Abstract
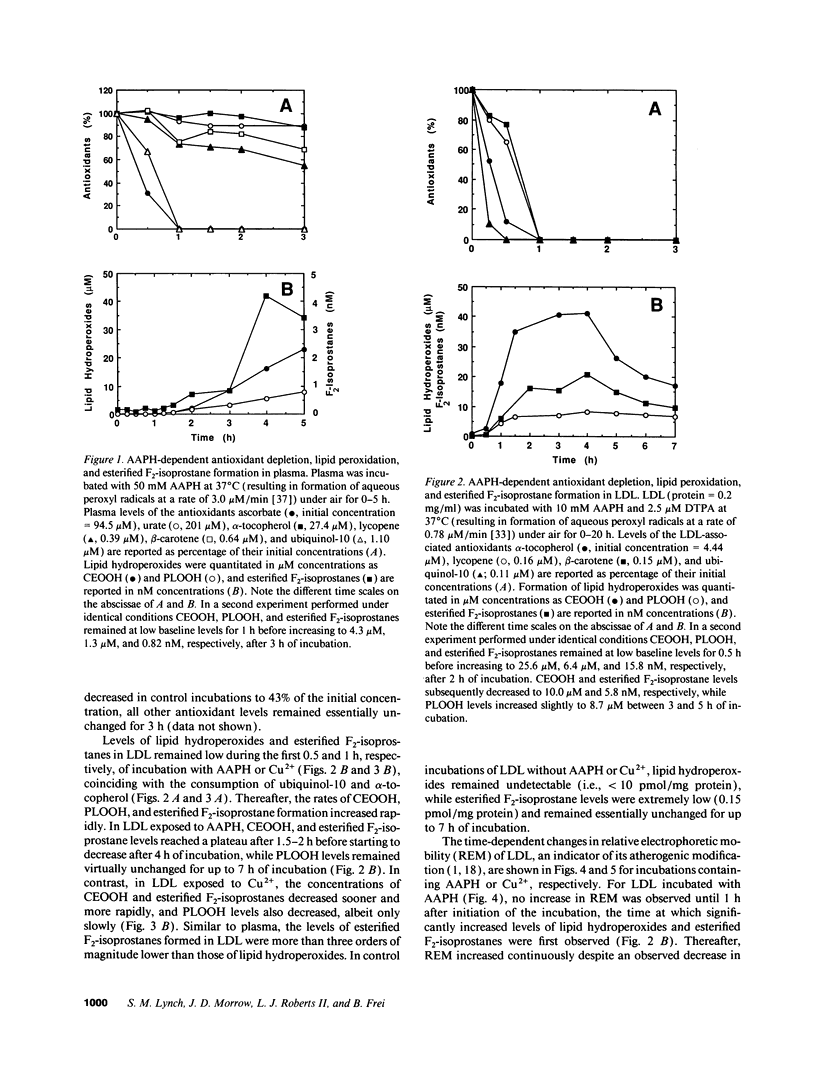
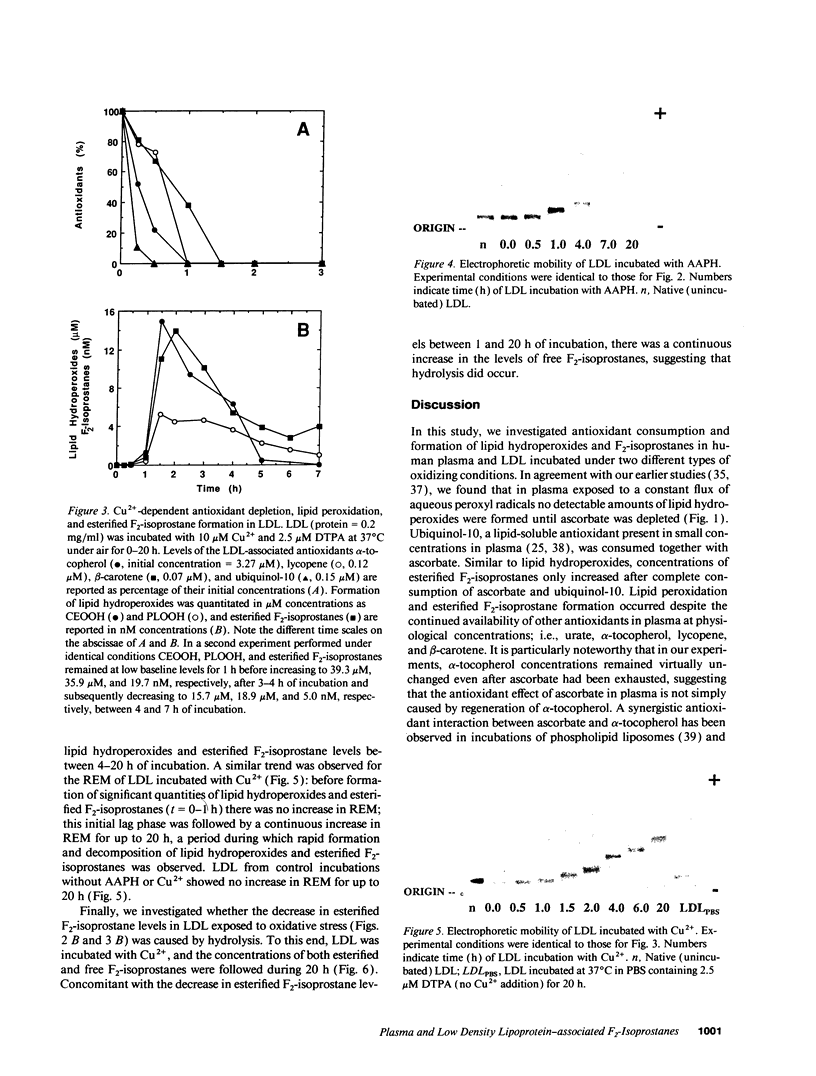
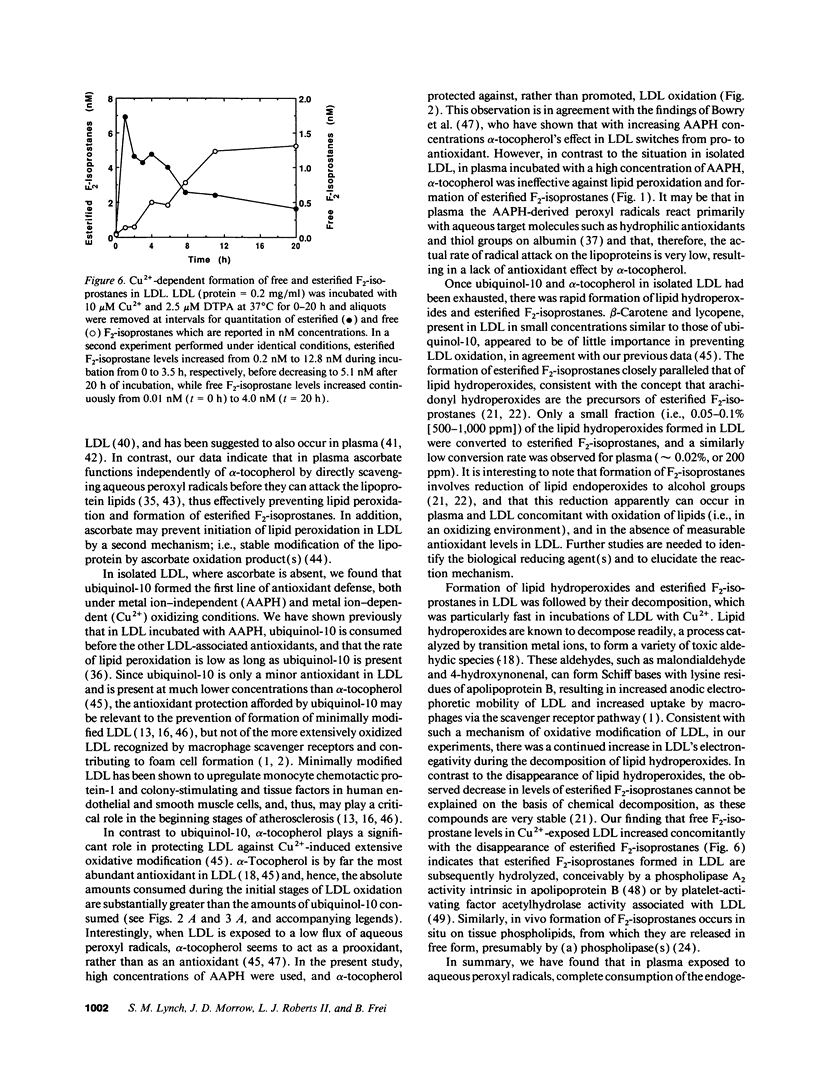
F2-isoprostanes are prostaglandin F2-like compounds that are known to be formed in vivo by free radical oxidation of arachidonyl-containing lipids, and their plasma levels have been suggested as indicators of in vivo oxidative stress. As oxidation of LDL, a likely causal factor in atherosclerosis, involves lipid peroxidation, we investigated whether F2-isoprostanes are formed in plasma and LDL exposed to oxidative stress, and how F2-isoprostane formation is related to endogenous antioxidant status. In plasma exposed to aqueous peroxyl radicals, lipid hydroperoxides and esterified F2-isoprostanes were formed simultaneously after endogenous ascorbate and ubiquinol-10 had been exhausted, despite the continued presence of urate, alpha-tocopherol, beta-carotene, and lycopene. In isolated LDL exposed to aqueous peroxyl radicals or Cu2+, consumption of endogenous ubiquinol-10 and alpha-tocopherol was followed by rapid formation and subsequent breakdown of lipid hydroperoxides and esterified F2-isoprostanes, and a continuous increase in LDL's electronegativity, indicative of atherogenic modification. In Cu(2+)-exposed LDL, the decrease in esterified F2-isoprostane levels was paralleled by the appearance of free F2-isoprostanes, suggesting that hydrolysis by an LDL-associated activity had occurred. Our data suggest that F2-isoprostanes are useful markers of LDL oxidation in vivo. As F2-isoprostanes are potent vasoconstrictors and can modulate platelet aggregation, their formation in LDL demonstrated here may also have important implications for the etiology of cardiovascular disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee M., Kang K. H., Morrow J. D., Roberts L. J., Newman J. H. Effects of a novel prostaglandin, 8-epi-PGF2 alpha, in rabbit lung in situ. Am J Physiol. 1992 Sep;263(3 Pt 2):H660–H663. doi: 10.1152/ajpheart.1992.263.3.H660. [DOI] [PubMed] [Google Scholar]
- Blanchard D. G., Ross J., Jr Hypertrophic cardiomyopathy: prognosis with medical or surgical therapy. Clin Cardiol. 1991 Jan;14(1):11–19. doi: 10.1002/clc.4960140105. [DOI] [PubMed] [Google Scholar]
