Abstract
Neurosteroid sensitivity of GABAA receptor mediated inhibition of the hippocampal dentate granule cells (DGCs) is reduced in animal models of temporal lobe epilepsy. However, the properties and subunit composition of GABAA receptors mediating tonic inhibition in DGCS of epileptic animals have not been described. In the DGCs of epileptic animals, allopregnanolone and L-65708 sensitivity of holding current was diminished and δ subunit was retained in the endoplasmic reticulum and its surface expression was decreased the in the hippocampus. Ro15–4513 and lanthanum had distinct effects on holding current recorded from DGCs of control and epileptic animals. The pharmacological properties of GABAA receptors maintaining tonic inhibition in DGCs of epileptic animals were similar to those containing the α4βxγ2 subunits. Furthermore, surface expression of the α4 subunit increased and a larger fraction of the subunit was co-immunoprecipitated with the γ2 subunit in hippocampi of epileptic animals. Together these studies revealed that functional α4βxδ and α5βxγ2 receptors were reduced in the hippocampi of epileptic animals, and that novel α4bxγ2 receptors contributed to the maintenance of tonic inhibition. The presence of α4βxγ2 receptors resulted in low GABA affinity and neurosteroid sensitivity of tonic currents in the DGCs of epileptic animals that could potentially increase seizure vulnerability. These receptors may represent a novel therapeutic target for anticonvulsant drugs without sedative actions.
Introduction
Temporal lobe epilepsy (TLE) is a common form of epilepsy and nearly a third of patients may be refractory to anticonvulsants. An array of pathological changes in neuronal circuits occur in the hippocampal dentate gyrus of experimental animals with TLE including, sprouting of mossy fibers and loss and dysfunction of GABAergic interneurons (Dudek and Sutula, 2007;Sutula and Dudek, 2007;Obenaus et al., 1993;Kobayashi and Buckmaster, 2003;Sun et al., 2007a;Zhang and Buckmaster, 2009). These changes render the hippocampus susceptible to generating and propagating seizures. However, there are endogenous molecules such as neurosteroids that can suppress seizures. Neurosteroids are molecules derived from circulating steroids and synthesized de novo from cholesterol. Neurosteroids modulate GABAA receptors and have an anticonvulsant action (Majewska et al., 1986;Kokate et al., 1994;Reddy et al., 2004;Lawrence et al., 2010).
There is growing evidence that neurosteroid modulation of GABAA receptors on hippocampal dentate granule cells (DGCs) is diminished in TLE (Mtchedlishvili et al., 2001;Peng et al., 2004;Zhang et al., 2007;Sun et al., 2007b). GABAA receptors are pentamers composed of subunits derived from α, β, γ, δ, ε, and π gene families, and the majority of receptors are composed of 2 α, 2 β, and a γ or δ subunit (Sieghart and Sperk, 2002). Receptors containing the δ subunit are particularly sensitive to neurosteroids (Mihalek et al., 1999;Wohlfarth et al., 2002;Belelli et al., 2006). In DGCs, GABAA receptors containing the α4 and δ subunit are targeted to the extrasynaptic membrane, where they contribute to neurosteroid-sensitive leak conductance often referred to as tonic inhibition (Wei et al., 2003;Stell and Mody, 2002;Sun et al., 2004;Mtchedlishvili and Kapur, 2006;Herd et al., 2007). In TLE, there is diminished expression of δ subunit mRNA and polypeptide in DGCs with associated diminished neurosteroid sensitivity of tonic currents (Zhang et al., 2007;Schwarzer et al., 1997a;Nishimura et al., 2005;Peng et al., 2004).
Previous studies investigating the neurosteroid modulation of tonic inhibition have not addressed several issues. Despite loss of neurosteroid sensitivity and reduced expression of the δ subunit, tonic inhibition of DGCs appears to be preserved in animals with TLE however; the identity and properties of these GABAA receptors have not been described. Studies in δ subunit knockout animals demonstrate a residual tonic current suggesting that GABAA receptors containing subunits other than the δ subunit can maintain tonic inhibition (Stell et al., 2003;Wei et al., 2004). Recent studies indicate that GABAA receptors containing the α5 subunit maintain residual tonic currents in DGCs (Glykys et al., 2008), suggesting the possibility that upregulation of α5 subunit-containing receptors can maintain tonic inhibition in DGCs of epileptic animals (Zhan and Nadler, 2009). Alternately, a previous study proposed that tonic inhibition of epileptic DGCs could be maintained by GABAA receptors containing the α4 and γ2 subunits (Zhang et al., 2007). These possibilities have not been tested directly, and assembly of the γ2 subunit with the α4 subunit remains untested.
