Abstract
Resistance to intracellular pathogens such as Mycobacterium leprae is dependent upon an effective T helper type 1 (Th1)-type immune response. On the other hand, intestinal helminths are known to subvert the host's immune response towards to either a Th2-type immune response or a regulatory T cell up-regulation, which may affect the host's ability to mount an effective response to mycobacteria. Here, we report a significant association between intestinal helminth infections and lepromatous leprosy [odds ratio (OR), 10·88; confidence interval (CI) 95%: 4·02–29·4; P < 0·001]. We also observed that the frequency of intestinal helminths correlated strongly with the mycobacterial index (r = 0·982, P < 0·01). Corroborating with our hypothesis, intracellular levels of interferon-γ were decreased significantly in leprosy patients co-infected with intestinal helminths when compared to leprosy patients without worms. Conversely, lepromatous leprosy patients with intestinal worms produced higher levels of both interleukin (IL)-4 and IL-10. Our results suggest that a pre-existing infection by intestinal helminths may facilitate the establishment of M. leprae infection or its progression to more severe forms of leprosy.
Keywords: cellular immunity, cytokines, intestinal helminths, multi-bacillary, Mycobacterium leprae
Introduction
Leprosy is a multi-spectral disease presenting, at opposing poles, the tuberculoid and lepromatous clinical forms. Cell-mediated immune response is an important aspect of host resistance to mycobacterial infection, and is regulated tightly by a balance between T helper type 1 (Th1), Th17 and Th2 cytokines. Interferon (IFN)-γ, produced by Th1 and natural killer (NK) cells in response to IL-12, activates macrophages (MΦ) and plays a pivotal role in anti-mycobacterial immune responses. In contrast, production of IL-10 during bacterial infection has been shown to suppress production of inflammatory mediators and to aid the development of Th2-type immunity. Th1 response predominates in patients with tuberculoid leprosy and has been associated with the observed resistance to Mycobacterium leprae displayed by these subjects. On the other hand, patients with the lepromatous form, characterized by an absent or inept cytotoxic T cell response, develop a Th2 immune response [1,2].
When individuals living in two distinct geographic areas with similar leprosy prevalence were compared, a higher frequency of lepromatous leprosy was observed among those residing in filarisis hyperendemic area [3]. Both human and experimental helminthic infections are characterized by elevated plasmatic IgE titres, peripheral blood and/or tissue eosinophilia and mastocytosis, and are associated usually with either a strong Th2, regulatory T cell (Treg) up-regulation or an impaired Th1/Th17 response [4–7]. In humans, helminthic infections have been also associated with an immunological hyporeactivity state [8–11]. Considering that an effective anti-Mycobacterium tuberculosis (MTB) immunity is dependent upon an intact Th1/Th17-type immunity, it is possible that a pre-existing infection with intestinal worms could facilitate a subsequent mycobacterial infection and/or its progression to more severe forms, via up-regulation of Treg cells or Th2-type cytokine production [2,4–10,12,13]. Active tuberculosis (TB) has been associated with the expansion of CD4+CD25high+, a subset of Treg cells, which may participate in the suppression of MTB-specific T cell responses, observed frequently among pulmonary TB patients [14]. Interestingly, it has been demonstrated that Treg frequencies are also augmented in nematode infections [15,16], and that antigen-specific Treg cells contribute to the immunosuppression associated with chronic onchocerciasis [16]. Recently, Babu et al. [16] demonstrated that Th-1- and Th-17-type responses were down-modulated by filarial co-infection in patients with pulmonary TB through an increase in the expression of programmed death-1 (PD-1) and cytotoxic T lymphocyte antigen-4 (CTLA-4), also associated with Treg cells and hyporeactive state in TB patients [10].
A significant association between intestinal helminthic infections and different bacterial infections, such as pulmonary tuberculosis, multi-bacillary leprosy and staphylococcal pyomiositis, was reported previously by our group [11,17–19]. We have demonstrated that multi-bacillary leprosy prevalence is significantly higher among patients with intestinal helminth infections when compared to patients without intestinal worms [17]. A direct correlation [odds ratio (OR) = 2·99, 95% confidence interval (CI) = 1·82–4·95, P = 0·000] between the presence of helminthic infection and multi-bacillary leprosy was also observed [17], corroborating the hypothesis that chronic immune activation caused by the intestinal worms may hinder the onset/maintenance of an effective immune response against mycobacteria. Data from these previous studies helped us to design a prospective study to investigate the prevalence of helminthic infections among leprosy patients and their healthy household contacts, and to evaluate if T cell activation status and cytokine profile from tuberculoid leprosy patients harbouring or not intestinal helminths were altered.