- Bowry V. W., Ingold K. U., Stocker R. Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem J. 1992 Dec 1;288(Pt 2):341–344. doi: 10.1042/bj2880341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., Morel D. W., Chisolm G. M., 3rd Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985 Aug;38(2):341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Segrest J. P., Ray M. J., Brunzell J. D., Hokanson J. E., Krauss R. M., Beaudrie K., Cone J. T. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986;128:181–209. doi: 10.1016/0076-6879(86)28068-4. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doba T., Burton G. W., Ingold K. U. Antioxidant and co-antioxidant activity of vitamin C. The effect of vitamin C, either alone or in the presence of vitamin E or a water-soluble vitamin E analogue, upon the peroxidation of aqueous multilamellar phospholipid liposomes. Biochim Biophys Acta. 1985 Jul 9;835(2):298–303. doi: 10.1016/0005-2760(85)90285-1. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Hannani K., Fei H. H., Lavi S., Berliner J. A. Minimally oxidized low-density lipoprotein induces tissue factor expression in cultured human endothelial cells. Am J Pathol. 1991 Mar;138(3):601–607. [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Gebicki J., Puhl H., Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992 Oct;13(4):341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- Frei B., England L., Ames B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Gaziano J. M. Content of antioxidants, preformed lipid hydroperoxides, and cholesterol as predictors of the susceptibility of human LDL to metal ion-dependent and -independent oxidation. J Lipid Res. 1993 Dec;34(12):2135–2145. [PubMed] [Google Scholar]
- Frei B., Kim M. C., Ames B. N. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4879–4883. doi: 10.1073/pnas.87.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Stocker R., England L., Ames B. N. Ascorbate: the most effective antioxidant in human blood plasma. Adv Exp Med Biol. 1990;264:155–163. doi: 10.1007/978-1-4684-5730-8_24. [DOI] [PubMed] [Google Scholar]
- Frei B., Yamamoto Y., Niclas D., Ames B. N. Evaluation of an isoluminol chemiluminescence assay for the detection of hydroperoxides in human blood plasma. Anal Biochem. 1988 Nov 15;175(1):120–130. doi: 10.1016/0003-2697(88)90369-7. [DOI] [PubMed] [Google Scholar]
- Galle J., Mülsch A., Busse R., Bassenge E. Effects of native and oxidized low density lipoproteins on formation and inactivation of endothelium-derived relaxing factor. Arterioscler Thromb. 1991 Jan-Feb;11(1):198–203. doi: 10.1161/01.atv.11.1.198. [DOI] [PubMed] [Google Scholar]