The current study demonstrates that receptors containing the α4 and γ2 subunit contribute to the maintenance of tonic inhibition of DGCs in epileptic animals. These receptors have a lower affinity for GABA and neurosteroids, which is likely to increase vulnerability to seizures.
Materials and Methods
Induction of TLE
All experimental procedures were performed on adult male Sprague-Dawley rats in accordance with the protocol approved by the University of Virginia Animal Use and Care Committee. TLE was induced in rats using the continuous hippocampal stimulation (CHS) protocol as described previously (Lothman et al., 1989). The rats were anesthetized with ketamine (50 mg/kg) and xylazine (40 mg/kg) and implanted with a pair of bipolar stimulating electrodes in the left posterior ventral hippocampus (AP 3.6, ML 4.0, DV 5.0 from dura; incisor bar at +5.0). After 1week of recovery, the left hippocampus was stimulated with 10s trains of 50 Hz, 1 ms, 400 mA biphasic square wave current pulses delivered every 13 s for 90 min to induce status epilepticus (SE) (Lothman et al., 1990). Approximately 4–6 weeks after stimulation, the rats developed spontaneous limbic seizures with a motor component. For this study, seizures were documented by either continuous EEG recording or by direct observation of the spontaneous seizure (Bertram et al., 1997). The epileptic animals were sacrificed at least 24 hours after the last seizure. All animals were housed individually under conditions of a 12h/12h light/dark cycle and had free access to food and water. Age-matched adult male Sprague-Dawley rats (150–200 g) were used as controls.
Electrophysiology
Animals were anesthetized with isoflurane and decapitated. The brain was dissected free and immersed in cold (2–4°C) ACSF composed of (in mM) 65.5 NaCl, 2 KCl, 5 MgSO4, 1.1 KH2PO4, 25 NaHCO3, 10 dextrose, 113 sucrose, and 1 CaCl2 (osmolarity 300 mOsm) saturated with 95%O2-5%CO2. Brains were mounted on a vibratome stage (Camden Instruments, UK) and 300 µM thick horizontal sections containing the right ventral hippocampus were cut. Slices were maintained in continuously oxygenated ACSF containing (in mM) contains (in mM) 127 NaCl, 2 KCl, 1.5 MgSO4, 25.7 NaHCO3, 10 Dextrose, 1.5 CaCl2 (pH 7.4; 300 mOsm) at 32°C in a holding chamber for 30–45 min and then at room temperature in a recording chamber mounted on the stage of an Olympus BX51 microscope equipped with a 40× water-immersion objective, IR-DIC optics, and video. Patch electrodes (final resistances 4–6 MΩ) were pulled from borosilicate glass (Sutter Instruments, Novato, CA) on a horizontal Flaming-Brown microelectrode puller (model P-97, Sutter Instruments), using a 2-stage pull protocol. Electrode tips were filled with a filtered internal recording solution consisting of (in mM): CsCl 153.3, MgCl2 1.0, N-[2-Hydroxyethyl] piperazine-N -[2-ethansulfonic acid] (HEPES) 10.0, and glycol-bis (α-aminoethyl ether) N,N,N ,N -tetraacetic acid (EGTA) 5.0, pH 7.2 (with CsOH), osmolarity was 285–295 mOsm. The electrode shank contained 3 mM ATP Mg2+ salt. GABAA receptor mediated currents were recorded from DGCs voltage-clamped to −65 mV with an Axopatch 200B amplifier (Molecular Devices, CA) in the presence of 50 µM DL-2-Amino-5-phosphonopentanoic acid (DL-AP5) and 20 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Tocris-Cookson Ellisville, MO). Whole cell capacitance and series resistance were compensated by 80% at a 7 µs lag. Recordings were performed when series resistance after compensation was less than 20 MΩ. Access resistance was frequently monitored with a 10 ms −5 mV test pulse. If the series resistance increased by 25% at any time during the experiment, the recording was terminated. Currents were filtered at 5kHz, digitized at 10 kHz using a Digidata 1440A digitizer, and acquired using Axoscope 10.2 software (Molecular Devices, CA) on an IBM PC-compatible computer hard drive.