Materials and methods
Subjects and samples used
One hundred and five adult, consecutive, treatment-naive leprosy patients, residents in the Metropolitan Region of Vitória, Espírito Santo State, attending the Leprosy Reference Center at the Santa Casa de Misericordia, Vitória, Espirito Santo state, Brazil, were invited to participate in the current study. A group of 146 age- and gender-matched household contacts from enrolled patients were also invited to participate as healthy controls (at least one household contact per leprosy patient was enrolled). Signed informed consent was obtained from all patients and household contacts prior to their enrolment. The present study was approved by the Internal Review Board at the Universidade Federal do Espírito Santo. Three consecutive stool samples from all patients and household contacts provided for intestinal helminth infection evaluation, which was carried out by three different methods: sedimentation (Hoffman, Ponz and Janner), Kato-Katz and Baerman techniques [20].
Leprosy patients were grouped according to their clinical form, following the criteria described at the Leprosy Control Manual from the Brazilian Ministry of Health [21]. Additionally, patients were also grouped according to their bacilloscopic index (Ridley and Joplin index) as paucibacillary (negative bacilloscopy results, including patients with either indeterminate or tuberculoid leprosy) or as multi-bacillary (positive bacilloscopy, including patients with dimorphous and lepromatous clinical forms) [22].
Assessment of intracellular cytokines in ex-vivo samples stimulated with polyclonal stimuli
Intracellular cytokine profiles were assessed using specific fluorescent staining and flow cytometry, as described elsewhere [23]. Briefly, 30 ml peripheral blood samples were collected from 24 leprosy patients, 12 with tuberculoid and 12 with lepromatous leprosy. In both patient groups, tuberculoid and lepromatous, half (six in each group) the patients were also positive for intestinal helminths and the other half helminth-negative. Peripheral blood mononuclear cells were separated and stimulated polyclonally in vitro (106 cells/well) with phorbol myristate acetate (PMA) (Sigma, St Louis, MO, USA) and ionomycin (Sigma) for 4 h at 37°C, 5% CO2. Intracytoplasmatic staining was achieved post-treatment with Brefeldin A and following cell permeabilization, through the use of fluorescein isothiocyanate (FITC)-labelled anti-IL-4 and anti-IFN-γ monoclonal antibodies (Becton & Dickinson, La Jolla, CA, USA) used in conjunction with surface staining phycoerythrin (PE)- and peridinin chlorophyll (PerCP)-labelled monoclonal antibodies specific for cell surface markers (CD3, CD4 and CD8). Fluorescence was assessed using a fluorescence activated cell sorter (FACS) Calibur (Becton & Dickinson) gated for lymphocytes.
Antigens
Preparations from both Ascaris lumbricoides crude soluble extract antigens (kindly donated by Dr Cristiano Lara, CPqRR- Fiocruz, Belo Horizonte, Minas Gerais, Brazil) and from M. leprae sonicate, cytosol and cell wall fractions (kindly donated by Dr John Spencer, Colorado State University, Fort Collins, CO, USA) were sonicated, sterilized by 0·2 µm filtration (Millipore, Bedford, MA, USA), checked for endotoxin presence, adjusted to a final concentration of 1 mg/ml, aliquoted and stored at −70°C until use as specific stimuli [24]. Staphylococcal enterotoxin B (SEB; 0·5 mg/ml) from Staphylococcus aureus was purchased from Sigma and used as polyclonal stimulus.
In vitro cell proliferation assays
Considering that the presence of intestinal helminths could interfere with Th-1-type immune responses in leprosy patients, fresh whole blood cells (WBC) from 14 tuberculoid leprosy patients, characterized by Th1-type immunity, were stimulated (5 × 106 cells/ml) in 15-ml polypropylene culture tubes (Falcon® tubes; Becton & Dickinson) in the presence of either media alone, SEB (1 µg/ml), Ascaris lumbricoides soluble antigens (10 µg/ml) or M. leprae cytosol fraction (10 µg/ml). Stimulated WBC samples were incubated at 37°C, 5%CO2 for 6 h. After 2 h incubation, 10 µl of a 1:10 dilution in 1× phosphate-buffered saline (PBS) of Brefeldin A (Sigma) was added to each tube and 50 µl of a 200-mM solution of ethylenediamine tetraacetic acid (EDTA) at the end of the incubation period.
