Abstract
Intestinal microflora play a critical role in the initiation and perpetuation of chronic inflammatory bowel diseases. In genetically susceptible hosts, bacterial colonization results in rapid-onset chronic intestinal inflammation. Nevertheless, the intestinal and systemic immune response to faecal bacteria and antigen exposure into a sterile intestinal lumen of a post-weaned animal with a mature immune system are not understood clearly. This study examined the effects of faecal bacteria and antigen exposure on the intestinal mucosal and systemic immune system in healthy axenic mice. Axenic wild-type mice were inoculated orally with a crude faecal slurry solution derived from conventionally raised mice and were analysed prior to and then at days 3, 7, 14 and 28 post-treatment. Ingestion of faecal slurry resulted in a transient, early onset of proinflammatory interferon (IFN)-γ, tumour necrosis factor (TNF)-α and interleukin (IL)-17 response that was maximal at day 3. In contrast, the transient release of the anti-inflammatory cytokines IL-10 and IL-4 occurred later and was maximal at day 7. Both responses subsided by day 14. This early cytokine imbalance was associated with a brief rise in colonic and caecal histopathological injury score at day 7. The bacterial antigen-specific systemic response was found to follow the intestinal immune response with a maximal release of both pro- and anti-inflammatory cytokines at day 7. Thus, first exposure of healthy axenic wild-type mice to normal faecal flora and antigens results in an early proinflammatory cytokine response and transient colonic inflammation that then resolves in conjunction with a subsequent anti-inflammatory cytokine profile.
Keywords: axenic, bacterial antigens, Crohn's disease, cytokines, endogenous bacteria, inflammatory bowel disease, ulcerative colitis
Introduction
An endogenous intestinal microflora is a natural constituent of all vertebrates [1,2]. Whereas commensal faecal bacteria live in symbiosis with normal hosts, in genetically susceptible hosts the same commensal faecal bacteria can induce and perpetuate chronic intestinal inflammation, such as in several experimental colitis models and in patients with ulcerative colitis and Crohn's disease, known collectively as chronic inflammatory bowel diseases (IBD) [3]. While the aetiology of IBD is not known, it is well established that endogenous bacteria, their components and/or antigenic products have a prevailing role in the initiation and perpetuation of the chronic inflammatory response. Indeed, in these genetically susceptible individuals there is loss of immune tolerance for commensal faecal bacteria and their antigens and a bacteria-specific mucosal and systemic immune response ensues subsequently [4]. In several animal models it has been demonstrated that genetically susceptible animals remain disease-free in a germ-free (axenic) state, but will develop rapid-onset chronic intestinal inflammation when associated with normal endogenous microflora [5–7].
We have demonstrated previously that the acquisition of commensal faecal bacteria in pre-weaned neonatal wild-type mice caused a transient release of cytokines, which was important subsequently for the establishment of tolerance to the individual endogenous microflora later in life [8]. Nevertheless, the intestinal immune and injury response and the systemic response to faecal bacteria and antigen exposure to a sterile intestinal lumen of a healthy post-weaned animal with a mature immune system are not understood clearly. Understanding the natural immune and injury response in the normal and immune competent animal can be key to understanding the disease state.
We thus examined the effects of normal faecal bacteria and antigen exposure on the intestinal mucosal and systemic immune system in wild-type axenic mice.
Materials and methods
Mice
Experiments were performed in two different mouse strains. Axenic Swiss Webster mice were purchased initially from Taconic Farm (Germantown, NY, USA) and were bred at the University of Alberta in specific sterile isolator bubbles. Axenic 129/SvEv mice were purchased from the Gnotobiotic Core Facility at North Carolina State University. The mice in this experiment were used at approximately 15 weeks of age. Results from analyses performed in both mouse strains had identical outcomes.
Inoculation of faecal material
Faecal material was collected from 129/SvEv mice housed under conventional conditions. For each preparation, 20 fresh faecal pellets were mashed into 3 ml of sterile distilled water. Axenic mice were removed from the sterile environment and 100 µl of this faecal slurry was given orally to the mice with a blue tip (Fisherbrand® General Purpose Redi-Tip™; Fisher Scientific, Ontario, Canada). Mice were forced to swallow by blocking their nasal airways temporarily, forcing the mice to gulp. An additional 100 µl was spread over their abdominal skin. Mice were held subsequently under conventional housing conditions.