Determination of tonic inhibition
To assess the potential alterations in tonic inhibition, we evaluated either the shift in baseline holding current (Ihold) (Mtchedlishvili et al., 2006; Rajasekaran et al., 2009), or changes in its root mean square amplitude of noise (RMS) (Mtchedlishvili et al., 2006) following application of antagonists or agonists. The GABAA receptor mediated component of the holding was confirmed by applying bicuculline and demonstrating a reduction in holding current. To determine the shifts in Ihold (IΔ), the digitized current traces were analyzed with Clampfit 10.2 (Molecular Devices, CA). The mean Ihold for each recording was determined by measuring the mean current (Iavg) sampled every 50 ms at 500 ms intervals. Fifty such data points were collected immediately before drug application and at least 10 min after drug application to allow steady state response after drug equilibration. To minimize the contribution of synaptically present receptors to our measures of Ihold, epochs containing synaptic events that could be visually identified or were larger than the RMS noise were eliminated from the analysis. Epochs having an unstable baseline were also eliminated from the analysis. The drug effects on individual neurons were assessed by comparing the distribution of the mean holding current before and after drug application by means of a Kolmogorov-Smirnov (KS) test (available online at http://www.physics.csbsju.edu/stats/KS-test.n.plot_form.html). The changes in Ihold between the experimental groups were not normalized to cell capacitance since we did not find any difference in whole cell capacitance between control and epileptic DGCs (data not shown). The difference in the mean Ihold after drug application relative to baseline was calculated and IΔ in the two experimental groups were then compared using an unpaired t test.
RMS noise (Irms) was defined by the equation
where Iavg is the average current amplitude, In is the amplitude of an individual point and n is the number of measurements in an epoch. Each epoch was 50 ms in duration and contained 2500 amplitude measurements. The time interval between 2 epochs was 500 ms. Sixty epochs were analyzed for each experimental condition (60 control and 60 after the drug application in each cell). In order to asses the effect of a drug on Irms in individual neurons, the distribution of Irms in epochs before application of the drug (during the baseline period) was compared to that following drug application by means of a KS test. In order to compare data obtained from a group of neurons, Irms values in individual epochs before and after drug application were averaged. Mean Irms values were compared using unpaired t-test.
Since differences in the washout of slices or pathophysiological changes leading to altered GABA synthesis, release, or clearance could potentially alter ambient GABA concentrations and confound experimental determination of changes in tonic currents (Glykys and Mody, 2007), most experiments were performed in the presence of 10 µM of the GABA uptake inhibitor, NO-711 (Sigma, St. Louis, MO), and 3 µM GABA (Sigma, St. Louis, MO). However, in experiments designed to measure RMS noise, the uptake blocker or GABA was not included in the ACSF.
A total of 40 epileptic animals and 30 age-matched controls were used for electrophysiological experiments. The age matched control group included animals that were implanted with electrodes but not stimulated and animals that were stimulated but did not develop acute status epilepticus since the physiological properties tested were similar to those of naïve control animals.
Biotinylation and western blotting
Whole hippocampus was used for all biochemical studies. Since stimulation and recording electrodes were implanted in the left ventral hippocampus, the tissue used for analysis consisted of the right hippocampus. Surface expression of GABAA receptor subunits was studied by biotinylation (Goodkin et al., 2008). The brain slices (300 µm thickness) were prepared as described above and incubated in oxygenated ACSF at 28°C for 1 hr. Surface proteins were biotinylated by incubating the slices in ice-cold ACSF containing 1 mg/ml sulfo-NHS-LC-biotin (Pierce Biotechnology, Rockford, IL) for 30 min at 4°C with gentle shaking. Unbound biotin was removed by washing the slices twice in TBS (25 mM Tris pH 7.4, 137 mM NaCl, 5 mM KCl, 2.3 mM CaCl2, and 0.5 mM MgCl2). Tissue was lysed in ice-cold RIPA lysis buffer containing 100 µM sodium orthovanadate and protease inhibitor cocktail set I (Calbiochem, San Diego, CA), and sonicated using a Branson Sonifier (5 pulses, output control 5, duty cycle 30%). The lysates were cleared by centrifugation at 14,000 × g for 15 min. Biotinylated proteins were purified by incubating lysates corresponding to 500 µg protein with 100 µl neutravidin-agarose beads (Pierce Biotechnology, Rockford, IL) overnight at 4°C, followed by extensive washing of the beads with RIPA-lysis buffer. The pulled-down proteins were eluted in non-reducing sample buffer, denatured at 95°C for 5 min, and separated on 10% SDS-PAGE gels. Expression of the δ and α4 subunits was detected by standard western blotting procedure using rabbit polyclonal anti-δ (1:1000 dilution) and anti-α4 (1:500 dilution) subunit antibodies (kind gift from Dr. Sieghart, Medical University, Vienna, Austria), directed against the N-terminal extracellular epitope of respective protein and which have been previously characterized for their specificity (Sperk et al, 1997). Expression of the δ and α4 subunits was also studied in 30 µg total protein for normalization. Blots were reprobed with the cytoplasmic protein β-actin (1:5000 dilution, Sigma, St. Louis, MO) to confirm specificity of the biotinylation reaction. Signal obtained in the western blots was quantified by scanning densitometry and the ratio of surface/total expression of the δ or α4 subunits was calculated. Total expression of the protein was normalized with β-actin expression. A total of 6 epileptic and age-matched control animals were used for the biochemical studies.
