Abstract
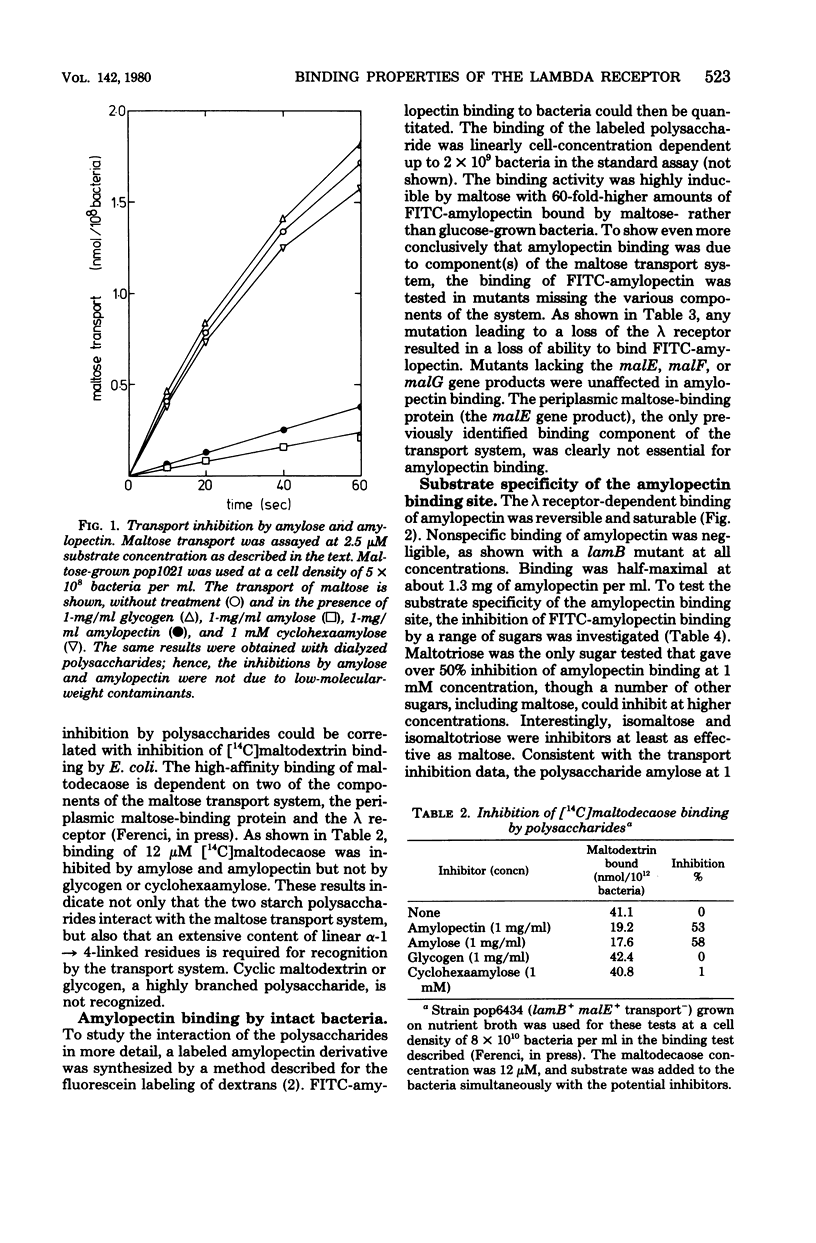
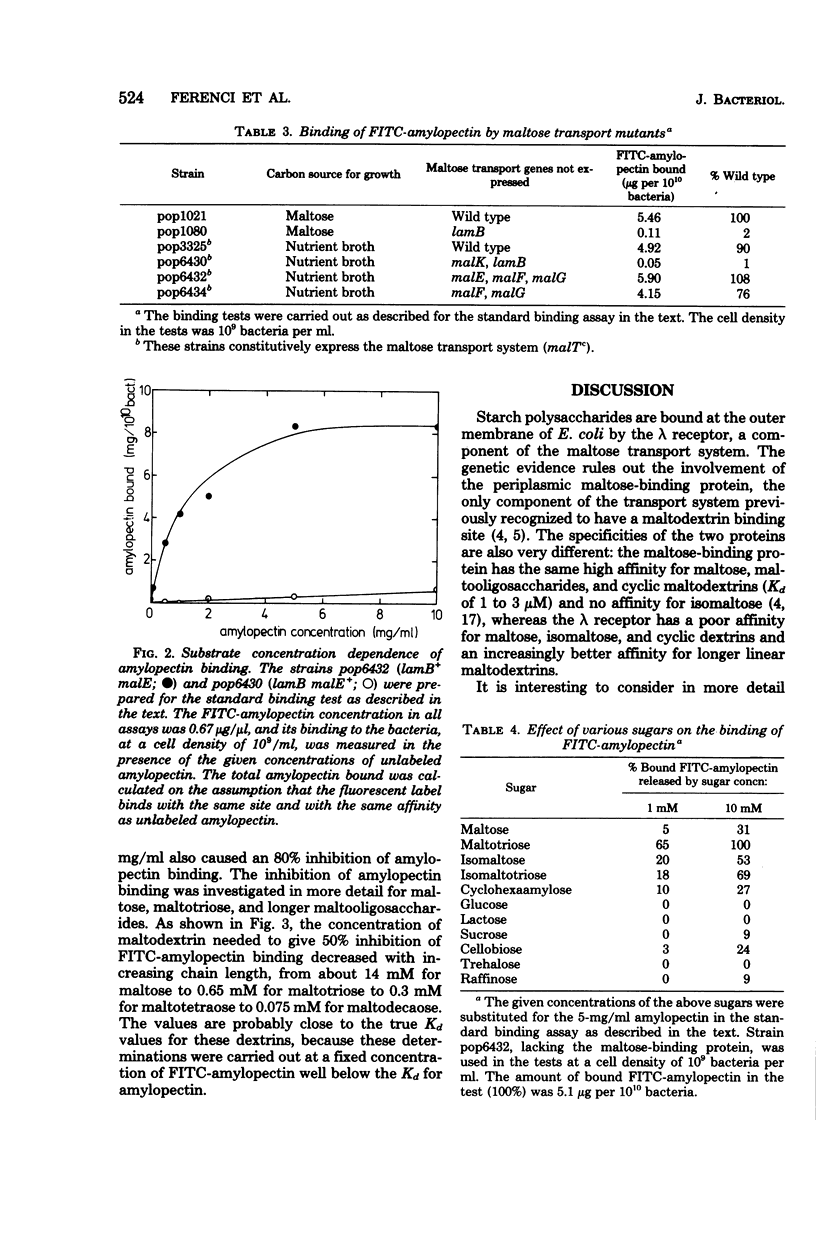
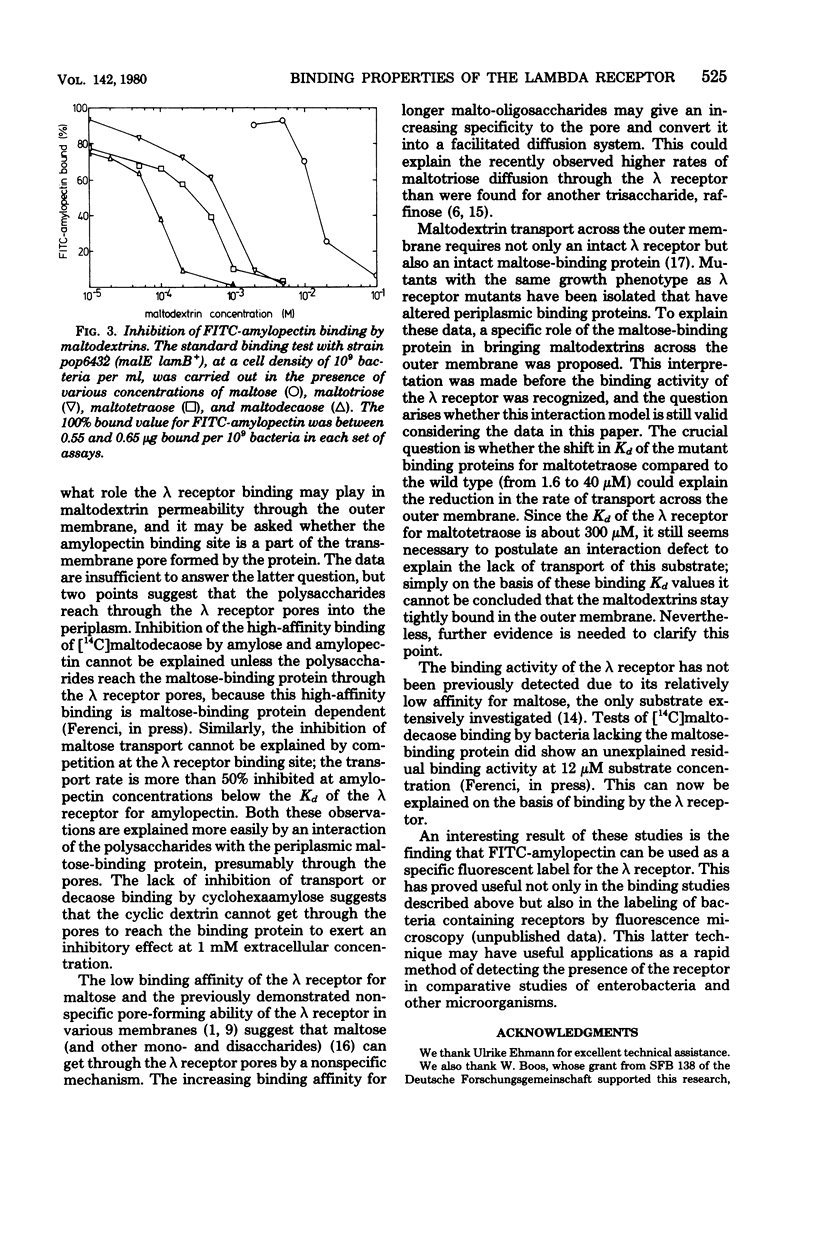
The starch polysaccharides amylose and amylopectin are not utilized by Escherichia coli, but are bound by the bacteria. The following evidence supports the view that the outer membrane λ receptor protein, a component of the maltose/ maltodextrin transport system is responsible for the binding. (i) Amylose and amylopectin both inhibit the transport of maltose into E. coli. (ii) Both polysaccharides prevent binding of non-utilizable maltodextrins by the intact bacterium, a process previously shown to be dependent on components of the maltose transport system (T. Ferenci, Eur. J. Biochem., in press). (iii) A fluorescent amylopectin derivative, O-(fluoresceinyl thiocarbamoyl)-amylopectin, has been synthesized and shown to bind to E. coli in a reversible, saturable manner. Binding of O-(fluoresceinyl thiocarbamoyl)-amylopectin is absent in mutants lacking the λ receptor, but mutations in any of the other components of the maltose transport system do not affect binding as long as λ receptor is present. (iv) Using the inhibition of λ receptor-dependent O-(fluoresceinyl thiocarbamoyl)-amylopectin binding as an assay, the affinities of the λ receptor for maltodextrins and other