Abstract
Incomplete or aberrant glycosylation leading to Tn antigen (GalNAcα1-Ser/Thr) expression on human glycoproteins is strongly associated with human pathological conditions, including tumors, certain autoimmune diseases, such as the idiopathic IgA nephropathy, and may modulate immune homeostasis. In addition, the Tn antigen is highly expressed by certain pathogens and plays a role in host–pathogen interactions. To enable experimental approaches to study interactions of the Tn antigen with the immune system and analyse anti-Tn antibody responses in infection or disorders, we generated a Tn-expressing resource that can be used for high-throughput screening. In consideration of IgA nephropathy in which the hinge region is incompletely glycosylated, we used this hinge sequence that encodes five potential glycosylation sites as the ideal template for the synthesis of a Tn antigen expressing glycopeptide. Inclusion of an N-terminal biotin in the peptide enabled binding to streptavidin-coated ELISA plates as monitored using Helix pomatia agglutinin or anti-Tn monoclonal antibody. We also found that the biotinylated IgA-Tn peptide is a functional acceptor for β1-3-galactosylation using recombinant T-synthase (β1-3-galactosyltransferase). Besides its immunochemical functionality as a possible diagnostic tool for IgA nephropathy, the peptide is an excellent substrate for glycan elongation and represents a novel template applicable for glycan–antigen-associated diseases.
Keywords: glycopeptide synthesis, IgA nephropathy, Tn-antigen
1. Introduction
Glycans covalently linked to proteins and lipids at the surface of cells play crucial roles in a wide variety of cellular recognition, signalling, and adhesion events. Abnormalities in the synthesis or presentation of the glycan moieties of the glycoconjugates can lead to loss-of-function of these molecules, or the acquisition of abnormal functional properties that may lead to debilitating diseases1–3.
The Tn antigen is an incompletely synthesized mucin-type O-glycan, consisting of only the sugar N-acetylgalactosamine α-linked to either a serine or threonine amino acid residue in a protein (GalNAcα1-O-Ser/Thr)4. In normal tissues, GalNAcα-O-Ser/Thr is elongated by a concerted process of stepwise addition of monosaccharides into distinct classes of O-glycans, reactions catalyzed by a large number of specific glycosyltransferases (see for review5). In humans, expression of the Tn antigen in several cases is due to loss of activity of T-synthase (core 1 β-(1→3)-galactosyltransferase), the enzyme responsible for addition of a Gal in β-(1→3)-linkage to the O-GalNAc, the start of elongation to core 1 and core 2 O-glycan structures. Remarkably, the loss of T-synthase activity can result from changes in expression or mutations in the gene encoding the molecular chaperone Cosmc6,7, which is required for expression of active T-synthase in vertebrates8,9.
The Tn antigen is found in the majority of human tumors4,7, where its presence is often associated with poor prognosis10,11. Since Tn antigens are rarely exposed in glycoproteins of healthy individuals, they represent excellent targets for diagnosis or cancer vaccine development. In addition, the Tn antigen is commonly found in certain human autoimmune diseases, such as Tn syndrome12,13 or the idiopathic autoimmune disease IgA nephropathy14,15. The Tn antigen is also a ligand for human macrophage galactose-binding lectin (MGL), a glycan-binding protein expressed on macrophages and immature dendritic cells16. MGL is involved in down regulation of effector T cell function by interaction with glycan antigens on CD45, which may be Tn antigens17, indicating that the presence of Tn antigen may lead to modulation of immune homeostasis. In addition to being an abnormal human antigen, the Tn antigen is also highly expressed by pathogens, such as a variety of parasitic helminths, and may play a role in host–pathogen interactions18,19. Whereas the occurrence of the Tn antigen has been widely described, its role in human biology or host pathogen interactions is poorly understood.
