Abstract
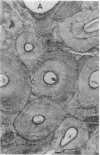
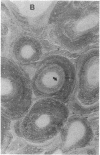
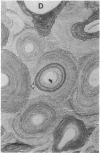
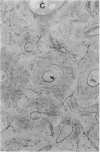
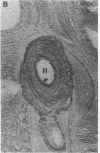
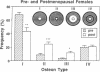
With increasing age, bone undergoes changes in remodeling that ultimately compromise the structural integrity of the skeleton. The presence of osteocalcin in bone matrix may alter bone remodeling by promoting osteoclast activity. Whether age- and/or gender-related differences exist in the distribution of osteocalcin within individual bone remodeling units is not known. In this study, we determined the immunohistochemical distribution of osteocalcin in the extracellular matrix of iliac crest bone biopsies obtained from normal male and female volunteers, 20-80 yr old. Four different distribution patterns of osteocalcin within individual osteons were arbitrarily defined as types I, II, III, or IV. The frequency of appearance of each osteon type was determined as a percent of the total osteons per histologic section. The proportion of osteons that stained homogeneously throughout the concentric lamellae (type I) decreased in females and males with increasing age. The proportion of osteons that lack osteocalcin in the matrix immediately adjacent to Haversian canals (type III) increased in females and males with age. Osteons staining intensely in the matrix adjacent to Haversian canals (type II) increased in females and was unchanged in aging males. Osteons that contained osteocalcin-positive resting lines (type IV) increased in bone obtained from males with increasing age but were unchanged in females. Sections of bone immunostained for osteopontin (SPP-I), osteonectin, and decorin did not reveal multiple patterns or alterations in staining with gender or increasing age. We suggest that the morphology of individual bone remodeling units is heterogeneous and the particular morphologic pattern of osteocalcin distribution changes with age and gender. These results suggest that differences in the distribution of osteocalcin in bone matrix may be responsible, in part, for the altered remodeling of bone associated with gender and aging.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990 Nov;38(11):1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Bolander M. E., Robey P. G., Fisher L. W., Conn K. M., Prabhakar B. S., Termine J. D. Monoclonal antibodies against osteonectin show conservation of epitopes across species. Calcif Tissue Int. 1989 Aug;45(2):74–80. doi: 10.1007/BF02561405. [DOI] [PubMed] [Google Scholar]
- Buchanan J. R., Myers C. A., Greer R. B., 3rd Effect of declining renal function on bone density in aging women. Calcif Tissue Int. 1988 Jul;43(1):1–6. doi: 10.1007/BF02555161. [DOI] [PubMed] [Google Scholar]
- Carlson C. S., Tulli H. M., Jayo M. J., Loeser R. F., Tracy R. P., Mann K. G., Adams M. R. Immunolocalization of noncollagenous bone matrix proteins in lumbar vertebrae from intact and surgically menopausal cynomolgus monkeys. J Bone Miner Res. 1993 Jan;8(1):71–81. doi: 10.1002/jbmr.5650080110. [DOI] [PubMed] [Google Scholar]
- Chan E. L., Lau E., Shek C. C., MacDonald D., Woo J., Leung P. C., Swaminathan R. Age-related changes in bone density, serum parathyroid hormone, calcium absorption and other indices of bone metabolism in Chinese women. Clin Endocrinol (Oxf) 1992 Apr;36(4):375–381. doi: 10.1111/j.1365-2265.1992.tb01463.x. [DOI] [PubMed] [Google Scholar]