Assessment of intracellular cytokine production after specific stimulation with M. leprae and A. lumbricoides antigens
Anti-human CD3 (clone SK7), CD4 (clone SK3), CD8 (clone SK1), CD69 (clone L78) and immunoglobulin (Ig)G1 (clone X40) monoclonal antibodies were obtained from Becton Dickinson Immunocytometry Systems (BDIS, San Jose, CA, USA). Antigen-stimulated WBCs (100 µl) were incubated at room temperature (RT) with a combination of monoclonal antibodies for 15 min protected from light, and then treated with a haemolytic buffer (FACS lysing solution; BDIS) for another 10 min. Cells were washed and resuspended in cold washing buffer [0·5% bovine serum albumin (BSA), 0·1% sodium azide, 1× PBS; pH 7·2] to remove cell debris and monocytes. Cells were fixed in 4% paraformaldehyde for 10 min and rinsed twice in cold washing buffer. Cytokine-specific monoclonal antibodies, IFN-γ (clone 25723.11), IL-10 (clone JES3-19F1), IL-4 (clone 3010.211) and IL-5 (clone JES1-39D10) were obtained from BDIS. Cell samples were analysed using FACScalibur flow cytometry (BDIS) equipped with an argon laser for four-colour detection. Acquisition and analysis were performed using CellQuest software (BDIS). Flow cytometry data (50 000 events per sample) were analysed using CellQuest software (BDIS).
Statistical analysis
Statistical analysis was performed using spss (Statistical Package for the Social Sciences), version 10·0 for Windows. ORs were calculated to evaluate the association of intestinal helminth and protozoan infection with different forms of leprosy compared to healthy household contacts. Logistic regression analysis was used to assess the association between leprosy patients and intestinal helminth infection, intestinal protozoan infection, age and gender. A P-value equal to or smaller than 0.05 was considered statistically significant in all analyses.
Results
Patients' demographics
Treatment-naive leprosy patients (n = 105) were separated according to their mycobacterial index and clinical form: (a) paucibacillary patients (n = 64), 20 (19%) indeterminate and 44 (41·9%) tuberculoid leprosy (TL) cases; and (b) multi-bacillary patients (n = 41), 14 (13·4%) dimorphous and 27 (25·7%) lepromatous leprosy (LL) cases (Table 1).
Table 1.
Gender and age distribution for both leprosy patient and healthy household contact (HHC) groups.
| Age |
||||
|---|---|---|---|---|
| Groups | n | Mean (±s.d.) | Median | Range |
| Healthy HHC | 146 | 40·60 ± 15.63 | 38·5 | 18–78 |
| Males | 73 | 41·05 ± 16.26 | 42·0 | 19–76 |
| Females | 73 | 40·15 ± 15.08 | 37·0 | 18–78 |
| Leprosy (all) | 105 | 42·51 ± 15.59 | 43·0 | 18–88 |
| Males | 53 | 40·75 ± 15.51 | 39·0 | 18–88 |
| Females | 52 | 44·31 ± 15.61 | 44·0 | 18–84 |
| Indeterminate | 20 | |||
| Males | 05 | 40·4 ± 12.22 | 46·0 | 22–51 |
| Females | 15 | 39·4 ± 14.47 | 41·0 | 18–60 |
| Tuberculoid | 44 | |||
| Males | 23 | 39·3 ± 14.71 | 38·0 | 19–64 |
| Females | 21 | 41·33 ± 13.61 | 39·0 | 18–63 |
| Dimorphous | 14 | |||
| Male | 07 | 49·71 ± 13.82 | 51·0 | 34–72 |
| Female | 07 | 54·14 ± 13.09 | 49·0 | 43–73 |
| Lepromatous | 27 | |||
| Male | 18 | 39·22 ± 17.74 | 35·0 | 18–88 |
| Female | 09 | 51·78 ± 19.48 | 46·0 | 18–84 |
s.d.: standard deviation.
The frequency of individuals harbouring at least one intestinal helminth was significantly higher among leprosy patients when compared to household contacts (Table 2). Infection with at least one intestinal helminth species was diagnosed in 24 (22%) of 105 leprosy patients. A. lumbricoides infection was reported in 15 patients, Strongyloides stercoralis in five and hookworms in four patients. By contrast, only 10 (6·8%) of 146 household contacts were parasitized, three with A. lumbricoides, three with S. stercoralis, three with Trichuris trichiura and one with Enterobius vermicularis. By contrast, frequency of infection by intestinal protozoans did not differ between the two groups (Table 2). Because the observed frequency of intestinal protozoan (Giardia lamblia and Entamoeba spp.) infection did not differ between leprosy patients and their household contacts, we may assume that both groups were living under the same risk of acquiring intestinal parasites.
Table 2.