To enable experimental approaches to study interactions of the Tn antigen with immune cells, or analyse anti-Tn antibody responses in infection or Tn-related disease, we set up the synthesis of Tn-containing glycopeptides as a tool for such studies. As proof of principle we synthesized the IgA nephropathy-associated IgA hinge region glycopeptide containing five Tn antigens and an N-terminal biotin. This glycopeptide can bind to streptavidin-coated ELISA plates, which allows opportunities for its use as a template for quantitative high-throughput screening of serum anti-Tn antibodies. Based on the prior method described for the synthesis of mucin-like glycopeptide, which features three consecutive O-glycosylated Thr residues,20 we have synthesized a glycopeptide using the building blocks Fmoc-Ser-(α-GalNAcAc3)-OH and Fmoc-Thr-(α-GalNAcAc3)-OH with O-glycosidic linkages and O-acetyl protection that are stable to both piperidine and TFA21. Subsequent N-terminal biotinylation using biotin p-nitrophenyl ester, which is optimal for solid-phase peptide synthesis (SPPS)22, resulted in the desired biotinylated glycopeptide. Characterization of the glycopeptide showed its immunochemical functionality as a tool to quantitatively determine the binding of Tn-recognizing lectin or antibodies. In addition, the peptide was found to be an excellent substrate for glycan elongation and a good template to synthesize glycopeptides with extended O-glycan structures applicable for the study of O-glycan-associated diseases.
2. Experimental
2.1. Solid-phase (glyco)peptide synthesis
IgA hinge region peptide PVPSTPPTPSPSTPPTPSPS (IgA peptide, Fig. 1) was prepared by solid-phase peptide synthesis (SPPS) using 9-fluorenylmethoxycarboyl (Fmoc) chemisty with a MilliGen 9050 synthesizer (MilliGen/Biosearch, Bedford, MA) according to the manufacturer’s procedures. Unless stated otherwise, peptide synthesis grade solvents were used directly as obtained from Biosolve (Valkenswaard, The Netherlands). The Nα-Fmoc-amino acids, side-chain protected by tert-butyl (tBu) were obtained from OrpegenPharma (Heidelberg, Germany). The solid phase Fmoc-L-Ser(tBu)-PEG-PS resin (0.1 mmol, loading 0.21 mmol/g; Applied Biosystems, Foster City, CA) in N-methyl-2-pyrrolidone (NMP) was applied to the column, and all steps were developed at a flow rate of 4 mL/min. Fmoc removal was achieved with piperidine (20% v/v) in NMP for 6 min. Fmoc-amino acids were dissolved at 4 times molar excess in N,N-dimethylformamide (DMF) containing 0.6 mM O-benzotiazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (HBTU) and 0.6 mM 1-hydroxybenzotriazole (HObt) and coupled in the presence of 0.45 M N,N-diisopropylethylamine (DIPEA) in NMP by recycling for 1.5 h. Washings between the reaction steps were carried out with NMP. The peptide was N-terminally biotinylated on the resin using 4 times molar excess biotin p-nitrophenylester (biotin-ONp) in the presence of HObt in NMP during 2 h22. Subsequently the biotin-labeled peptide was detached from the resin and deprotected with 14 mL of trifluoroacetic acid 85:5:5:5 (TFA)–phenol–thioanizole–H2O in a 25 mL syringe applied with a frit under gentle shaking during at least 2 h. Next, N2 was flushed through the reaction mixture to reduce volume to less than 2 mL. The reaction mixture was purged from the syringe into 30 mL of ice-cold Et2O in a 50-mL tube, and the remaining resin was washed twice with 2 mL of TFA. The precipitated peptide was washed 4 times with ice-cold Et2O using a magnetic driven centrifuge (Christ RVC 2-25, Osterode, Germany) at 230g for 5 min, subsequently dissolved in 10 mL H2O, flushed with N2 to remove excess ether and lyophilized.
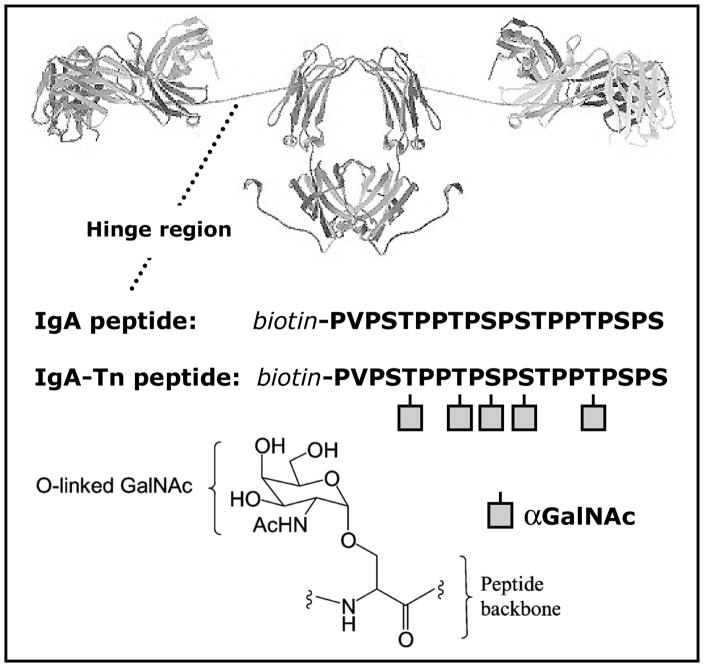
Fig. 1.