Isolation of microsomes
Whole hippocampus was used to isolate microsomal membranes and tissue from one animal represents single replicate. Hippocampal tissue was dissected out in ice-cold oxygenated ACSF and homogenized in homogenization buffer (0.32M sucrose, 10 mM HEPES-NaOH pH 7.2) containing protease inhibitor cocktail set I (Calbiochem, San Diego, CA) in a glass douncer with 10 strokes. All the steps were carried out at 4°C. Homogenates were first cleared twice at 1000 × g for 10 min and then at 9000 × g for 15 min. The resulting supernatant (S2) was centrifuged at 200,000 × g for 1 h to pellet out total microsomal fraction. The microsomal fractions were suspended in a non-reducing sample buffer, denatured at 95°C for 5 min, and separated on 9% SDS-PAGE gels. Presence of the δ subunit was detected by Western blotting as described above. Expression of the endoplasmic reticulum (ER) marker proteins calnexin and Glucose responsive protein (Grp78/BIP) was also studied by Western blotting. Four pairs of epileptic and control animals were used.
Co-immunoprecipitation
Whole hippocampus was used in immunoprecipitation studies and each animal represents a single replicate. Six pairs of epileptic and control animals were used to study co-immunoprecipitaiton between the α4 or α1 and γ2 subunits. Co-immunoprecipitation of the α4 and γ2 subunit was performed in the whole hippocampus using the ProFound mammalian co-immunoprecipitation kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Anti-GABAA receptor γ2 subunit antibody (50 µg, Alpha Diagnostics, San Antonio, TX, directed against the N-terminal extracellular domain) was used to pull-down the γ2 subunit and associated proteins. The expression of the α4, α1, and γ2 subunits was studied using standard SDS-PAGE and Western blotting procedures. The intensity of the α4 and γ2 signals in pull-down and total protein was determined by densitometric scanning and the pull-down signal was normalized with total expression of the respective subunit. A ratio of normalized α4 to γ2 was obtained and compared between control and epileptic animals. Association of the α4 subunit with the γ2 subunit was expressed as percent fraction of that in naïve animals. As a negative control co-immunoprecipiation between the δ and γ2 subunits which are mutually exclusive in vivo (Arajuo et al, 1998) was also studied.
Results
Neurosteroid modulation of GABA mediated tonic currents is diminished in TLE
We investigated the role of allopregnanolone modulation of tonic currents in epileptic DGCs. Physiological GABAA receptor modulating concentrations (30 and 60 nM) of allopregnanolone were bath-applied in separate experiments to control and epileptic DGCs. Modulation of Ihold was studied with an elevated extracellular GABA concentration obtained by blocking GABA uptake and including 3 µM GABA in the ACSF. Allopregnanolone (30 nM) increased Ihold in control but not epileptic DGCs (Fig 1A). In all control DGCs, the mean Ihold during 50 epochs after drug application was larger than that at baseline (p < 0.05, KS test, n = 8 cells / 4 animals). In contrast, drug application did not change (p > 0.05, KS test) the mean Ihold in 5 out of 7 epileptic DGCs (n = 5 animals). Allopregnanolone caused a smaller change in IΔ in epileptic DGCs (0.10 ± 0.9 pA) than in control DGCs (6.61 ± 2.0 pA; p = 0.01, unpaired t test).
Figure 1.
Modulation of tonic inhibition by allopregnanolone is diminished in epileptic DGCs. A. (a) Representative current traces recorded from DGCs of control and epileptic animals before and after application of 30 nM allopregnanolone. (b) Decimated traces of (a) for better temporal resolution. In this and all subsequent traces, arrowheads represent the beginning of drug application. The straight dotted lines in these and subsequent traces indicate the mean holding current for the respective recordings. (c) Quantitative data of mean shift in holding current (IΔ) in recordings from control and epileptic DGCs following application of 30 nM allopregnanolone. B. (a) Representative current traces recorded from DGCs of control and epileptic animals before and after application of 60 nM allopregnanolone. (b) Decimated traces of (i) for better temporal resolution. (c) Quantification of the mean IΔ in recordings from control and epileptic DGCs following application of 60 nM allopregnanolone. All recordings were performed in the presence of 10 µM NO-711 and 3 µM GABA. *p<0.05, **p<0.01 compared to control, unpaired t test.