Experimental outline
Our study was performed twice with germ-free Swiss Webster mice. In one experimental outline of the Swiss Webster study the mice were fed on the same day and analysed on different days post-treatment. In the second experiment mice were fed on days 3, 7 and 14 prior to the analysis and mice were then all analysed on the same day. Both experimental designs yielded results that were indistinguishable; therefore, data from both Swiss Webster experiments were combined. The experiment with 129/SvEv mice was staggered so that mice belonging to one experimental time-point group were analysed on two different days.
Histopathological injury score
Mice were killed by cervical dislocation prior to faecal slurry inoculation (axenic) and on days 3, 7, 14 and 28 post-inoculation. Colon, caecum and ileum were excised, cut longitudinally and half of each organ was prepared in paraffin with haematoxylin and eosin staining for light-microscopic examination as detailed previously [8]. The slides were reviewed in a blinded fashion and were assigned a histological score for intestinal inflammation ranging from 0 (no injury) to 10 (maximal injury). The histological inflammation scale represents the numerical sum of four scoring criteria: mucosal ulceration, epithelial hyperplasia, lamina propria mononuclear infiltration and lamina propria neutrophilic infiltration [8].
Analysis of epithelial permeability
To study epithelial barrier function a segment of colon was assayed in Ussing chambers, as described previously [9]. In the chambers the flux of [3H]-labelled mannitol from the mucosal to the serosal side was monitored as an indicator for the permeability of the intestinal epithelial layer.
In vitro cytokine secretion by intestinal tissue
Parts of colon, caecum and ileum were cultured for 24 h in 1 ml complete RPMI-1640 medium, as described previously [8]. Cytokine release in the supernatants was quantified using standard sandwich enzyme-linked immunosorbent assay (ELISA) techniques. For the ELISA the following antibodies were used: anti-interferon (IFN)-γ (clone R4-6A2), anti-tumour necrosis factor (TNF)-α (clone G281-2626), anti-interleukin (IL)-17 (clone eBioTC11-18H10·1), anti-IL-10 (clone JES5-2A5) and anti-IL-4 (clone 11B11) as capture antibodies and biotinylated anti-IFN-γ (clone XMG1·2), anti-TNF-α (clone MP6-XT3), anti-IL-17 (clone eBioTC11-8H4), anti-IL-10 (clone JES5-16E3) and anti-IL-4 (clone BVD6-24G2) as detection antibodies. All antibodies and recombinant cytokine standards were purchased from PharMingen Canada (Mississauga, Ontario, Canada) except for the anti-IL-17 monoclonal antibodies, which were obtained from eBiosciences (San Diego, CA, USA). All antibodies and standards were used at pre-titred concentrations to give optimal results. For the detection of IL-6 and granulocyte-colony stimulating factor (G-CSF) commercially available kits (R&D Systems, Minneapolis, MN, USA) were used.
In vitro systemic T cell responses
Spleens were removed from the killed mice, minced into a single-cell suspension in complete RPMI-1640 with 10% fetal calf serum (FCS) and depleted of red blood cells by osmotic shock. Spleen cell numbers were enumerated with trypan blue. To assess changes in the amount of inflammation-induced leucocytes, 5 × 106 washed spleen cells were stained with the following fluorescence-coupled monoclonal antibodies anti-CD11b-phycoerythrin (PE) or -allophycocyanin (APC), granulocyte-differentiation antigen-1 (Gr-1)-PE, B220-fluorescein isothiocyanate (FITC), anti-CD4-PE, anti-CD25-FITC and biotinylated anti-CD3ε followed by incubation with streptavidin-PE-Cy5 (PharMingen Canada for conjugated monoclonal antibodies, and Cedarlane, Hornby, Ontario, Canada for streptavidin) for flow cytometry according to published procedures. The remaining splenic lymphocytes were placed into the wells of 96-well plates at a concentration of 2 × 105 cells per well. Cultures were stimulated with either sterile sonicates prepared from pure strains of selected endogenous bacteria, as detailed in Sydora et al.[8], or with sterile lysates prepared from faecal material using glass beads as described in Sydora et al.[9]. Bacterial sonicates and faecal lysates were added at a protein concentration of 50 µg/ml, which was found to be optimal for cytokine production. Control stimuli included plate-bound anti-CD3ε clone 145-2C11 (PharMingen Canada) and medium alone. After 48 h of incubation at 37°C in a humidified incubator at 5% CO2, the plates were centrifuged, and the amounts of the indicated cytokines in the supernatants were quantified using standard ELISA techniques, as described above.
Statistical analysis
Data are expressed as means ± standard error of the mean (s.e.m.) or means ± standard deviation (s.d.) dependent upon whether data were combined from both experiments of the same mouse strain or whether they were derived from only one experimental group, respectively. Differences between mean values were evaluated using analysis of variance or paired t-tests, where appropriate (SigmaStat; Jandel Corporation, San Rafael, CA, USA).