A ribbon view of immunoglobulin A1 (IgA1). The IgA peptide corresponds to the amino acid sequence of the hinge region, and the IgA-Tn peptide is the same peptide but featured with five Tn-antigens, depicted as squared symbols, resembling O-αGalNAc residues. Both peptides are biotinylated at their N-temini.
The corresponding IgA hinge region glycopeptide PVPST-O-GalNAcPPT-O-GalNAcPS-O-GalNAcPS-O-GalNAcTPPT-O-GalNAcPSPS, featured with five Tn antigens (IgA-Tn peptide, Fig. 1) was synthesized manually using similar protocols but downscaled 25 times in volume based on the low amounts (~50 nmol) of the expensive Tn antigen building blocks, Fmoc-O-β-(2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-D-galactopyranosyl)-L-Ser/Thr (OrpegenPharma). Fmoc-L-Ser(tBu)-Wang resin (12 nmol, loading 0.62 mmol/g, Novabiochem, Darmstadt, Germany) was loaded into a small column running in NMP and processed by hand. During reaction steps the column was shaken rigorously, followed by washings under gentle nitrogen pressure. This peptide was also biotinylated with biotin-ONp in synthesis and subsequently cleaved from the resin as described above. The TFA-stable O-acetyl protection groups of the Ser/Thr(GalNAcAc3) residues were removed with sodium methoxide. The lyophilized glycopeptide was dissolved in MeOH (~5mg/mL) and 0.3 M NaOMe was added dropwise to a final concentration of 30 mM. Removal of the O-acetyl protection groups was monitored by RP-HPLC. After completion in about 1 h, the O-deacetylation reaction mixture was neutralized with dry ice, and the MeOH was removed by evaporation under reduced pressure (Christ RVC 2-25).
2.2. Peptide purification and analysis
IgA-peptide and IgA-Tn peptide were purified by semipreparative RP-HPLC (Jasco Corporation, Tokyo, Japan) on a VYDAC C18-column (218MS510, Vydac, Hesperia, CA) Peptides were dissolved in H2O, containing 5% MeCN and 0.1% TFA. Elution was performed with a linear gradient from 15 to 45% MeCN containing 0.1% TFA in 20 min at a flow rate of 4 mL/min. The absorbance of the column effluent was monitored at 214 nm, and peak fractions were pooled and lyophilized. Re-analysis by RP-HPLC on an analytical Vadac C18-column (218MS54) developed with a similar gradient at a flow rate of 1 mL/min revealed a purity of at least 95%.
Removal of O-acetyl protecting groups of the IgA-Tn peptide by NaOMe was monitored by RP-HPLC of 10-μL aliquots of the reaction mixture using a Vadac C18-column (218MS54) column developed with a linear gradient from 5 to 40% MeCN containing 0.1% TFA in 20 min at a flow rate of 1 mL/min. Elution of peptides at lower MeCN percentage corresponded with less hydrophobicity due to O-acetyl group removal.
2.3. Galactosylation of Tn antigen by T-synthase
Human recombinant soluble HPC4-tagged T-synthase was expressed in insect Hi-5 cells as previously described23 and absorbed from the culture media using an anti-HPC4 column. The activity of T-synthase on the beads was 2 μmol/h-mL. The IgA1 hinge region glycopeptide was galactosylated overnight at 37 °C in 100–200 nmol aliquots by using a 6-fold molar excess of UDP-Gal (Sigma) in the presence of 50-μL beads (100 nmol/h T-synthase) in a total volume of 200 μL of reaction mixture containing 50 mM MES, pH 6.8, 15 mM MnCl2. As a control, the IgA1 hinge region peptide was also setup for the galactosylation reaction as above. After purifying the peptide and glycopeptides using a C18 cartridge, the purified materials were analyzed by MALDI-TOFMS using an Ultraflex II MALDI-TOF/TOF instrument (Bruker Daltonics).