A higher concentration of allopregnanolone (60 nM) robustly enhanced Ihold in control DGCs but had minimal effect in epileptic DGCs (Fig 1B). In all control DGCs (n=6 cells / 3 animals) 60 nM allopregnanolone increased Ihold (p < 0.05, KS test), whereas in epileptic DGCs (n = 6 cells / 4 animals), it caused minimal (4 / 6 cells, p < 0.05, KS test) or no change in Ihold (2 / 6 cells, p > 0.05, KS test). The mean population IΔ in epileptic DGCs (3.18 ± 1.6 pA) was significantly less than that in control DGCs (25.60 ± 8.3 pA; p = 0.02, unpaired t-test).
Role of the δ subunit
GABAA receptors containing the δ subunit are particularly sensitive to neurosteroids. Based on our initial findings with allopregnanolone, we tested whether the expression of this subunit was diminished in the hippocampi of epileptic animals by Western blot technique. There expression of the δ subunit was reduced in the membranes prepared from the hippocampi of epileptic animals (Fig. 2A). The expression of β-actin was similar between control and epileptic animals confirming equal protein loading. The δ subunit band was normalized to the β-actin band and the δ subunit protein level in the hippocampi of epileptic animals was 80 ± 11% of that in the hippocampi of controls (n = 7, p < 0.001, t-test). Loss of principal neurons in epileptic animals may have contributed to decreased expression of the δ subunit. In addition, the diminution of total δ subunit expression was modest in comparison to loss of allopregnanolone sensitivity observed in the physiological studies. Furthermore, the δ subunit immunoreactivity in tissue lysates is likely to detect intracellular polypeptides besides that on surface, which are physiologically active.
Figure 2.
Surface expression of the δ subunit was reduced in chronic epileptic animals and more of it was retained in the ER. A. Surface expression of δ subunit was studied in control (lane C) and epileptic (lane E) animals by a biotinylation assay (lanes marked “Surface”). Expression of δ subunit was also studied using total cell lysates for normalization (lanes marked “Total”). The same blots were used for expression of β-actin used as a marker for cytoplasmic proteins. Absence of a β-actin signal in surface samples indicates specificity of the biotinylation assay. B. The total microsomal fraction was isolated from control (lane C) and epileptic (lane E) animals and blotted for expression of δ subunit (lanes marked “Microsomal Fraction”). Expression in total cell lysates was studied for normalization (lanes marked “Total”). A stronger signal in the microsomal fraction from epileptic animals indicates ER retention of δ subunit. Blots were also reprobed with the ER markers calnexin and Grp78/Bip to confirm equal loading of ER membranes.
We therefore tested whether δ subunit surface expression was reduced in epileptic animals. In biotin-labeled hippocampal membrane proteins obtained from TLE animals, the δ protein signal was weaker than that in the membrane proteins from control animals (Fig. 2 A). To quantify surface expression of the δ subunit, expression of the δ subunit was determined by scanning densitometry. Intensity of the surface signal was 19486 ± 2934 units in control rats and 6191 ± 532 units in epileptic rats (n=5) and expressed as a fraction of total δ expression. In controls, the ratio of surface to total expression was 0.34 ± 0.05 (n=5), whereas in epileptic animals it was 0.13 ± 0.01 (n=5). Thus the surface expression of the δ subunit in epileptic animals was 60% less than that in controls (p < 0.01, t-test).
The extent of reduction in the surface fraction of the δ subunit was higher than the decline in the total δ subunit expression, suggesting altered trafficking of the receptor. Defects in trafficking of receptor subunits to the cell surface could lead to their accumulation in the microsomal fraction, which contains the ER, Golgi apparatus, and endosomes. Expression of the δ subunit in the microsomal fraction isolated from hippocampi of control and epileptic animals was compared; and it was more in epileptic animals than that in controls (Fig 2B). Isolation of the microsomal fraction was confirmed by the expression of the ER marker proteins, calnexin and Grp78/BIP, which were similar in the hippocampi of epileptic and control animals indicating equal loading of proteins. The δ subunit in microsomal fraction isolated from control animals was 129214 ± 82768 units (n=4) less than that in epileptic animals (244227 ±175212 units; n=4). The δ subunit expression in microsomal fraction was normalized to total δ protein. This was 65 ± 8% higher in epileptic animals (0.89±0.07, n=4) than in controls (0.54 ± 0.05, n=4, p< 0.05, t-test). The microsomal δ subunit expression was also normalized to the ER marker protein calnexin, which revealed a ratio 0.7 ± 0.2 in control and 1.2 ± 0.3 in epileptic animals, demonstrating a 75 ±27% higher expression in the epileptic animals (n=4, p=0.05). Taken together, these studies demonstrated altered trafficking of the δ subunit in epilepsy.