Frequency of intestinal parasites in the studied groups.
| Helminths |
Protozoans |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | n | Pos | Neg | OR (CI 95%)†‡ | P‡ | Pos | Neg | OR (CI 95%)† | P |
| Healthy HHC§ | 146 | 10 | 136 | 26 | 120 | ||||
| Leprosy (all forms) | 105 | 24 | 81 | 4·03 (1·83–8·85) | 0·000 | 16 | 89 | 0·83 (0·42–1·63) | 0·59 |
| Indeterminate leprosy | 20 | 05 | 15 | 4·53 (1·36–15·03) | 0·02 | 0 | 20 | 0·82 (0·76–0·88) | 0·046 |
| Tuberculoid leprosy | 44 | 05 | 39 | 1·74 (0·56 –5·40) | 0·34 | 10 | 34 | 1·35 (0·59–3·09) | 0·46 |
| Dimorphous leprosy | 14 | 02 | 12 | 2·26 (0·44–11·55) | 0·28 | 02 | 12 | 0·76 (0·16–3·64) | 1·000 |
| Lepromatous leprosy | 27 | 12 | 15 | 10·88 (4·02–29·40) | 0·000 | 04 | 23 | 0·80 (0·25–2·51) | 1·000 |
Odds ratio (OR) [confidence interval (CI) 95%] compared to healthy HHC group.
Data statistically different (P < 0·05) are represented in bold.
HHC: healthy household contacts. Statistical significance is depicted by bold types.
Considering that intestinal helminths were more prevalent among patients with leprosy when compared to household contacts, the correlation of bacillary load with intestinal worm prevalence was investigated. Frequency of intestinal helminthic infection was significantly higher among multi-bacillary patients (34·1%) when compared to either paucibacillary patients (15·6%; OR = 3·78; CI 95% 1·23–11·90; P = 0·000) or household contacts (6·8%; OR = 7·62; CI 95% 2·79–21·08; P = 0·000) (Table 2). As expected, when compared to healthy household contacts, lepromatous patients presented the highest frequency of intestinal helminth infection (50%; OR = 10·88; CI 95% 4·02–29·40; P = 0·000), followed by patients with the indeterminate form of the disease (20·8%; OR = 4·53; CI 95% 1·36–15·03; P = 0·02) (Table 2). In order to rule out both age and gender influence, logistic regression analysis was performed, demonstrating that co-infection with intestinal helminths was the only variable associated significantly with leprosy (OR = 3·955, CI 95% 1·772–8·827, P = 0·001) (Table 3). Additionally, a significant correlation was observed between bacillary load and the presence of intestinal helminths (Fig. 1a).
Table 3.
Logistic regression analysis of intestinal parasite infection, age and gender among leprosy patients and HHC groups.
| Variables | Leprosy patients (n = 105) | Healthy HHC† (n = 143) | OR (CI 95%)‡ | P§ |
|---|---|---|---|---|
| Age | ||||
| 18–30 | 43 | 32 | 1·322 (0·674–2·594) | 0·417 |
| 31–50 | 68 | 46 | 0·898 (0·481–1·676) | 0·735 |
| >50 | 32 | 27 | Reference | |
| Gender | ||||
| Female | 52 | 73 | 1·058 (0·628–1·783) | 0·831 |
| Intestinal parasites | ||||
| Protozoans | 16 | 26 | 1·056 (0·526–2·121) | 0·878 |
| Helminths | 24 | 10 | 3·955 (1·772–8·827) | 0·001 |
HHC: healthy household contacts.
Odds ratio (OR) for age was calculated considering individuals older than 50 years as reference.
Data statistically different (P < 0·05) are represented in bold type. CI: confidence interval. Statistical significance is depicted by bold types.
Fig. 1.
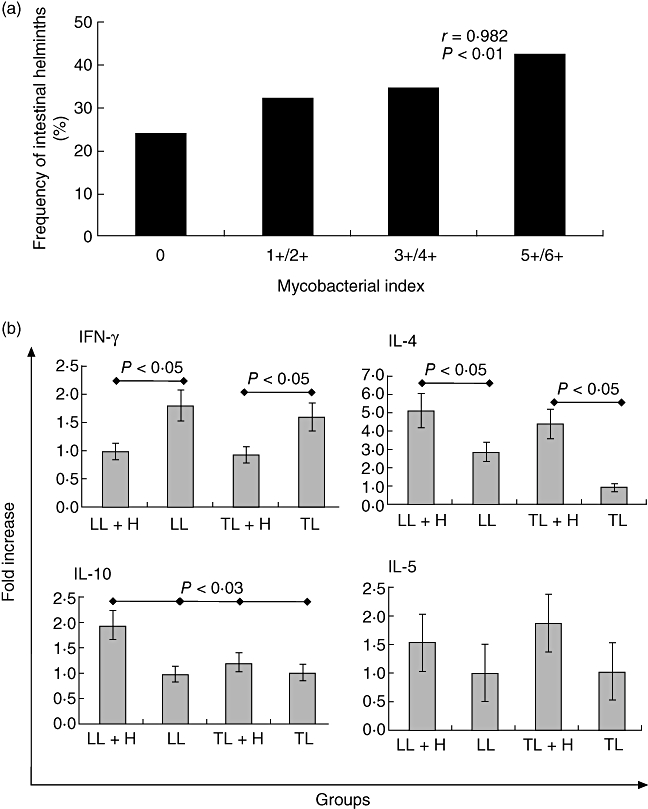
(a) Correlation between mycobacterial index and the frequency of intestinal helminths in leprosy patients (n = 105); and (b) assessment of intracellular interferon (IFN)-γ, interleukin (IL)-4, IL-10 and IL-5 in phytohaemagglutinin (PHA) + ionomycin-stimulated peripheral blood mononuclear cells (PBMC) culture from leprosy patients co-infected or not with intestinal helminths by flow cytometry. LL: lepromatous leprosy patients; LL+H: intestinal helminth-infected lepromatous leprosy patients; TL: tuberculoid leprosy patients; TL+H: intestinal helminth-infected tuberculoid leprosy patients.