sugars have been estimated. The affinity for dextrins increases with increasing degree of polymerization (Kd for maltose, 14 mM; for maltotetraose, 0.3 mM; for maltodecaose, 0.075 mM). Maltose and some other di- and trisaccharides are inhibitory to amylopectin binding, but only at concentrations above 1 mM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boehler-Kohler B. A., Boos W., Dieterle R., Benz R. Receptor for bacteriophage lambda of Escherichia coli forms larger pores in black lipid membranes than the matrix protein (porin). J Bacteriol. 1979 Apr;138(1):33–39. doi: 10.1128/jb.138.1.33-39.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Boos W., Schwartz M., Szmelcman S. Energy-coupling of the transport system of Escherichia coli dependent on maltose-binding protein. Eur J Biochem. 1977 May 2;75(1):187–193. doi: 10.1111/j.1432-1033.1977.tb11516.x. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Klotz U. Affinity chromatographic isolation of the periplasmic maltose binding protein of Escherichia coli. FEBS Lett. 1978 Oct 15;94(2):213–217. doi: 10.1016/0014-5793(78)80940-5. [DOI] [PubMed] [Google Scholar]
- Kellermann O., Szmelcman S. Active transport of maltose in Escherichia coli K12. Involvement of a "periplasmic" maltose binding protein. Eur J Biochem. 1974 Aug 15;47(1):139–149. doi: 10.1111/j.1432-1033.1974.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. A porin activity of purified lambda-receptor protein from Escherichia coli in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1979 Jun 13;88(3):774–781. doi: 10.1016/0006-291x(79)91475-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Roa M., Braun-Breton C., Schwartz M. Structure of the malB region in Escherichia coli K12. I. Genetic map of the malK-lamB operon. Mol Gen Genet. 1979 Jul 24;174(3):241–248. doi: 10.1007/BF00267796. [DOI] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Brickman E., Bassford P. J., Jr, Casadaban M. J., Shuman H. A., Schwartz V., Guarente L., Schwartz M., Beckwith J. R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979 Jul 24;174(3):249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Wandersman C., Schwartz M., Ferenci T. Escherichia coli mutants impaired in maltodextrin transport. J Bacteriol. 1979 Oct;140(1):1–13. doi: 10.1128/jb.140.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Nikaido H. Outer membrane of gram-negative bacteria. XVII. Secificity of transport process catalyzed by the lambda-receptor protein in Escherichia coli. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1100–1107. doi: 10.1016/0006-291x(77)90534-4. [DOI] [PubMed] [Google Scholar]