GABAA receptors containing the α5 subunit were not responsible for modulating tonic currents in epileptic DGCs.
We then compared bicuculline sensitivity of Ihold in control and epileptic DGCs. Bicuculline was bath-applied after 5 minutes of baseline recording, which caused a slow outward current, accompanied by loss of spontaneous inhibitory post synaptic currents. Bicuculline reduced the mean Ihold in each control (n = 9 cells / 5 animals, p < 0.05, KS test) and epileptic DGC tested (n = 6 cells / 5 animals, p < 0.05, KS test, Fig 3a). The mean IΔ in epileptic DGCs (50.86 ± 7.80 pA) was similar to that in control DGCs (46.59 ± 5.1 pA, p=0.33, unpaired t test, Fig 3b). Another competitive antagonist, SR 95531 (gabazine), similarly reduced the mean Ihold in both control and epileptic DGCs (data not shown).
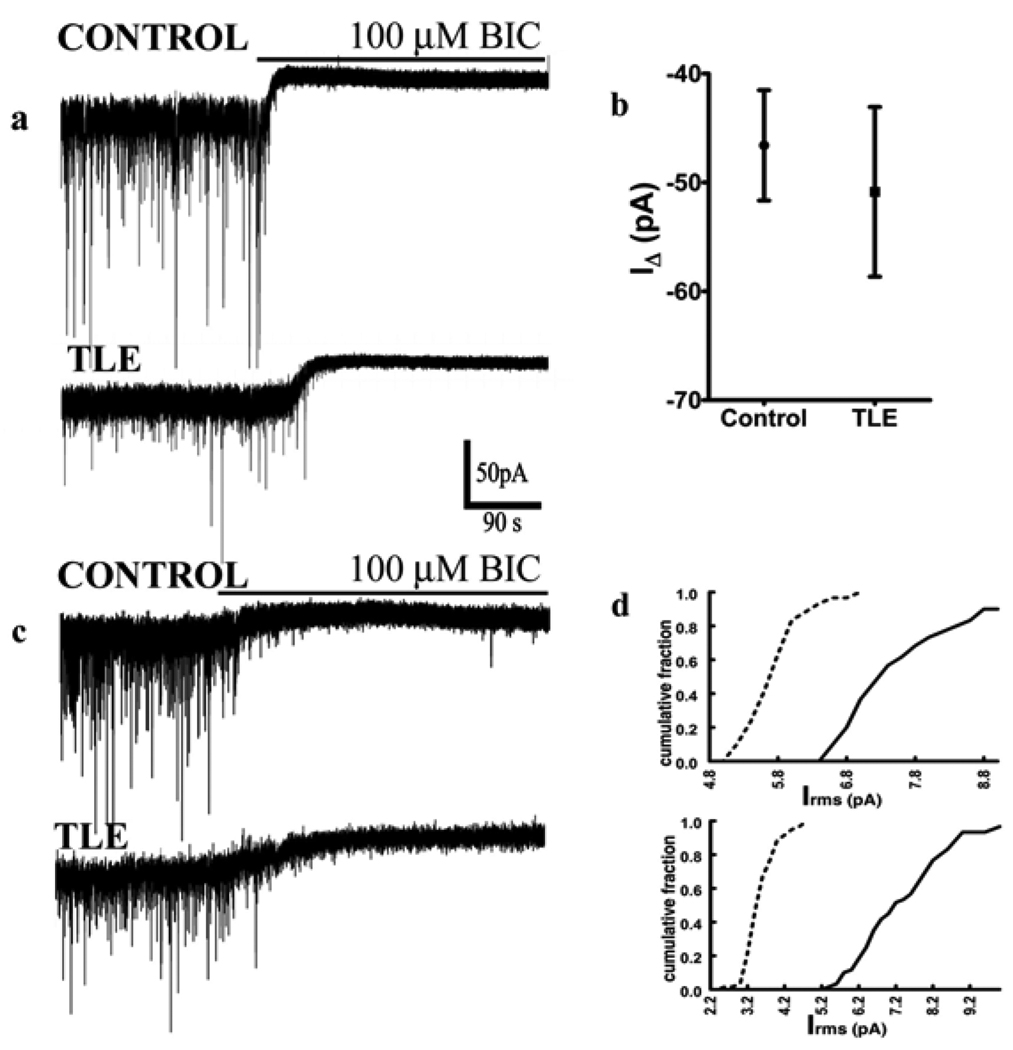
Figure 3.