- Chapuy M. C., Durr F., Chapuy P. Age-related changes in parathyroid hormone and 25 hydroxycholecalciferol levels. J Gerontol. 1983 Jan;38(1):19–22. doi: 10.1093/geronj/38.1.19. [DOI] [PubMed] [Google Scholar]
- Clemens T. L., Zhou X. Y., Myles M., Endres D., Lindsay R. Serum vitamin D2 and vitamin D3 metabolite concentrations and absorption of vitamin D2 in elderly subjects. J Clin Endocrinol Metab. 1986 Sep;63(3):656–660. doi: 10.1210/jcem-63-3-656. [DOI] [PubMed] [Google Scholar]
- Conn K. M., Termine J. D. Matrix protein profiles in calf bone development. Bone. 1985;6(1):33–36. doi: 10.1016/8756-3282(85)90404-1. [DOI] [PubMed] [Google Scholar]
- DeFranco D. J., Glowacki J., Cox K. A., Lian J. B. Normal bone particles are preferentially resorbed in the presence of osteocalcin-deficient bone particles in vivo. Calcif Tissue Int. 1991 Jul;49(1):43–50. doi: 10.1007/BF02555901. [DOI] [PubMed] [Google Scholar]
- Dickson I. R., Bagga M. K. Changes with age in the non-collagenous proteins of human bone. Connect Tissue Res. 1985;14(1):77–85. doi: 10.3109/03008208509089845. [DOI] [PubMed] [Google Scholar]
- Finkelman R. D., Linkhart T. A., Mohan S., Lau K. H., Baylink D. J., Bell N. H. Vitamin D deficiency causes a selective reduction in deposition of transforming growth factor beta in rat bone: possible mechanism for impaired osteoinduction. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3657–3660. doi: 10.1073/pnas.88.9.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L. W., Hawkins G. R., Tuross N., Termine J. D. Purification and partial characterization of small proteoglycans I and II, bone sialoproteins I and II, and osteonectin from the mineral compartment of developing human bone. J Biol Chem. 1987 Jul 15;262(20):9702–9708. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D. Noncollagenous proteins influencing the local mechanisms of calcification. Clin Orthop Relat Res. 1985 Nov;(200):362–385. [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Glowacki J., Lian J. B. Impaired recruitment and differentiation of osteoclast progenitors by osteocalcin-deplete bone implants. Cell Differ. 1987 Sep;21(4):247–254. doi: 10.1016/0045-6039(87)90479-9. [DOI] [PubMed] [Google Scholar]
- Groessner-Schreiber B., Krukowski M., Lyons C., Osdoby P. Osteoclast recruitment in response to human bone matrix is age related. Mech Ageing Dev. 1992 Feb;62(2):143–154. doi: 10.1016/0047-6374(92)90051-e. [DOI] [PubMed] [Google Scholar]
- Halloran B. P., Portale A. A., Lonergan E. T., Morris R. C., Jr Production and metabolic clearance of 1,25-dihydroxyvitamin D in men: effect of advancing age. J Clin Endocrinol Metab. 1990 Feb;70(2):318–323. doi: 10.1210/jcem-70-2-318. [DOI] [PubMed] [Google Scholar]
- Hartwell D., Riis B. J., Christiansen C. Changes in vitamin D metabolism during natural and medical menopause. J Clin Endocrinol Metab. 1990 Jul;71(1):127–132. doi: 10.1210/jcem-71-1-127. [DOI] [PubMed] [Google Scholar]
- Hauschka P. V., Lian J. B., Gallop P. M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V., Wians F. H., Jr Osteocalcin-hydroxyapatite interaction in the extracellular organic matrix of bone. Anat Rec. 1989 Jun;224(2):180–188. doi: 10.1002/ar.1092240208. [DOI] [PubMed] [Google Scholar]
- Helfrich M. H., Nesbitt S. A., Dorey E. L., Horton M. A. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin, via a beta 3 integrin. J Bone Miner Res. 1992 Mar;7(3):335–343. doi: 10.1002/jbmr.5650070314. [DOI] [PubMed] [Google Scholar]
