lo were similar in SVR and non-SVR. Values did not change during therapy regardless of treatment outcome, so monitoring of Treg cells would not predict a response to therapy and these cells are probably not involved in suppressing antigen-specific responses by PBMC. However, there may be modifications to the percentages or functions of intrahepatic Treg cells.
There are several limitations to this study. First, only 19 of the 34 patients could be evaluated during therapy. However, similar results were obtained when the cohort was restricted to patients with samples available before and on therapy (data not shown). Secondly, we could not study intrahepatic T cells because liver biopsies are not taken on therapy. HCV-specific T cells may be sequestered to the site of infection during IFN-α treatment and released when therapy is stopped [15]. CXCR3 is implicated in the recruitment of T cells to the liver. Chronically HCV-infected patients who achieved an SVR after 24 weeks of therapy had higher expression of CXCR3 on CD8+ T cells [38]. As responses to CMV were also affected by pegIFN-α therapy, it may be important to investigate chemokine receptors on other antigen-specific T cells to determine if sequestration of these cells also occurs. Furthermore, the association between CXCR3 expression on CD4+ T cells and cellular immune responses in HCV-infected patients have not been evaluated. In HIV/HCV co-infected patients, decreased proportions of CD4+ T cells expressing CXCR3 [39] could account for the diminished HCV-specific CD4+ T cell responses when compared to patients with HCV mono-infection [40].
In summary, we describe decreased CD4+ T cell responses to HCV proteins and an unrelated common antigen, CMV, during pegIFN-α/ribavirin therapy in patients with SVR. This suggests global suppression of antigen-specific CD4+ T cell responses. Lower frequencies of circulating TEM and TEMRA CD4+ T cells and/or increased expression of PD-1 may contribute to this finding.
Acknowledgments
The authors thank the patients who donated blood for this study and Saroj Nazareth and Marion McInerney who assisted in the collection of samples. This work received support from Schering-Plough Pty Limited, North Ryde, NSW. This is publication 2009-23 (Clinical Immunology and Immunogenetics, RPH).
Disclosure
None of the authors have any conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Comparison of CD45RA and CCR7 or CD57 to identify T-cell differentiation status.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Deutsch M, Hadziyannis SJ. Old and emerging therapies in chronic hepatitis C: an update. J Viral Hepat. 2008;15:2–11. doi: 10.1111/j.1365-2893.2007.00887.x. [DOI] [PubMed] [Google Scholar]
- 2.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–72. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 3.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang KM, Thimme R, Delpolder JJ, et al. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–76. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 6.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–79. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 7.Nisii C, Tempestilli M, Agrati C, et al. Accumulation of dysfunctional effector CD8+ T cells in the liver of patients with chronic HCV infection. J Hepatol. 2006;44:475–83. doi: 10.1016/j.jhep.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–62. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 9.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–55. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuti S, Rosa D, Valiante NM, et al. Dynamics of intra-hepatic lymphocytes in chronic hepatitis C: enrichment for Valpha24+ T cells and rapid elimination of effector cells by apoptosis. Eur J Immunol. 1998;28:3448–55. doi: 10.1002/(SICI)1521-4141(199811)28:11<3448::AID-IMMU3448>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Giovannetti A, Mazzetta F, Coviello R, et al. T-cell immune activation in children with vertically transmitted hepatitis C virus infection. Viral Immunol. 2001;14:169–79. doi: 10.1089/088282401750234547. [DOI] [PubMed] [Google Scholar]
- 12.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–58. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabrera R, Tu Z, Xu Y, et al. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 14.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123:40–9. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes E, Gelderblom HC, Humphreys I, et al. Cellular immune responses during high-dose interferon-alpha induction therapy for hepatitis C virus infection. J Infect Dis. 2009;199:819–28. doi: 10.1086/597072. [DOI] [PubMed] [Google Scholar]