2.4. ELISA using biotinylated IgA-peptide and IgA-Tn-peptide
Streptavidin-coated high binding capacity black plates (Pierce, Rockford, IL) were washed three times with 200 μL of TSM (20 mM Tris-HCl pH 7.4, containing 150 mM NaCl, 2 mM CaCl2 and 2 mM MgCl2) and coated with biotinylated (glyco)peptides in TSA, in duplicate at 4 °C by overnight incubation at concentrations as indicated. After washing three times with TSM, bound (glyco)peptides were incubated with anti-Tn monoclonal antibody (mouse IgM, CA3638, clone 12A8-C7-F5)7,24, followed by peroxidase-labeled goat-antimouse IgM, with peroxidase-labeled Helix pomatia agglutinin (HPA, from E-Y Laboratories, 2 μg/mL) or in the experiments with human sera with peroxidase-labeled goat-antihuman IgG, for 1 h at room temperature. Human sera were derived from 16 healthy individuals and diluted 1:100 in PBS. The sera 1–8 were derived from adults aged 18–63, whereas the sera 9–16 were derived from children aged 5–17. Bound peroxidase-labeled molecules were detected after incubation with a solution containing 3,3′,5,5′-tetramethylbenzidine (10 mg/mL) and 0.5 μL hydrogen peroxide (30%) in 0.1 M NaOAc and 0.1 M citric acid at pH 4 (100 μl/well). The color reaction was stopped by adding 25 μL of 4 M H2SO4, and the absorbance was read at 450 nm with a microplate reader. The assays were performed twice in triplicate, and background reaction was subtracted from each sample.
3. Results
3.1. Solid-phase synthesis of a biotinylated IgA hinge region glycopeptide, expressing five Tn antigens
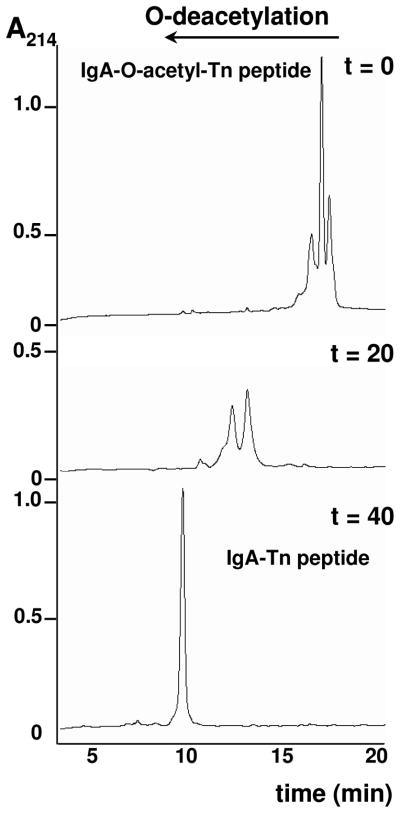
The IgA hinge region peptide (IgA peptide, Fig. 1) was synthesized by classical SPPS. The corresponding IgA hinge region glycopeptide (IgA-Tn peptide, Fig. 1), featured with five Tn antigens was synthesized manually using similar protocols but downscaled 25 times in volume to meet the use of small amounts of the expensive Tn antigen building blocks, Fmoc-O-β-(2-acetamido-3,4,6-tri-O-acetyl-2-deoxy-α-D-galactopyranosyl)-L-Ser/Thr. Desired low volumes were also a motivation for the resin choice. The Wang resin was used because it combines a high capacity with reduced swelling properties, allowing lower reaction volumes. Both peptides were prepared to contain biotin at their N-termini with biotin-ONp as the final step in the SPPS, to get a 1:1 stoichiometry, and to be analyzed by RP-HPLC (Fig. 2). The non-glycosylated peptide eluted as one main peak (not shown), while the glycopeptide, which contained the O-acetyl protecting groups, revealed a cluster of peaks (Fig. 2). Removal of the O-acetyl groups with sodium methoxide resulted in a peak shift corresponding to reduced hydrophobicity concomitant with a gradual reduction of the number of peaks with increasing time of treatment, ending with one final peak indicating the completion of deprotection and a single product (Fig. 2).
Fig. 2.