- Horst R. L., Goff J. P., Reinhardt T. A. Advancing age results in reduction of intestinal and bone 1,25-dihydroxyvitamin D receptor. Endocrinology. 1990 Feb;126(2):1053–1057. doi: 10.1210/endo-126-2-1053. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Davies J. Perspectives: adhesion receptors in bone. J Bone Miner Res. 1989 Dec;4(6):803–808. doi: 10.1002/jbmr.5650040603. [DOI] [PubMed] [Google Scholar]
- Huffer W. E. Morphology and biochemistry of bone remodeling: possible control by vitamin D, parathyroid hormone, and other substances. Lab Invest. 1988 Oct;59(4):418–442. [PubMed] [Google Scholar]
- Hultenby K., Reinholt F. P., Oldberg A., Heinegård D. Ultrastructural immunolocalization of osteopontin in metaphyseal and cortical bone. Matrix. 1991 Jun;11(3):206–213. doi: 10.1016/s0934-8832(11)80160-5. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A. TGF-beta and extracellular matrix related influences on gene expression and phenotype. Crit Rev Eukaryot Gene Expr. 1991;1(2):75–84. [PubMed] [Google Scholar]
- Ingram R. T., Clarke B. L., Fisher L. W., Fitzpatrick L. A. Distribution of noncollagenous proteins in the matrix of adult human bone: evidence of anatomic and functional heterogeneity. J Bone Miner Res. 1993 Sep;8(9):1019–1029. doi: 10.1002/jbmr.5650080902. [DOI] [PubMed] [Google Scholar]
- Kahn A. J., Teitelbaum S. L., Malone J. D., Krukowski M. The relationship of monocytic cells to the differentiation and resorption of bone. Prog Clin Biol Res. 1982;110(Pt B):239–248. [PubMed] [Google Scholar]
- Lian J. B., Marks S. C., Jr Osteopetrosis in the rat: coexistence of reductions in osteocalcin and bone resorption. Endocrinology. 1990 Feb;126(2):955–962. doi: 10.1210/endo-126-2-955. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Tassinari M., Glowacki J. Resorption of implanted bone prepared from normal and warfarin-treated rats. J Clin Invest. 1984 Apr;73(4):1223–1226. doi: 10.1172/JCI111308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J., Stewart C., Puchacz E., Mackowiak S., Shalhoub V., Collart D., Zambetti G., Stein G. Structure of the rat osteocalcin gene and regulation of vitamin D-dependent expression. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1143–1147. doi: 10.1073/pnas.86.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone J. D., Teitelbaum S. L., Griffin G. L., Senior R. M., Kahn A. J. Recruitment of osteoclast precursors by purified bone matrix constituents. J Cell Biol. 1982 Jan;92(1):227–230. doi: 10.1083/jcb.92.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazess R. B. On aging bone loss. Clin Orthop Relat Res. 1982 May;(165):239–252. [PubMed] [Google Scholar]
- McKee M. D., Farach-Carson M. C., Butler W. T., Hauschka P. V., Nanci A. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. J Bone Miner Res. 1993 Apr;8(4):485–496. doi: 10.1002/jbmr.5650080413. [DOI] [PubMed] [Google Scholar]
- McKee M. D., Glimcher M. J., Nanci A. High-resolution immunolocalization of osteopontin and osteocalcin in bone and cartilage during endochondral ossification in the chicken tibia. Anat Rec. 1992 Dec;234(4):479–492. doi: 10.1002/ar.1092340404. [DOI] [PubMed] [Google Scholar]
- Ninomiya J. T., Tracy R. P., Calore J. D., Gendreau M. A., Kelm R. J., Mann K. G. Heterogeneity of human bone. J Bone Miner Res. 1990 Sep;5(9):933–938. doi: 10.1002/jbmr.5650050906. [DOI] [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteoblast-like cells by parathyroid hormone. J Cell Biol. 1989 Feb;108(2):713–718. doi: 10.1083/jcb.108.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M. Transcriptional regulation of osteocalcin production by transforming growth factor-beta in rat osteoblast-like cells. Endocrinology. 1989 Feb;124(2):612–617. doi: 10.1210/endo-124-2-612. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos J. M., Amado J. A., Riancho J. A., Albájar M., González-Macías J. Sex and age distribution of 1,25(OH)2D3 receptors in peripheral blood mononuclear cells from normal human subjects. Bone. 1990;11(6):407–409. doi: 10.1016/8756-3282(90)90135-l. [DOI] [PubMed] [Google Scholar]