The magnitude of tonic inhibition recorded in the presence or absence of 10 µM NO-711 and 3 µM GABA in control and epileptic DGCs was similar. (a) Representative current traces recorded from DGCs of control and epileptic animals before and after application of 100 µM bicuculline in slices bathed in ACSF with uptake blocker NO-711 and GABA. Bicuculline application is shown by same bar as in control. (b) Quantification of the mean IΔ in recordings from control and epileptic DGCs following application of bicuculline. (c) Representative current traces recorded from DGCs of control and epileptic animals before and after application of 100 µM bicuculline in slices bathed in ACSF without uptake blocker NO-711 and GABA. Bicuculline application is shown by the same bar as in control. (d) Cumulative frequency plot of Irms distribution before (solid line) and after (dotted line) application of bicuculline in control (upper panel) and epileptic (lower panel) DGC. Note the leftward shift of distribution plot in presence of bicuculline.
To account for potential changes in the basal level of extracellular GABA and uptake mechanisms between control and epileptic DGCs, we compared bicuculline induced shift in Ihold in the absence of exogenous GABA and NO-711. In these conditions also, bicuculline induced a similar shift (p > 0.05) in the Gaussian mean of all-point histogram in both control (214.3 ± 11.3, n = 5 cells / 3 animals) and epileptic DGCs (217.8 ± 30.2 pA, n = 5 cells / 3 animals, Fig 3c). Bicuculline also caused similar reductions in Irms in epileptic DGCs and control DGCs (p > 0.05). In epileptic DGCs, it caused an Irms shift from 4.21 ± 0.21 pA to 2.67 ± 0.07 pA (n = 5, p < 0.0001, KS test) and in control DGCs, it shifted Irms from 7.449 ± 0.08 pA to 6.083 ± 0.03 pA (n = 5, D = 0.69, p < 0.0001, KS test). These findings are consistent with those reported in DGCs in epileptic mice and in CA1 pyramidal neurons of rats with TLE (Zhang et al., 2007;Scimemi et al., 2005).
Although tonic inhibition in hippocampal DGCs is mainly mediated by the α4δ subunit-containing GABAA receptors, it can also be maintained by the α5γ2 subunit-containing GABAA receptors (Caraiscos et al., 2004; Glykys and Mody, 2008). Modulation of tonic currents in epileptic DGCs despite reduced surface expression of the δ subunit suggested that α5γ2 subunit-containing GABAA receptors may contribute to maintenance of tonic currents in these neurons.
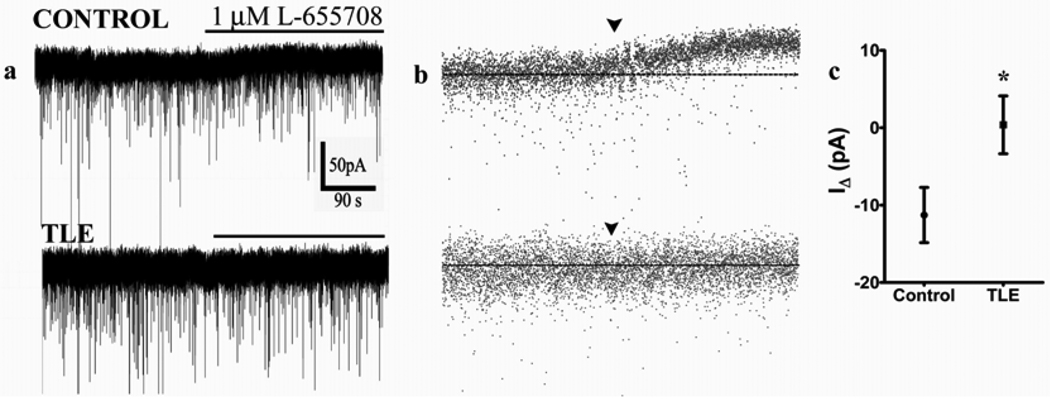
We therefore compared the modulation of Ihold using the α5 subunit-specific benzodiazepine site inverse agonist, L-655,708 (Caraiscos et al., 2004; Scimemi et al., 2005). L-655,708 inhibited the Ihold in each control DGC (n = 6 cells / 5 animals; p < 0.05, KS test) but it did not do so in 4 out of 6 epileptic DGCs (n = 5 animals, p > 0.05, KS test, Fig 4). In data pooled from all cells, L-655,708 caused a larger IΔ in control (11.2 ± 3.6 pA) compared to epileptic DGCs (0.38 ± 3.7 pA; p = 0.04, unpaired t test). Among the 2 epileptic DGCs in which L-655,708 altered the mean Ihold, the response in 1 epileptic DGC (mean IΔ = 14.4 pA) was similar to that observed in control DGC; however the response of the other cell was lower (mean IΔ = 5.6 pA) compared to that of control values. These results suggested diminished contribution of α5 subunit-containing GABAA receptors to the maintenance of tonic currents in the epileptic DGCs.