Results
Inoculation of faecal slurry stimulates release of cytokines in colon and caecum of axenic mice
In axenic mice, spontaneous release of cytokines from colonic and caecal mucosal tissue was low (Fig. 1, day 0), similar to cytokine release in wild-type mice raised under conventional, non-pathogenic conditions in the presence of commensal intestinal bacteria [8]. However, inoculation of the axenic mice with faecal bacteria slurry resulted in a significant colonic and caecal immune response of proinflammatory cytokines, IFN-γ, TNF-α and IL-17 that peaked at 3–7 days after faecal slurry exposure (Fig. 1 and data not shown). Similarly, there was a significant increase in G-CSF 3 days post-faecal slurry feeding. In contrast, colonic and caecal immune response of anti-inflammatory cytokines, IL-4 and IL-10, followed that of the proinflammatory cytokines and peaked at day 7 (Fig. 1). While small increases in production of IL-6 were noted on days 3 and 7, these increases were not significant (data not shown). By day 14 following faecal slurry exposure, production of all cytokines was diminished and reached background levels by 28 days (Fig. 1 and data not shown). In caecal tissues release of pro- and anti-inflammatory cytokines was generally less abundant than in the colonic tissue, with the exception of IFN-γ. In contrast to colonic IFN-γ release, caecal IFN-γ was maximal at day 7 (Fig. 1). No significant changes in cytokine production were noted in small intestinal tissues (data not shown). The results shown are derived from experiments with 129/SvEv mice; however, results indistinguishable from these were also produced with Swiss Webster mice.
Fig. 1.
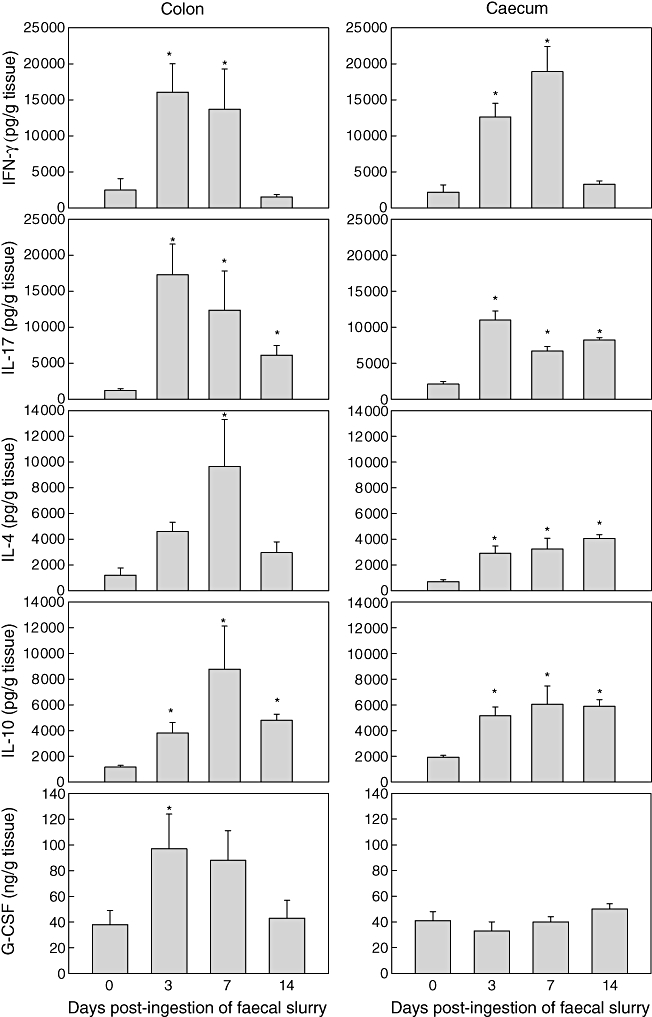
Kinetics of cytokine release from colonic and caecal mucosal explant cultures derived from axenic 129/SvEv wild-type mice following ingestion of faecal slurry. Spontaneous release of interferon (IFN)-γ, interleukin (IL)-17, IL-4, IL-10 and granulocyte-colony stimulating factor (G-CSF) was assessed in supernatants after 24 h using standard enzyme-linked immunosorbent assay (ELISA) techniques. Production of proinflammatory cytokines (IFN-γ, IL-17) as well as production of G-CSF preceded the secretion of anti-inflammatory cytokines (IL-14, IL-10). Bars represent mean values ± standard error of the mean for four to six mice per group; *P > 0·05.