Assessment of intracellular cytokines in ex-vivo samples stimulated with polyclonal stimuli
Peripheral blood mononuclear cells (PBMC) were stimulated polyclonally in vitro, and the frequency of cells expressing intracellular IFN-γ, IL-10, IL-4 and IL-5 was assessed (Fig. 1b). Data from cytokine intracellular staining indicate that leprosy patients, both tuberculoid and lepromatous co-infected with intestinal helminths (TL+H and LL+H, respectively), regardless of their clinical form, produce significantly lower levels of IFN-γ (P < 0·005) when compared to leprosy patients without helminths (TL or LL) (Fig. 1b). Conversely, both TL+H and LL+H patients displayed a significant increase of intracellular IL-4 (P < 0·005) when compared to TL and LL, respectively (Fig. 1b). Intracellular IL-10 levels were close to twofold higher in LL+H patients (P < 0·003) when compared to LL, TL and TL+H patients (Fig. 1b). As opposed to the observed pattern for both IL-4 and IL-10 levels, elevated in leprosy patients co-infected with intestinal worms, IL-5 levels did not differ significantly (Fig. 1b).
Phenotypical evaluation of the early activation marker CD69 on either CD4+ or CD8+ T cells after specific stimulation with M. leprae and A. lumbricoides antigens
Considering that both human and experimental helminthic infections are associated with an up-regulation of Th2 immunity, and that an effective immune response against leprosy is dependent upon Th1 immunity, we decided to investigate whether the presence of intestinal worms could interfere on the activation status and the production of Th-1 cytokine in TL patients. Initially, CD4+ and CD8+ cell frequencies as well as the expression of CD69 after stimulation with either M. leprae or A. lumbricoides antigens were evaluated in TL and TL+H patients (Fig. 2a). Although the frequency of activated CD4+ T cells increased after stimulation with M. leprae antigens, it was only significant among TL+H patients (Fig. 2b). On the other hand, frequency of activated CD8+ T cells was not affected, regardless of antigen or patient group.
Fig. 2.

Phenotypic analysis of whole blood cultures from tuberculoid leprosy (TL) and tuberculoid leprosy patients co-infected with intestinal helminths (TL+H) by flow cytometry. (a) Scatter plots of CD4+CD69+ expression on T cells from TL patients stimulated with either medium alone, Staphylococcal enterotoxin B (SEB), Mycobacterium leprae or Ascaris lumbricoides antigens. Numbers in quadrants depict the observed cell frequency; and (b) frequencies of CD4+, CD8+, CD4+CD69+ and CD8+CD69+ T cells from both TL and TL+H patients after stimulation with SEB, M. leprae (ML) or A. lumbricoides (AL) antigens.
Although WBC stimulation with M. leprae or A. lumbricoides antigens led to a reduction in CD69 expression by CD4+ cells on both TL and TL+H patients, this reduction was significant only for A. lumbricoides antigen (Fig. 2b). Conversely, CD69 expression by CD8+ cells was not affected by A. lumbricoides antigen; however, a significant reduction of CD8+CD69+ cell frequency was observed among TL patients stimulated with M. leprae antigen (Fig. 2b).
Assessment of intracellular cytokine production after specific stimulation with M. leprae and A. lumbricoides antigens
Because stimulation with A. lumbricoides antigen altered the activation status of CD4+ T cells (Fig. 2b). The effect of specific stimulation with either M. leprae or Ascaris lumbricoides antigens on cytokine production was assessed in WBC from TL and TL+H patients (Fig. 3a). In contrast to TL patients, TL+H patients displayed a weakened capacity to produce IFN-γ when stimulated, regardless of stimuli (Fig. 3b).