- 16.Bullock GC, Bruns DE, Haverstick DM. Hepatitis C genotype determination by melting curve analysis with a single set of fluorescence resonance energy transfer probes. Clin Chem. 2002;48:2147–54. [PubMed] [Google Scholar]
- 17.Castelain S, Descamps V, Thibault V, et al. TaqMan amplification system with an internal positive controls for HCV RNA quantitation. J Clin Virol. 2004;31:227–34. doi: 10.1016/j.jcv.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 19.Watson MW, Jaksic A, Price P, et al. Interferon-gamma response by peripheral blood mononuclear cells to hepatitis C virus core antigen is reduced in patients with liver fibrosis. J Infect Dis. 2003;188:1533–6. doi: 10.1086/379252. [DOI] [PubMed] [Google Scholar]
- 20.Kohler S, Thiel A. Life after the thymus–CD31+ and CD31–human naïve CD4+ T-cell subsets. Blood. 2009;113:769–74. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y, Albelda SM, Horgan KJ, et al. CD31 expressed on distinctive T cell subsets is a preferential amplifier of b1 integrin-mediated adhesion. J Exp Med. 1992;176:245–53. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capa L, Soriano V, García-Samaniego J, et al. Evolution of T-cell responses to hepatitis C virus (HCV) during pegylated interferon plus ribavirin treatment in HCV-monoinfected and in HCV/HIV-coinfected patients. Antivir Ther. 2007;12:459–68. [PubMed] [Google Scholar]
- 23.Burton JR, Jr, Klarquist J, Im K, et al. Prospective analysis of effector and regulatory CD4+ T cells in chronic HCV patients undergoing combination antiviral therapy. J Hepatol. 2008;49:329–38. doi: 10.1016/j.jhep.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Kamal SM, Fehr J, Roesler B, Peters T, Rasenack JW. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology. 2002;123:1070–83. doi: 10.1053/gast.2002.36045. [DOI] [PubMed] [Google Scholar]
- 25.Barnes E, Harcourt G, Brown D, et al. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743–54. doi: 10.1053/jhep.2002.35344. [DOI] [PubMed] [Google Scholar]
- 26.Neau-Cransac M, Foucher J, Ledinghen VD, Bernard PH, Legrand E, Lafon ME. Modifications of T-lymphocyte subsets before and during interferon and ribavirin treatment for chronic hepatitis C infection. Viral Immunol. 2005;18:197–204. doi: 10.1089/vim.2005.18.197. [DOI] [PubMed] [Google Scholar]
- 27.Appasamy R, Bryant J, Hassanein T, Van Thiel DH, Whiteside TL. Effects of therapy with interferon-alpha on peripheral blood lymphocyte subsets and NK activity in patients with chronic hepatitis C. Clin Immunol Immunopathol. 1994;73:350–7. doi: 10.1006/clin.1994.1209. [DOI] [PubMed] [Google Scholar]
- 28.Neau D, Galperine T, Legrand E, et al. T-lymphocyte populations in hepatitis C and HIV co-infected patients treated with interferon-alfa-2a and ribavirin. HIV Med. 2003;4:120–6. doi: 10.1046/j.1468-1293.2003.00140.x. [DOI] [PubMed] [Google Scholar]
- 29.Arizcorreta A, Marquez M, Ferandez-Gutierrez C. T-cell receptor excision circles (TRECs), CD4+, CD8+ and their CD45RO+ and CD45RA+ subpopulations in hepatitis C virus (HCV)–HIV-co-infected patients during treatment with interferon alpha plus ribavirin :analysis in a population on effective antiretroviral therapy. Clin Exp Immunol. 2006;146:270–77. doi: 10.1111/j.1365-2249.2006.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 31.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175:8415–23. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand J, Cornberg M, Aslan N, et al. Fate and function of hepatitis-C-virus-specific T-cells during peginterferon-alpha2b therapy for acute hepatitis C. Antivir Ther. 2007;12:303–16. [PubMed] [Google Scholar]
- 33.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatila TA. Role of regulatory T cells in human diseases. J Allergy Clin Immunol. 2005;116:949–59. doi: 10.1016/j.jaci.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 35.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 38.Larrubia JR, Calvino M, Benito S, et al. The role of CCR5/CXCR3 expressing CD8+ cells in liver damage and viral control during persistent hepatitis C virus infection. J Hepatol. 2007;47:632–41. doi: 10.1016/j.jhep.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Roe B, Coughlan S, Dean J, et al. Phenotypic characterization of lymphocytes in HCV/HIV co-infected patients. Viral Immunol. 2009;22:39–48. doi: 10.1089/vim.2008.0074. [DOI] [PubMed] [Google Scholar]
- 40.Harcourt G, Gomperts E, Donfield S, Klenerman P. Diminished frequency of hepatitis C virus specific interferon gamma secreting CD4+ T cells in human immunodeficiency virus/hepatitis C virus coinfected patients. Gut. 2006;55:1484–7. doi: 10.1136/gut.2005.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