Inoculation of faecal slurry causes a transient inflammation in colon and caecum of axenic mice
The imbalance in intestinal cytokine release with a maximal production of proinflammatory cytokines prior to production of anti-inflammatory cytokines was associated subsequently with a transient intestinal histopathological injury at day 7 post-faecal slurry exposure (Fig. 2a). The increase in intestinal injury scores was seen in both colonic and caecal tissues and involved mainly an influx in lamina propria mononuclear cells (Fig. 2b). However, not all mice developed colonic or caecal injury; the injury score among individual mice ranged from 1 to 8 in colon and from 1 to 7 in the caecum. Higher scores were found primarily among the Swiss Webster mice, whereas 129/SvEv mice scored generally lower. However, even those mice that were found to be microscopic disease-limited (i.e. histopathological injury score of 1 at day 7) demonstrated increased proinflammatory mucosal cytokine production. Colonic and caecal injury had subsided in most mice by day 14 (Fig. 2) and returned to base levels by day 28 (data not shown).
Fig. 2.
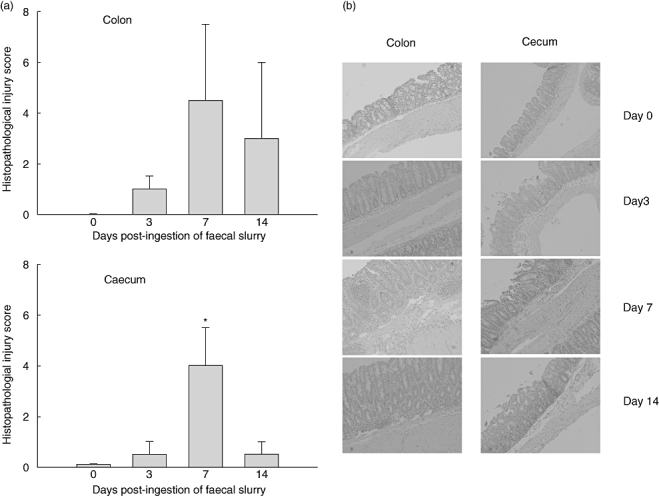
Histological injury assessment in Swiss Webster mice prior to and on days 3, 7 and 14 post-faecal slurry ingestion. Injury score (a) and histological inflammation (b) in both colon and caecum is maximal at day 7 and declines thereafter. Values represent mean values ± standard error of the mean for five mice per group. Fig. 2b shows haematoxylin and eosin staining of a histology preparation from a representative mouse for each time-point.
Inoculation of faecal slurry does not compromise intestinal epithelial barrier function
Colonic epithelial permeability was not altered significantly in these mice when tested at days 3, 7 and 14 post-faecal slurry exposure. In fact, we observed a slight reduction in mannitol flux in colonic tissue when subjected to Ussing chamber analysis (Fig. 3). Thus, despite the temporary cytokine imbalance and brief inflammatory response in the large bowel, the intestinal epithelial barrier function appeared to be intact.
Fig. 3.
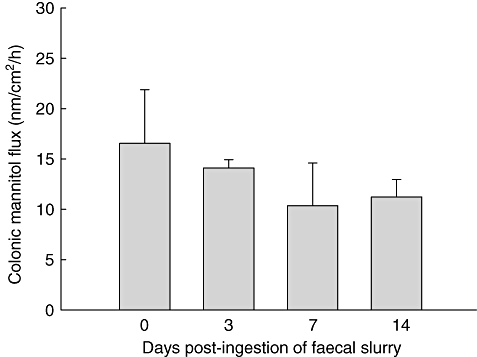
Epithelial barrier integrity as measured by colonic transmural mannitol flux in Ussing chambers pre- and post-faecal slurry ingestion. Bars represent mean values ± standard error of the mean for three Swiss Webster mice per group. All mice were assessed in duplicate.
Faecal slurry inoculation stimulates a unique systemic response
To investigate systemic immune responses to ingestion of faecal slurry in these axenic mice we assessed cytokine release in unseparated splenocytes stimulated with faecal lysates derived from specific pathogen-free (SPF)-raised mice. Maximal release of IFN-γ, IL-17 and IL-10 was measured at day 7 post-bacterial treatments (Fig. 4a, shaded bars). No increase in either TNF-α or IL-4 production was noted in any of these antigen-stimulated spleen cell cultures. As expected, cytokine release following spleen cell stimulation with lysates from axenic mice that are devoid of bacterial components remained at baseline level (Fig. 4a, solid bars).
Fig. 4.