Fig. 3.
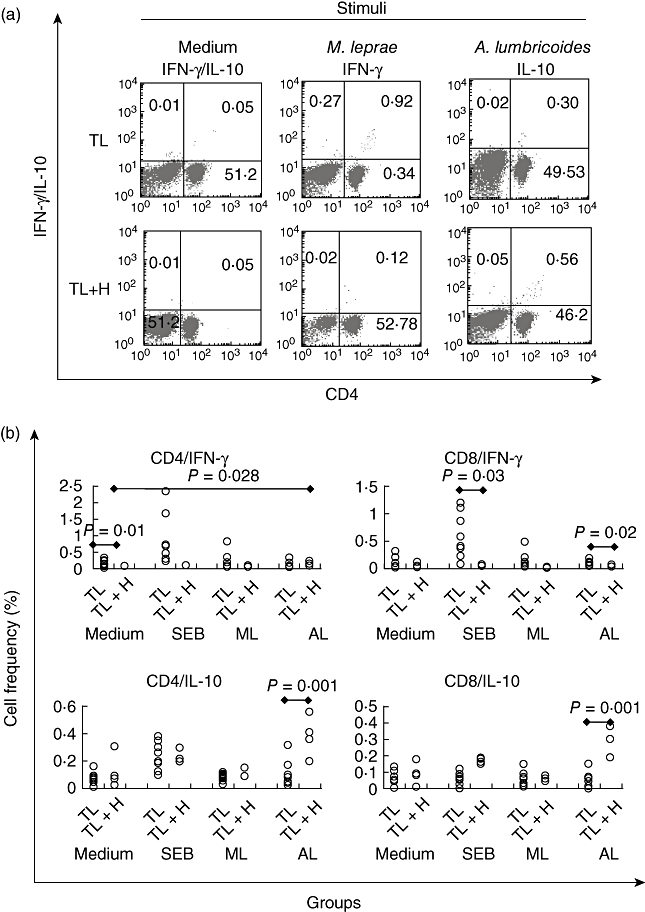
Intracellular assessment of interferon (IFN)-γ and interleukin (IL)-10 on whole blood cultures from tuberculoid leprosy (TL) and tuberculoid leprosy patients co-infected with intestinal helminths (TL+H) by flow cytometry frequencies. (a) Scatter plots of intracellular assessment of IFN-γ and IL-10 on whole blood cultures from tuberculoid leprosy (TL) and tuberculoid leprosy patients co-infected with intestinal helminths (TL+H) stimulated with either medium alone, Mycobacterium leprae or Ascaris lumbricoides antigens by flow cytometry. Numbers in quadrants depict the observed cell frequency. Numbers in quadrants depict the observed cell frequency; and (b) frequencies of both CD4+ and CD8+ T cells from both TL and TL+H patients producing IFN-γ or IL-10 after stimulation with Staphylococcal enterotoxin B (SEB), M. leprae (ML) or A. lumbricoides (AL) antigens.
On the other hand, a significant increase of both CD4+IL-10+ and CD8+IL-10+ T cells was the hallmark when WBCs from TL+H patients were stimulated with A. lumbricoides antigen. Interestingly, the observed frequency of CD4+IL-10+ and CD8+IL-10+ cells after stimulation with A. lumbricoides antigen was greater than when SEB was used (Fig. 3b).
Discussion
In the present work, we report a significant age- and gender-independent association between intestinal helminthic infection and either multi-bacillary or lepromatous leprosy patients, confirming previous observations [3,17]. A similar frequency of intestinal protozoan infection was observed for leprosy patients and their household contacts, indirect evidence that both groups were living under the same risk of acquiring intestinal parasites.
Data presented here support the hypothesis that intestinal helminths may disturb the immune regulation through the onset of a hypoergic/anergic state, which in turn could facilitate subsequent infections or disease progression to severe forms [23,24]. Intestinal helminths are known to elicit a strong systemic Th2 response or to up-regulate Treg activity, which are normally associated with a weakened Th1 immunity [4,8–10,14,25,26]. Activation and maintenance of a Th1-type immune response, pivotal for a successful response against mycobacteria, may be hindered in co-infected patients by a strong Th2 response and/or Treg up-regulation mediated by intestinal worms. Therefore, it is possible that an existing infection with intestinal helminths may facilitate a subsequent infection by M. leprae or its progression to more severe forms of leprosy [2,13]. Data presented here demonstrated that the frequency of cells expressing intracellular Th2 cytokines, such as IL-4 and IL-10, in WBC from leprosy patients co-infected with intestinal helminths (LL+H and TL+H) were higher than those from patients without worms (LL and TL). Interestingly, although the frequency of cells expressing intracellular IL-4 and IL-10 were elevated in SEB-stimulated cultures from LL patients, these patients presented the lowest intracellular IFN-γ level.