- Omdahl J. L., Garry P. J., Hunsaker L. A., Hunt W. C., Goodwin J. S. Nutritional status in a healthy elderly population: vitamin D. Am J Clin Nutr. 1982 Dec;36(6):1225–1233. doi: 10.1093/ajcn/36.6.1225. [DOI] [PubMed] [Google Scholar]
- Orwoll E. S., Meier D. E. Alterations in calcium, vitamin D, and parathyroid hormone physiology in normal men with aging: relationship to the development of senile osteopenia. J Clin Endocrinol Metab. 1986 Dec;63(6):1262–1269. doi: 10.1210/jcem-63-6-1262. [DOI] [PubMed] [Google Scholar]
- Ozono K., Liao J., Kerner S. A., Scott R. A., Pike J. W. The vitamin D-responsive element in the human osteocalcin gene. Association with a nuclear proto-oncogene enhancer. J Biol Chem. 1990 Dec 15;265(35):21881–21888. [PubMed] [Google Scholar]
- Pankovich A. M., Simmons D. J., Kulkarni V. V. Zonal osteons in cortical bone. Clin Orthop Relat Res. 1974 May;(100):356–363. [PubMed] [Google Scholar]
- Poser J. W., Price P. A. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J Biol Chem. 1979 Jan 25;254(2):431–436. [PubMed] [Google Scholar]
- Price P. A., Otsuka A. A., Poser J. W., Kristaponis J., Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976 May;73(5):1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada J. M., Coopmans W., Ruiz B., Aljama P., Jans I., Bouillon R. Influence of vitamin D on parathyroid function in the elderly. J Clin Endocrinol Metab. 1992 Aug;75(2):494–501. doi: 10.1210/jcem.75.2.1639950. [DOI] [PubMed] [Google Scholar]
- Reinholt F. P., Hultenby K., Oldberg A., Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs B. L., Wahner H. W., Dunn W. L., Mazess R. B., Offord K. P., Melton L. J., 3rd Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest. 1981 Feb;67(2):328–335. doi: 10.1172/JCI110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Sandberg M. M. Matrix in cartilage and bone development: current views on the function and regulation of major organic components. Ann Med. 1991 Aug;23(3):207–217. doi: 10.3109/07853899109148050. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Lian J. B., Owen T. A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990 Oct;4(13):3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Belcourt A. B., Conn K. M., Kleinman H. K. Mineral and collagen-binding proteins of fetal calf bone. J Biol Chem. 1981 Oct 25;256(20):10403–10408. [PubMed] [Google Scholar]
- Termine J. D. Cellular activity, matrix proteins, and aging bone. Exp Gerontol. 1990;25(3-4):217–221. doi: 10.1016/0531-5565(90)90055-7. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Thompson D. D. Age changes in bone mineralization, cortical thickness, and haversian canal area. Calcif Tissue Int. 1980;31(1):5–11. doi: 10.1007/BF02407161. [DOI] [PubMed] [Google Scholar]
- Tsai K. S., Heath H., 3rd, Kumar R., Riggs B. L. Impaired vitamin D metabolism with aging in women. Possible role in pathogenesis of senile osteoporosis. J Clin Invest. 1984 Jun;73(6):1668–1672. doi: 10.1172/JCI111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb M., Shinar D., Rodan G. A. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J Bone Miner Res. 1990 Aug;5(8):831–842. doi: 10.1002/jbmr.5650050806. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]