RP-HPLC of IgA-Tn peptide before and during NaOMe treatment to remove the O-acetyl groups. Removal was monitored by analyzing 10-μL aliquots from the reaction mixture on a Vydac C18-column (218MS54) column developed with a linear gradient from 5 to 40% MeCN containing 0.1% TFA in 20 min at a flow rate of 1 mL/min. The shift in retention time corresponded with reduced hydrophobicity caused by O-acetyl group removal. After 40 min of treatment, no further shift occurred.
3.2. Structural analysis of the IgA peptide and IgA-Tn peptide
The authenticity of the purified peptide and glycopeptide obtained after semipreparative RP-HPLC was confirmed by MALDI-TOF/TOFMS analysis. Mass spectra indicated major peaks at m/z 2176.2 and m/z 3191.7 identical to the calculated [M + Na]+ of the IgA-peptide and IgA-Tn peptide, respectively (Fig. 3a,b). The mass difference between the two samples exactly matched the addition of five α-GalNAc residues O-linked to the IgA-peptide, which was termed the IgA-Tn peptide.
Fig. 3.
The Tn-moieties of the IgA-Tn peptide were modified to T antigens with recombinant human T-synthase (core 1 β-(1→3)-galactosyltransferase), and the products analyzed by MS. Five products were detected, each differing by a mass of 162 due to the addition of a Gal residue. The non-glycosylated IgA peptide was not modified by T-synthase. Thus, IgA-Tn peptides were modified with one to five Gal residues, indicating that all 5 Tn-antigens of the peptide were accessible to galactosylation.
The accessibility of each of the Tn-antigens within the IgA-Tn peptide was analyzed by testing its function as an acceptor substrate for enzymatic elongation of the glycan residue. Recombinant T-synthase (core 1 β1,3-galactosyltransferase) was used to catalyze the transfer of a Gal residue from UDP-Gal to α-GalNAc residues on the glycopeptide25. The enzyme products were analyzed by MALDI-TOF/TOFMS analysis. The mass spectrum showed five peaks at m/z values each with an interval of approximately 162 Da (Fig. 3d). The absence of a peak at m/z 3191.7 suggested that 100% conversion of the starting material was achieved. Although all the IgA-Tn peptides contained at least one Gal residue, the presence of a subpopulation containing five Gal residues demonstrated the possibility of elongating each of the five α-GalNAc residues. The conditions presently used for β-(1→3)-galactosylation resulted in a mixed population of IgA hinge region glycopeptides expressing one to four Tn-antigens in combination with five to one T-antigens (Galβ1-3GalNAcα-Ser/Thr), respectively. IgA control peptide without α-GalNAc residues was not a substrate for T-synthase (Fig. 3c).
3.3. Recognition of the Tn-antigen by Helix pomatia agglutinin and anti-Tn monoclonal antibody
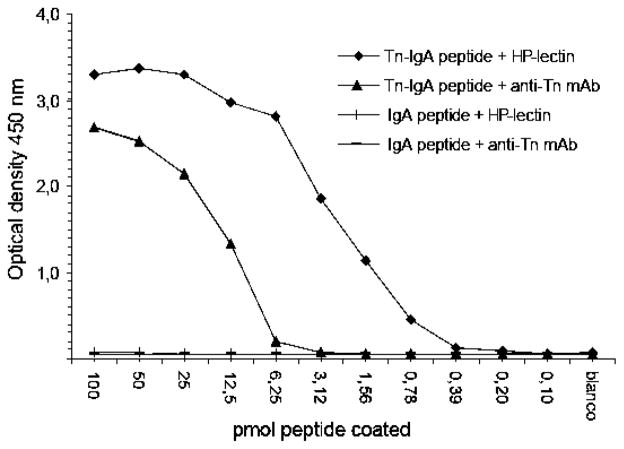
The applicability of the IgA-Tn peptide in an immunochemical assay was tested in ELISA using anti-Tn mAb as well as Helix pomatia agglutinin (HPA), a lectin that recognizes the Tn-glycan9,26,27 (Fig. 4). The N-terminally biotinylated IgA peptide and IgA-Tn peptide were serially diluted and loaded onto streptavidin-coated 96-well plates, to allow quantitative measurement of bound molecules. Upon probing with anti-Tn antibodies in sera or Helix pomatia lectin, the IgA-Tn peptide was recognized in a dose-dependent manner, whereas no reactivity was found with the IgA peptide not carrying Tn antigen. The anti-Tn antibody used recognized at least 6.25 pmoles of IgA-Tn peptide, whereas HPA recognized as little as 0.4 pmol of the antigen. These data show that the IgA-Tn peptide can be used in assays to determine quantitatively the serum levels of anti-Tn antibodies, or to evaluate the binding of specific lectins to Tn-antigens.