The frequency of intracellular IFN-γ+ cells in TL and LL patients PBMC cultures stimulated with SEB were approximately twofold higher than those TL+H and LL+H patients, respectively, further evidence that Th1 immunity is down-modulated during intestinal helminthic infection. On the other hand, frequency of intracellular IL-4+ cells in LL+H and TL+H patients were twofold higher than LL and TL patients, respectively.
Recently, Babu et al. [12] reported that the presence of an active larial infection was associated with a diminished production of IL-17 and IL-23 in response to mycobacterial antigens. According to these authors filaria-induced CTLA-4 and PD-1, which are associated closely with Treg cells, appear to exert a potent bystander effect on immune responses against mycobacterial antigens in patients with latent TB. Data from these authors concur with previous results from Borkow et al. [10], who demonstrated that immune cells from highly immune-activated individuals (harbouring intestinal helminths) were defective in several signalling responses, all of which gradually restored following anti-helminthic treatment. Peripheral blood mononuclear cells (PBMCs) from these individuals showed poor transmembrane signalling, increased expression of CTLA-4, decreased beta-chemokine secretion by CD8+ cells after stimulation and reduced proliferation to recall antigen stimulation. The data provided here add further evidence of impaired T cell activation, through the observed CD69 down-regulation in WBC from both TL and TL+H patients when stimulated with A. lumbricoides antigen, which may be due to a Th2 and/or Treg effect resulting from exposure to intestinal helminth antigens.
The existence of a hypoergic state in co-infected patients is supported by our data, demonstrating that IFN-γ+CD4+ or IFN-γ+CD8+ T cell frequencies were reduced dramatically among TL+H patients, even when SEB, a polyclonal stimulus, was used as opposed to the observed increased CD4+IL-10+ and CD8+IL-10+ cell frequencies (Fig. 2b). Although an increased CD8+IL-10+ frequency was not expected, naive CD8 T cells activated in the presence of basophils differentiate efficiently into IL-10-producing cells [27].
It has been reported that Tregs can be induced to regulate responses to pathogens such as Leishmania, helminths and M. tuberculosis[16,28–31]. It is possible that intestinal helminth infections, in addition to promoting a strong up-regulation of Th2-type immune responses, also stimulate Tregs, which could interfere with T cell activation through the suppression of Th1-type responses. Although the role of Tregs was not investigated in the present study, indirect evidence, such as IL-10 levels in LL+H patients, may indicate a possible role for Tregs, suggesting that Treg participation in leprosy should be evaluated further. We have demonstrated that intestinal helminth infection have a negative impact on M. tuberculosis-specific immune responses during active tuberculosis [11]. Decreased CD4+ T cell frequencies accompanied by lower IFN-γ, elevated IL-10 levels in WBC from TB patients co-infected with intestinal helminths compared to TB patients, were demonstrated by some of us. In addition to a depressed anti-MTB immunity, TB patients co-infected with intestinal helminths also presented severe radiological pulmonary disease, indicating that concomitant intestinal helminth infection in TB patients skewed their immune response towards a Th2 profile, favouring a persistent MTB infection and a more protracted clinical course of the disease.
Data presented suggest that infection by intestinal helminths could facilitate infection by M. leprae and/or disease progression towards the lepromatous pole, the more severe form of leprosy. Although data discussed here represent groups of patients and controls from a single endemic area, it indicates the existence of a significant association between the presence of intestinal helminths and multi-bacillary leprosy. Our results support the implementation of anti-helminthic strategies in endemic areas, which may improve both general health and reduce the burden of mycobacterial infections. Investigations on Th2/Treg up-regulation in co-infected patients (leprosy + intestinal helminthes) are being conducted, and may further the knowledge about the negative impact of intestinal worms in mycobacterial infections.
Disclosure
Authors' potential conflicts of interest: none.