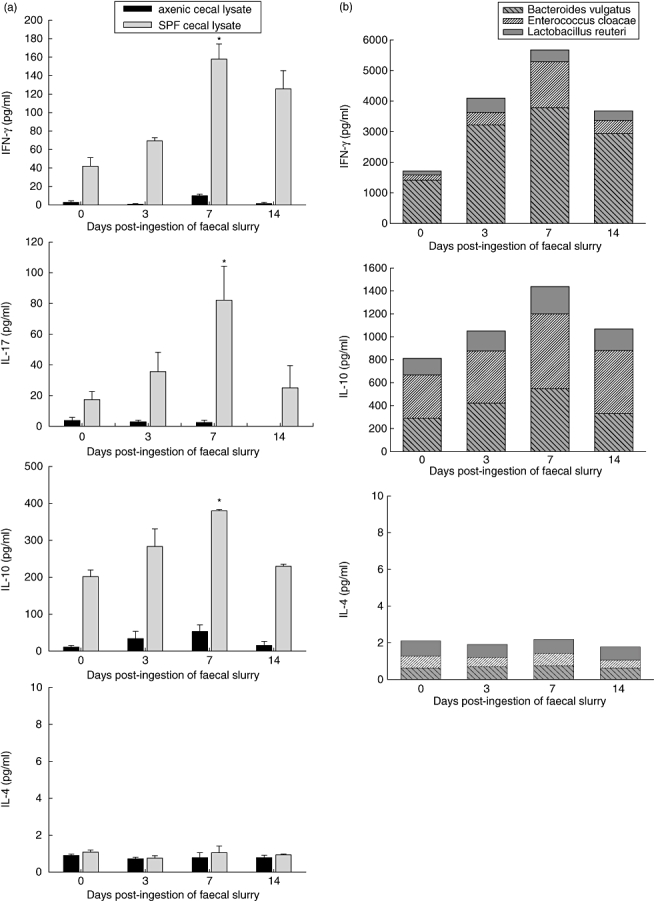
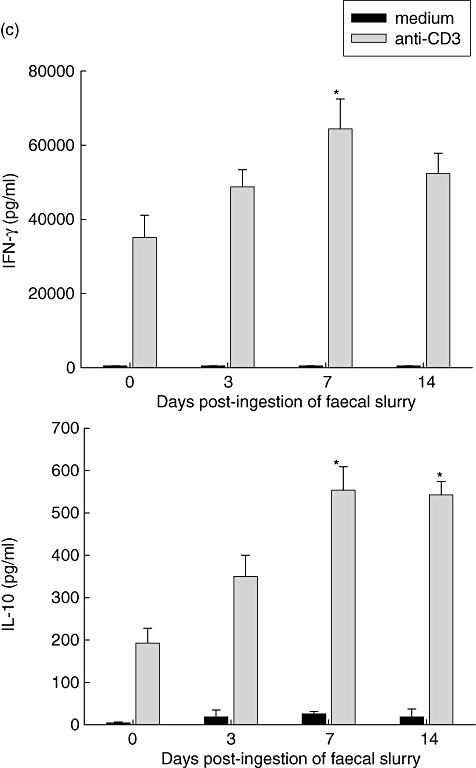
Systemic immune response prior to and on days 3, 7 and 14 following faecal slurry ingestion as measured by cytokine release in 24-h antigen-stimulated cultures of spleen cells from 129/SvEv mice. At day 7 a temporary maximal increase in cytokine production [interferon-γ, interleukin (IL)-17 and IL-10, but not IL-4] was observed in response to (a) sterile lysates prepared from caecal matter of specific pathogen-free mice containing several endogenous intestinal bacteria to (b) sterile bacterial antigens prepared from pure cultures of Bacteroides vulgatus, Enterococcus cloacae and Lactobacillus reuteri and to (c) cross-linked anti-CD3 monoclonal antibodies. The results from stimulation with the three bacterial antigens are combined in one bar. Values represent mean values ± standard error of the mean for five to 10 mice per group; *P > 0·05.
Consistent with these results from stimulation with faecal lysates, we observed a similar increase in production of IFN-γ and IL-10 at day 7 in cultures stimulated with sonicates derived from pure cultures of three endogenous bacterial strains: Bacteroides vulgatus, Enterobacter cloacae and Lactobacillus reuteri (Fig. 4b).