Fig. 4.
Streptavidin-coated ELISA plates were incubated with serially diluted biotinylated (glyco)peptides and probed with anti-Tn antibody or HPA. IgA-Tn peptide was recognized in a dose-dependent manner, showing that the method allows quantitative measurement of serum antibodies.
3.4. IgG recognizing IgA hinge region in serum of healthy individuals
The presence of IgG antibodies recognizing the IgA hinge region was evaluated using serum from a panel of 16 healthy individuals (Fig. 5). Remarkably, the presence of IgG antibodies recognizing the peptides was highly variable between different individuals, and several of the sera samples tested showed relatively high IgG antibody levels recognizing the IgA- and/or IgA-Tn-peptides. Comparison of the serum antibody levels to the IgA-peptide versus the IgA-Tn-peptide within a particular serum sample demonstrated that a few sera showed an elevated response to the IgA-Tn-peptide, suggesting the presence of Tn-specific antibodies. Several sera showed a higher response to the IgA-peptide compared to the IgA-Tn-peptide, suggesting that the presence of the GalNAc residues blocked access of the antibodies to the peptide sequence.
Fig. 5.
IgG antibody levels against IgA hinge region and Tn-antigen in serum of healthy individuals. Streptavidine-coated ELISA plates were incubated with biotinylated IgA-peptide and IgA-Tn-peptide (100 pmol). After washing, they were incubated with 1:100 diluted serum of healthy individuals, varying in age from 5–63 years. Bound IgG was detected using peroxidase-labeled goat-antihuman IgG.
4. Discussion
Here we describe the synthesis of a pentavalent glycopeptide containing five O-GalNAc residues (Tn antigens) as a tool to study the role of this antigen in disease or host–pathogen interactions. For the template we chose the human IgA1-hinge region that contains several potential glycosylation sites, five of which are normally occupied in human IgA1. This glycopeptide with five O-GalNAc residues allowed us to determine the feasibility of synthesizing multiple O-glycan peptides by SPPS, constructed with repeated Ser and Thr residues often interspersed with one or more Pro residues. Moreover, we showed that the GalNAc residues on this glycopeptides could be used to evaluate the recognition and elongation of each of the GalNAc residues by a specific glycosyltransferase, thus allowing the possibility to produce more complex glycopeptides by a combined organic chemical and enzymatic approach. The conditions used for β-(1→3)-galactosylation resulted in a mixed population of glycopeptides expressing one to four Tn-antigens in combination with five to one T-antigens, respectively. If complete galactosylation is desired, raising the enzyme and substrate concentrations and extending the incubation time would be obvious steps. On the other hand, as partial galactosylation might occur in vivo, its modulating effects on the accessibility of residual Tn-antigens or the formation of anti-Tn-antibodies can be explored with these partially galactosylated glycopeptides. Such glycopeptides have been shown to be extremely useful tools to evaluate the biological function of specific glycan antigens28.
The Tn-antigen is a special glycan antigen consisting of only a GalNAc directly linked to a Ser/Thr of a protein, and existing evidence indicates that recognition of the GalNAc by the immune system or lectins may occur in the context of the carrier peptide backbone. In addition, the peptide also determines the multivalency of presentation of GalNAc residues by the repetitive and spatial occurrence of Ser/Thr residues, such as in mucins. This implies that commercially available neoglycoconjugates such as GalNAcα-BSA, or GalNAcα-PAA, have serious limitations for studying the biological function of Tn-antigens. For example, several monoclonal antibodies recognize Tn-antigens in different contexts (unpublished data). The synthesis of Tn-peptides as described here allows a sequence-specific approach to synthesize specific Tn-peptides, such as the IgA1 hinge region glycopeptide, mimicking the in vivo situation.