References
- 1.Verhagen CE, Van Der P, Kraan TCTM, et al. Type 1 and Type 2-like lesional skin-derived Mycobacterium leprae – responsive T cell clones are characterized by coexpression of IFN-γ/TNF-α and IL-4/IL-5/Il-13, respectively. J Immunol. 1998;160:2380–7. [PubMed] [Google Scholar]
- 2.Goulart IMB, Penna GO, Cunha G. Imunopatologia da hanseníase: a complexidade dos mecanismos da resposta imune do hospedeiro ao Mycobacetrium leprae (Immunopathology of leprosy: the complexity of the host's immune response to Mycobacterium leprae) Rev Soc Brazil Medical Trop. 2002;35:365–75. doi: 10.1590/s0037-86822002000400014. [DOI] [PubMed] [Google Scholar]
- 3.Prost A, Nebout M, Rougemont A. Lepromatous leprosy and onchocerciasis. BMJ. 1979;1:589–90. doi: 10.1136/bmj.1.6163.589-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper PJ, Chico ME, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis. 2000;182:1207–13. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 5.Finkelman FD, Shea-Donohue T, Goldhill J, et al. Regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 6.Stewart GR, Boussinesq M, Coulson T, Elson L, Nutman T, Bradley JE. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol. 1999;117:517–23. doi: 10.1046/j.1365-2249.1999.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for TH2 responses. Eur J Immunol. 2000;30:1977–87. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Bentwich Z, Weisman Z, Moroz C, Bar-Yehuda S, Kalinkovich A. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections? Clin Exp Immunol. 1996;103:239–43. doi: 10.1046/j.1365-2249.1996.d01-612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalinkovich A, Weisman Z, Greenberg Z, Nahmias J, Eitan S, Stein M. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114:414–21. doi: 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borkow G, Leng Q, Weisman Z, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000;106:1053–60. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Có TR, Hirsch C, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu S, Bhat SQ, Kumar NP, et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J Infect Dis. 2009;200:288–98. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abulafia J, Vignale RA. Leprosy: pathogenesis updated. Int J Dermatol. 1999;38:321–34. doi: 10.1046/j.1365-4362.1999.00650.x. [DOI] [PubMed] [Google Scholar]
- 14.Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy. Clin Microbiol Rev. 2004;17:1012–30. doi: 10.1128/CMR.17.4.1012-1030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoguina J, Mempel M, Larbi J, et al. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis) Microbes Infect. 2002;4:1291–300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 16.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–31. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 17.Diniz LM, Zandonade E, Dietze R, Pereira FEL, Rodrigues RR. Short report: do intestinal nematodes increase the risk for multibacillary leprosy? Am Soc Trop Med Hyg. 2001;65:852–4. doi: 10.4269/ajtmh.2001.65.852. [DOI] [PubMed] [Google Scholar]
- 18.Tristao-Sa R, Ribeiro-Rodrigues R, Johnson LT, Pereira FE, Dietze R. Intestinal nematodes and pulmonary tuberculosis. Rev Soc Bras Med Trop. 2002;35:533–5. doi: 10.1590/s0037-86822002000500020. [DOI] [PubMed] [Google Scholar]
- 19.Moreira-Silva SF, Leite ALA, Brito EF, Pereira FEL. Nematode infections are risk factors for staphylococcal infection in children. Mem Inst Oswaldo Cruz. 2002;97:395–9. doi: 10.1590/s0074-02762002000300021. [DOI] [PubMed] [Google Scholar]
- 20.Pereira Júnior DB. 3rd edn. Brazil: Vitória; 2002. Principais Técnicas Laboratoriais em Exames Parasitológicos de Fezes (Major laboratorial techniques for parasitological examination of stool samples) [Google Scholar]
- 21.Brazilian Ministry of Health. Brazilian National Health Foundation, National Epidemiology Center, National Sanitary Dermatology Coordination. Leprosy control guidelines. 2nd edn. Brazil: Brasília; 1994. [Google Scholar]
- 22.Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev. 1962;33:119–28. doi: 10.5935/0305-7518.19620014. [DOI] [PubMed] [Google Scholar]
- 23.Baran J, Kowalczyk D, Ozog M, Zembala M. Three-color flow cytometry detection of intracellular cytokines in peripheral blood mononuclear cells: comparative analysis of phorbol myristate acetate–ionomycin and phytohemagglutinin stimulation. Clin Diagn Lab Immunol. 2001;8:303–13. doi: 10.1128/CDLI.8.2.303-313.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 25.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–91. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 26.Bentwich Z, Kalinkovich A, Weisman Z, Borkov G, Beyers N. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol Today. 1999;20:485–7. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol. 2009;183:3033–9. doi: 10.4049/jimmunol.0900332. [DOI] [PubMed] [Google Scholar]
- 28.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factor for developing active tuberculosis? Trop Med Intern Health. 2005;11:551–8. doi: 10.1111/j.1365-3156.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- 29.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro-Rodrigues R, Resende Co T, Rojas R, et al. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borkow G, Teicher C, Bentwich Z, Helminth-HIV Coinfection: should we deworm? PLoS Negl Trop Dis. 2007;1:e160–2. doi: 10.1371/journal.pntd.0000160. [DOI] [PMC free article] [PubMed] [Google Scholar]