Stimulation with cross-linked anti-CD3 resulted in a similar cytokine boost with a peak at day 7 (Fig. 4c). While anti-CD3-stimulated IL-10 secretion was at the same magnitude as bacterial antigen-stimulated secretion, the release of IFN-γ was between 16-fold (day 7) and 30-fold (day 0) higher for anti-CD3 stimulation compared to bacterial stimulation, suggesting that the potential repertoire of IFN-γ-producing T cells was higher than the repertoire stimulated by bacterial antigens alone. In contrast, the stimulation of IL-10 secreting T cells was linked tightly to bacterial antigen stimulation. It is possible that some of the cytokine production could also be a result of activation of other monocytic spleen cells via their Toll-like-receptors or through a downstream bystander effect.
To test for a possible regulatory mechanism for the decline in cytokine production after day 7 post-injection, we examined the amount and composition of a variety of cells within the spleen cell population. No significant change in the percentage of CD25-positive cells was detected (Fig. 5a), suggesting that regulatory T cells within this population were not instrumental in the down-regulation of the immune response. However, concomitant with the increase of cytokine release at day 7 we found an increase in the number of CD11b-positive leucocytes (Fig. 5b). An overlap of CD11b staining with markers for T cells (anti-CD3), B cells (B220) and dendritic cells (anti-CD11c) was less than 2%, while on average more than 68% of these cells also stained for Gr-1, suggesting a myeloid-derived suppressor cell phenotype (data not shown). There was no significant change in total number of spleen cells recovered from mice at the various time-points post-faecal ingestion (Fig. 5c). Similarly, changes in numbers and percentages of both CD3-positive T cells and B220-positive B cells were not significant. However, the ratio of B220/CD3-positive cells was reduced significantly from 1·54 ± 0·14 (day 0) to 1·02 ± 0·03 (day 14) as a consequence of a slight increase in percentage of T cells and a concomitant decrease in the percentage of the B cell population at days 7–14.
Fig. 5.
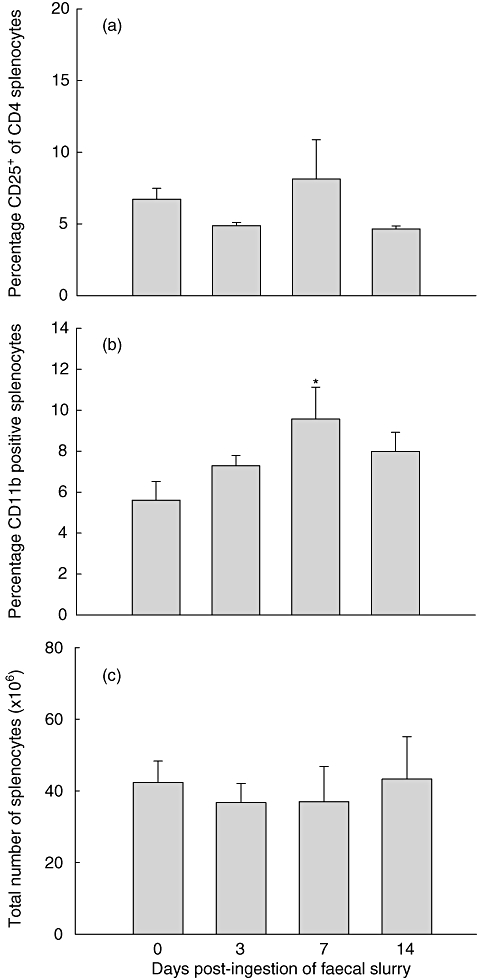
Flow cytometric assessment and enumeration of spleen cells pre- and post-faecal slurry ingestion. No significant increase in the population of CD25-positive cells was noted (a). At day 7 CD11b-positive leucocytes increased significantly in the spleen cell population (b). The total number of spleen cells remained similar at various time-points post-feeding (c). Values represent mean values ± standard error of the mean (s.e.m.) for three to four mice per group (a) and mean values ± s.e.m. for five to 10 mice per group (b,c); *P > 0·05. Results for CD25 staining were derived from one experiment with Swiss Webster mice. Results for CD11b staining as well as spleen cell enumeration data were derived from experiments with 129/SvEv mice; however, data from Swiss Webster mice generated similar results.
Discussion
In this study we have investigated the impact of commensal faecal flora and antigen acquisition in an immune environment that developed in the absence of an enteric bacterial influence.