In IgA nephropathy (IgAN), the most common glomerulonephritis in the world, aberrant glycosylation patterns of the O-glycans in the hinge-region of IgA1 is a hallmark of the disease15. These abnormal O-glycans may be manifested by a defective β-(1→3)-galactosylation of the O-glycans of the proline-rich hinge region of IgA114, resulting in terminal Tn antigens as well as its sialylated form, sialyl-Tn. These epitopes are recognized by IgG or IgA1 antibodies, which may lead to the formation of macromolecular immune complexes that are prone to mesangial trapping and subsequent chronic inflammation in the kidney29. In addition, the presence of serum antibodies against the abnormal glycosylated IgA1 hinge region has been reported in IgAN patients30,31. In this respect, our finding that healthy individuals show highly variable levels of antibodies recognizing the IgA hinge region is remarkable and not completely understood. Studies directed toward analyzing serum antibodies recognizing altered carbohydrate moieties should therefore be carefully controlled. Understanding the molecular basis for variations in carbohydrate components in the O-glycans of IgA1 and especially in IgA nephropathy patients may be related to altered balances of individual glycosyltransferases. As such, the Tn-glycopeptides reported here may be useful as tools for identification of the distinct roles of such glycosyltransferases in the events of defective O-glycosylation, or to determine serum antibodies in IgAN patients, which is essential for pinpointing putative abnormalities in the synthesis of O-glycans in IgAN.
The Tn antigen is found in many human cancers and is used and/or explored as a target for immunodiagnosis and prophylactic vaccination strategies. For example, the cancer-associated mucin MUC-1 carrying Tn, STn, and Thomsen–Friedenreich (TF) antigens is found in patient sera and is used as a marker for breast cancer32. Interestingly, glycopeptides carrying the Tn-antigen have been used to induce a carbohydrate-specific cytotoxic T-cell response in mice33. In addition, several helminth parasites synthesize Tn and/or T antigens, such as described for Echinococcus spp., Mesocestoides spp., Taenia hydatigena, Fasciola hepatica, S. mansoni, Nippostrongylus brasiliensis, and Toxocara canis18,34–37. The Tn antigen also appears to be a common “helminth” antigen found across the phyla boundaries of Platyhelminthes and Nematodes.
In summary, glycopeptides exposing Tn-antigens in specific peptide sequences, or forms that are modified by enzymatic synthesis to glycopeptides carrying T-antigen or sialyl-Tn, can be applied in many ways. They may be useful as template for high-throughput quantitative measurements of serum antibodies, such as in IgAN patients, cancer patients or helminth-infected individuals, or provide a basis to develop vaccine components targeting Tn-exposing cancer cells or pathogens.
Acknowledgments
We thank W. van ’t Hof for helpful discussion and “The Netherlands Kidney Foundation (NSN)” for financial support of this research. This project is also supported by NIH RO1 grant (RO1DK80876) to T.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brockhausen I. Biochim Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 2.Freeze HH, Aebi M. Curr Opin Struct Biol. 2005;15:490–498. doi: 10.1016/j.sbi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Wopereis S, Lefeber DJ, Morava E, Wevers RA. Clin Chem. 2006;52:574–600. doi: 10.1373/clinchem.2005.063040. [DOI] [PubMed] [Google Scholar]
- 4.Springer GF. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- 5.Brockhausen I. Methods Mol Biol. 2000;125:273–293. doi: 10.1385/1-59259-048-9:273. [DOI] [PubMed] [Google Scholar]
- 6.Schietinger A, Philip M, Yoshida BA, Azadi P, Liu H, Meredith SC, Schreiber H. Science. 2006;314:304–308. doi: 10.1126/science.1129200. [DOI] [PubMed] [Google Scholar]
- 7.Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE, Laszik Z, Benbrook DM, Hanigan MH, Cummings RD. Cancer Res. 2008;68:1636–1646. doi: 10.1158/0008-5472.CAN-07-2345. [DOI] [PubMed] [Google Scholar]