- Harats D., Ben-Naim M., Dabach Y., Hollander G., Havivi E., Stein O., Stein Y. Effect of vitamin C and E supplementation on susceptibility of plasma lipoproteins to peroxidation induced by acute smoking. Atherosclerosis. 1990 Nov;85(1):47–54. doi: 10.1016/0021-9150(90)90181-h. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehr H. A., Hübner C., Nolte D., Finckh B., Beisiegel U., Kohlschütter A., Messmer K. Oxidatively modified human low-density lipoprotein stimulates leukocyte adherence to the microvascular endothelium in vivo. Res Exp Med (Berl) 1991;191(2):85–90. doi: 10.1007/BF02576662. [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Awad J. A., Boss H. J., Blair I. A., Roberts L. J., 2nd Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Awad J. A., Kato T., Takahashi K., Badr K. F., Roberts L. J., 2nd, Burk R. F. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992 Dec;90(6):2502–2507. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Harris T. M., Roberts L. J., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990 Jan;184(1):1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Hill K. E., Burk R. F., Nammour T. M., Badr K. F., Roberts L. J., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Minton T. A., Roberts L. J., 2nd The F2-isoprostane, 8-epi-prostaglandin F2 alpha, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins. 1992 Aug;44(2):155–163. doi: 10.1016/0090-6980(92)90077-7. [DOI] [PubMed] [Google Scholar]
- Morrow J. D., Roberts L. J., 2nd Quantification of noncyclooxygenase derived prostanoids as a marker of oxidative stress. Free Radic Biol Med. 1991;10(3-4):195–200. doi: 10.1016/0891-5849(91)90076-f. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Barnett J. Phospholipase A2 activity of low density lipoprotein: evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9741–9745. doi: 10.1073/pnas.87.24.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Printz D. J., Boyd D., Joy L., Steinberg D. Macrophage oxidation of low density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis. 1986 Sep-Oct;6(5):505–510. doi: 10.1161/01.atv.6.5.505. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Porter N. A., Funk M. O. Letter: Peroxy radical cyclization as a model for prostaglandin biosynthesis. J Org Chem. 1975 Nov 28;40(24):3614–3615. doi: 10.1021/jo00912a037. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Stanley J. P. Letter: A suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975 Nov 28;40(24):3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Retsky K. L., Freeman M. W., Frei B. Ascorbic acid oxidation product(s) protect human low density lipoprotein against atherogenic modification. Anti- rather than prooxidant activity of vitamin C in the presence of transition metal ions. J Biol Chem. 1993 Jan 15;268(2):1304–1309. [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Ylä-Herttuala S., Yamamoto R., Butler S., Korpela H., Salonen R., Nyyssönen K., Palinski W., Witztum J. L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992 Apr 11;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Sato K., Niki E., Shimasaki H. Free radical-mediated chain oxidation of low density lipoprotein and its synergistic inhibition by vitamin E and vitamin C. Arch Biochem Biophys. 1990 Jun;279(2):402–405. doi: 10.1016/0003-9861(90)90508-v. [DOI] [PubMed] [Google Scholar]
- Sharma M. K., Buettner G. R. Interaction of vitamin C and vitamin E during free radical stress in plasma: an ESR study. Free Radic Biol Med. 1993 Jun;14(6):649–653. doi: 10.1016/0891-5849(93)90146-l. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Pritchard P. H. Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J Lipid Res. 1989 Mar;30(3):305–315. [PubMed] [Google Scholar]
- Stocker R., Bowry V. W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nammour T. M., Fukunaga M., Ebert J., Morrow J. D., Roberts L. J., 2nd, Hoover R. L., Badr K. F. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest. 1992 Jul;90(1):136–141. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner F. C., Noll G., Boulanger C. M., Lüscher T. F. Oxidized low density lipoproteins inhibit relaxations of porcine coronary arteries. Role of scavenger receptor and endothelium-derived nitric oxide. Circulation. 1991 Jun;83(6):2012–2020. doi: 10.1161/01.cir.83.6.2012. [DOI] [PubMed] [Google Scholar]
- Weis J. R., Pitas R. E., Wilson B. D., Rodgers G. M. Oxidized low-density lipoprotein increases cultured human endothelial cell tissue factor activity and reduces protein C activation. FASEB J. 1991 Jul;5(10):2459–2465. doi: 10.1096/fasebj.5.10.2065893. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Frei B., Ames B. N. Assay of lipid hydroperoxides using high-performance liquid chromatography with isoluminal chemiluminescence detection. Methods Enzymol. 1990;186:371–380. doi: 10.1016/0076-6879(90)86130-n. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]