Generally the mammalian gastrointestinal tract is populated with a highly diverse microbial flora immediately after birth. Studies employing gnotobiotic rodent colonies have shown that microbial colonization affects the general morphology, gut motility and differentiation of epithelial cell lineages [10–12]. In addition, acquisition of intestinal microflora is vital for the development of immunity. Gene expression profiling has revealed that the residential microbiota modifies genes significantly, including those involved in immune function [13,14]. Expression of several activation markers on intestinal immune cells is greatly reduced in axenic mice [11]. Similarly, expression of major histocompatibility complex (MHC) class II molecules is greatly dependent upon the presence of intestinal microorganisms [15]. The number of intestinal intraepithelial lymphocytes (IEL) expressing the αβ T cell receptor (TCR) is greatly reduced in axenic mice in addition to a reduced cytotoxic ability of these cells, although no difference was found in the number of γδ TCR-positive IELs [16–18].
While the intestinal microflora has essential beneficial functions, this same endogenous non-pathogenic microflora and/or its antigens are also implicated in the pathogenesis of chronic intestinal inflammation during inflammatory bowel diseases [19]. Several axenic rodent models of chronic intestinal inflammation have demonstrated that disease development is dependent upon bacterial colonization [6,7,20]. While healthy wild-type animals have developed tolerance to their endogenous intestinal microflora, animals that are genetically prone to develop chronic intestinal inflammation lack this tolerance and mount an uncontrolled immune response to enteric bacteria and/or their components. This response is apparent locally in the mucosal, gastrointestinal compartment as well as systemically and involves both humoral and cellular immune responses [21,22].
Our results indicate that acquisition of the normal faecal endogenous flora later in life can induce a transient intestinal inflammation. Mice that are kept in axenic conditions while their immune system matures without exposure to bacterial antigens lack tolerance to endogenous microflora. Thus, without previous exposure to luminal microflora, if faecal and bacterial antigens are encountered in the presence of a mature immune system a rapid-onset mucosal and systemic immune response ensues. The first response appears to be dominated by a local intestinal innate response that is skewed towards T helper type 1 (Th1) proinflammatory cytokine production. Early transient activation of proinflammatory gene expression and innate signal transduction has been demonstrated in intestinal epithelial cell lines and naive epithelial cells isolated following monoassociation of axenic rats with probiotic Bifidobacterium lactis, suggesting a role for activation of proinflammatory transcription factors in initiating epithelial cell homeostasis at an early stage of bacterial colonization [23]. Here we show that the initial proinflammatory response is followed by a response that appears to be dominated by the adaptive immune system characterized by systemic activation of antigen-specific lymphocytes and a subsequent infiltration of immune cells in the intestinal tissue. The latter may be facilitated by the increase in intestinal G-CSF. The initial relative abundance of mucosal proinflammatory cytokines instigates a transient colonic inflammation that then resolves, in conjunction with a subsequent anti-inflammatory response and establishment of a homeostatic cytokine balance. A similar transient mucosal immune response has been recorded in wild-type C3H/HeN axenic mice following colonization with Morganella morganii, a Gram-negative murine commensal organism. Those authors hypothesized that a state of unresponsiveness to the endogenous microflora may be apparent only after a transient mucosal immune response has occurred [24]. The response to bacteria and bacterial antigens we observed in our experiment might be elevated due in part to a transient unphysiological high load of bacteria in the axenic mice; however, it might mimic a response that occurs on a frequent basis, albeit less pronounced, whenever a new bacterial strain is introduced in the intestinal lumen.
The changes in the intestinal milieu with regard to cytokine and chemokine secretion as well as expression of cell surface antigens may instigate the generation of immune-regulatory cells. A crucial role for the presence of a microflora in the induction of regulatory T cells has been demonstrated in a murine transfer model of colitis [25]. Protective T cells showed reduced efficacy in preventing colitis development and demonstrated reduced release of IL-10 and IFN-γ when derived from axenic mice as opposed to those derived from conventionally housed mice. While we did not detect a significant increase in systemic T cells with a common regulatory phenotype, such as CD25-positive T cells, we cannot exclude the generation of a specific population of cells with regulatory function in mucosal tissues and/or systemically. The increased CD11b-positive leucocyte population may be involved in the suppression of activated T cell responses. Myeloid-derived suppressor cells with a CD11b-positive, Gr-1-positive phenotype and immunosuppressive function have been described and have been implicated in the protection of T cell-mediated chronic enterocolitis [26,27].
We have demonstrated previously a similar rapid onset of proinflammatory cytokine and intestinal injury in adult axenic IL-10 gene-deficient mice following colonization with commensal faecal flora [8]. A similar uncontrolled proinflammatory cytokine response to commensal bacterial antigens has also been found to play a crucial role in the human leucocyte antigen-B27 (HLA-B27) transgenic rat colitis model [28].