- 8.Ju T, Cummings RD, Canfield WM. J Biol Chem. 2002;277:169–177. doi: 10.1074/jbc.M109056200. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, He M, Cummings RD. Proc Natl Acad Sci USA. 2010;107:9228–33. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumacher U, Higgs D, Loizidou M, Pickering R, Leathem A, Taylor I. Cancer. 1994;74(3):104–3107. doi: 10.1002/1097-0142(19941215)74:12<3104::aid-cncr2820741207>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Laack E, Nikbakht H, Peters A, Kugler C, Jasiewicz Y, Edler L, Hossfeld DK, Schumacher U. Am J Pathol. 2002;160:1001–1008. doi: 10.1016/S0002-9440(10)64921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger EG. Biochim Biophys Acta. 1999;1455:255–268. doi: 10.1016/s0925-4439(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 13.Ju T, Cummings RD. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 14.Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, Shinzato T, Kobayashi Y, Maeda K. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 15.Narita I, Gejyo F. Clin Exp Nephrol. 2008;12:332–338. doi: 10.1007/s10157-008-0054-5. [DOI] [PubMed] [Google Scholar]
- 16.van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, Appelmelk B, Irimura T, Geijtenbeek TB, Blixt O, Alvarez R, van Die I, van Kooyk Y. Int Immunol. 2005;17:661–669. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 17.van Vliet SJ, van Liempt E, Geijtenbeek TB, van Kooyk Y. Immunobiology. 2006;211:577–585. doi: 10.1016/j.imbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Casaravilla C, Freire T, Malgor R, Medeiros A, Osinaga E, Carmona C. J Parasitol. 2003;89:709–714. doi: 10.1645/GE-2970. [DOI] [PubMed] [Google Scholar]
- 19.van Die I, Cummings RD. Glycobiology. 2009;20:2–12. doi: 10.1093/glycob/cwp140. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Barany G, Live D. Carbohydr Res. 2005;340:2111–2122. doi: 10.1016/j.carres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Kihlberg J. In: Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Chan WC, White PD, editors. Oxford University Press; Oxford, UK: 2000. pp. 195–213. [Google Scholar]
- 22.Baumeister B, Beythien J, Ryf J, Schneeberger P, White PD. Int J Pept Res Ther. 2005;11:139–141. [Google Scholar]
- 23.Ju T, Cummings RD. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avichezer D, Springer GF, Schechter B, Arnon R. Int J Cancer. 1997;72:119–27. doi: 10.1002/(sici)1097-0215(19970703)72:1<119::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM. J Biol Chem. 2002;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 26.Newman RA, Uhlenbruck GG. Eur J Biochem. 1977;76:149–155. doi: 10.1111/j.1432-1033.1977.tb11580.x. [DOI] [PubMed] [Google Scholar]
- 27.Hammarström S, Murphy LA, Goldstein IJ, Etzler ME. Biochemistry. 1977;16:2750–5. doi: 10.1021/bi00631a025. [DOI] [PubMed] [Google Scholar]
- 28.Leppanen A, Mehta P, Ouyang YB, Ju T, Helin J, Moore KL, van Die I, Canfield WM, McEver RP, Cummings RD. J Biol Chem. 1999;274:24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- 29.Mestecky J, Suzuki H, Yanagihara T, Moldoveanu Z, Tomana M, Matousovic K, Julian BA, Novak J. Contrib Nephrol. 2007;157:56–63. doi: 10.1159/000102305. [DOI] [PubMed] [Google Scholar]
- 30.Kokubo T, Hashizume K, Iwase H, Arai K, Tanaka A, Toma K, Hotta K, Kobayashi Y. Nephrol Dial Transplant. 2000;15:28–33. doi: 10.1093/ndt/15.1.28. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Moldoveanu Z, Hall S, Brown R, Julian BA, Wyatt RJ, Tomana M, Tomino Y, Novak J, Mestecky J. Contrib Nephrol. 2007;157:129–133. doi: 10.1159/000102454. [DOI] [PubMed] [Google Scholar]
- 32.Hattrup CL, Gendler SJ. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Gendler SJ, Franco A. J Exp Med. 2004;199:707–716. doi: 10.1084/jem.20031865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez Errico D, Medeiros A, Miguez M, Casaravilla C, Malgor R, Carmona C, Nieto A, Osinaga E. Exp Parasitol. 2001;98:100–109. doi: 10.1006/expr.2001.4620. [DOI] [PubMed] [Google Scholar]
- 35.Freire T, Fernandez C, Chalar C, Maizels RM, Alzari P, Osinaga E, Robello C. Biochem J. 2004;382:501–510. doi: 10.1042/BJ20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medeiros A, Chiribao ML, Ubillos L, Festari MF, Saldana J, Robello C, Dominguez L, Calvete JJ, Osinaga E. Int J Parasitol. 2008;38:265–276. doi: 10.1016/j.ijpara.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Nyame K, Cummings RD, Damian RT. J Parasitol. 1988;74:562–572. [PubMed] [Google Scholar]