Our results demonstrate for the first time that bacterial colonization in wild-type mice initially triggers a similar proinflammatory immune response, causing temporary intestinal inflammation. Endogenous bacterial antigens are treated as ‘foreign’ and stimulate an antigen-specific systemic immune response. However, in contrast to colitis susceptible rodents, wild-type mice are able to down-regulate the initial proinflammatory immune response and establish mucosal as well as systemic tolerance. Acquisition of immunological homeostasis appears to follow a defined inflammatory response pattern when first exposed to faecal bacteria and antigens, which probably plays an important role in the induction of tolerance to the endogenous microflora.
Acknowledgments
The authors would like to acknowledge the University of Alberta Health Sciences Laboratory Animal Services for taking care of the axenic mouse colony and Mrs Dorothy Rutkowski for help with flow cytometry. These studies were supported by the Crohn's and Colitis Foundation of Canada.
Disclosure
The authors have no conflict of interest to report with regard to this manuscript.
References
- 1.Ley RE, Lozupone CA, Hamady M, Knight R, Gordon JI. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6:776–88. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes KL. Recent knowledge of the strict anaerobes of the gut. Aust Vet J. 1972;48:508–14. doi: 10.1111/j.1751-0813.1972.tb02311.x. [DOI] [PubMed] [Google Scholar]
- 3.Sartor RB. Targeting enteric bacteria in treatment of inflammatory bowel diseases: why, how, and when. Curr Opin Gastroenterol. 2003;19:358–65. doi: 10.1097/00001574-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onderdonk AB, Franklin ML, Cisneros RL. Production of experimental ulcerative colitis in gnotobiotic guinea pigs with simplified microflora. Infect Immun. 1981;32:225–31. doi: 10.1128/iai.32.1.225-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–53. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sydora BC, Tavernini MM, Wessler A, Jewell LD, Fedorak RN. Lack of interleukin-10 leads to intestinal inflammation, independent of the time at which luminal microbial colonization occurs. Inflamm Bowel Dis. 2003;9:87–97. doi: 10.1097/00054725-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Sydora BC, Martin SM, Lupicki M, et al. Bacterial antigens alone can influence intestinal barrier integrity, but live bacteria are required for initiation of intestinal inflammation and injury. Inflamm Bowel Dis. 2006;12:429–36. doi: 10.1097/00054725-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–70. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Kelly D, King T, Aminov R. Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res. 2007;622:58–69. doi: 10.1016/j.mrfmmm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Mutch DM, Simmering R, Donnicola D, et al. Impact of commensal microbiota on murine gastrointestinal tract gene ontologies. Physiol Genomics. 2004;19:22–31. doi: 10.1152/physiolgenomics.00105.2004. [DOI] [PubMed] [Google Scholar]
- 14.Fukushima K, Ogawa H, Takahashi K, et al. Non-pathogenic bacteria modulate colonic epithelial gene expression in germ-free mice. Scand J Gastroenterol. 2003;38:626–34. doi: 10.1080/00365510310000376. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto S, Setoyama H, Umesaki Y. Differential induction of major histocompatibility complex molecules on mouse intestine by bacterial colonization. Gastroenterology. 1992;103:1777–82. doi: 10.1016/0016-5085(92)91434-6. [DOI] [PubMed] [Google Scholar]
- 16.Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993;79:32–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi M, Nanno M, Umesaki Y, et al. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc Natl Acad Sci USA. 1993;90:8591–4. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandeira A, Mota-Santos T, Itohara S, et al. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172:239–44. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–70. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996;38:365–75. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchmann R, May E, Heike M, Knolle P, Neurath M, Meyer zum Buschenfelde KH. T cell specificity and cross reactivity towards enterobacteria, bacteroides, bifidobacterium, and antigens from resident intestinal flora in humans. Gut. 1999;44:812–18. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz PA, Hoffmann M, Szcesny S, Blaut M, Haller D. Innate mechanisms for Bifidobacterium lactis to activate transient pro-inflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology. 2005;115:441–50. doi: 10.1111/j.1365-2567.2005.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shroff KE, Meslin K, Cebra JJ. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–13. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauch UG, Obermeier F, Grunwald N, et al. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54:1546–52. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Haile LA, von Wasielewski R, Gamrekelashvili J, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–81. doi: 10.1053/j.gastro.2008.06.032. 81 e1–5. [DOI] [PubMed] [Google Scholar]
- 28.Dieleman LA, Hoentjen F, Qian BF, et al. Reduced ratio of protective versus proinflammatory cytokine responses to commensal bacteria in HLA-B27 transgenic rats. Clin Exp Immunol. 2004;136:30–9. doi: 10.1111/j.1365-2249.2004.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