Figure 4.
Tonic inhibition in epileptic DGCs is not maintained by α5 subunit GABAA receptors. (a) Representative current traces recorded from control and epileptic DGCs before and after application of the selective α5 subunit antagonist L-655708 (1 µM). (b) Decimated traces of (a) for better temporal resolution. (c) Quantification of the mean IΔ in recordings from control and epileptic DGCs following application of L-655708. All recordings were performed in the presence of 10 µM NO-711 and 3 µM GABA. *p<0.05 compared to control, unpaired t test.
Diminished modulation of tonic currents by drugs acting on putative α4δ- and the α5γ2- containing GABAA receptors in epileptic DGCs suggested that in these neurons, tonic currents were maintained by GABAA receptors containing novel subunit composition(s). We have previously demonstrated using electron microscopic studies that the α4 subunit expression increased in epileptic DGCs and with a specific increase in localization at synapses (Sun et al., 2007b). Using similar techniques, it has also been demonstrated in a mouse model of TLE that there is an increased localization of the γ2 subunit of the GABAA receptor in peri- and extra-synaptic sites (Zhang et al., 2007). Thus, GABAA receptors containing the α4γ2 subunits may potentially contribute to maintaining tonic currents in epileptic DGCs (Zhang et al., 2007).
Properties of GABAA receptors contributing to tonic currents in epileptic DGCs
To test whether GABAA receptors in epileptic DGCs have properties similar to those containing α4γ2 subunits, we tested the effect of lanthanum (LaCl3) and Ro15–4513 on Ihold in control and epileptic DGCs. These drugs have distinct effects on GABA currents in recombinant receptors containing the α4δ or α4γ2 subunits (Brown et al., 2002).
Lanthanum (LaCl3) potently inhibits GABAA receptor currents mediated by recombinant receptors containing the α4δ subunit, but has minimal effects on α4γ2 subunit-containing receptors (Brown et al., 2002). LaCl3 significantly decreased the mean Ihold in all control DGCs (n = 10 cells / 5 animals, p < 0.05, KS testFig 5A)). In contrast, in epileptic DGCs, LaCl3 modestly decreased (3 / 7 cells, p < 0.05, KS test) or had no effect (4 / 7 cells, p > 0.05, KS test) on the mean Ihold (n = 5 animals). The mean population IΔ in control DGCs (12.60 ± 2.9 pA) was significantly different from that obtained in epileptic DGCs (0.95 ± 6.3 pA, p = 0.047, unpaired t-test). These data suggest a reduction of GABAA receptors with pharmacological properties of the α4δ subunit-containing assembly, and the presence of GABAA receptors with pharmacological properties similar to those that contain α4γ2 subunits.
Figure 5.
Tonic inhibition in epileptic DGCs is mediated by GABAA receptors assembled with α4γ2 subunits. A. (a) Representative current traces recorded from control and epileptic DGCs before and after application of 100 µM lanthanum (LaCl3). (b) Decimated traces of (a) for better temporal resolution. (c) Quantitative analysis of the mean shift in holding current (IΔ) in recordings from control and epileptic DGCs following application of lanthanum. B. (a) Representative current traces recorded from control and epileptic DGCs before and after application of Ro-154513 (300 nM), a partial inverse agonist against non-α4 subunit-containing GABAA receptors. (b) Decimated traces of (i) for better temporal resolution. (c) Quantification of the mean IΔ in recordings from control and epileptic DGCs following application of Ro-154513. All recordings were performed in the presence of 10 µM NO-711 and 3 µM GABA. *p<0.05 compared to control, unpaired t test.
To further confirm the presence of putative α4γ2 subunit-containing GABAA receptors in epileptic DGCs, we tested the effect of the imidazobenzodiazepine, Ro15–4513 (ethyl-8-azido-6-dihydro-5-methyl-6-oxo-4H–imidazo[1,5α] [1,4]benzodiazepine-3-carboxylate). Ro15–4513 binds to GABAA receptors containing α4γ2 subunit combinations, but not to the α4δ–containing receptors (Scholze et al., 1996). Application of Ro15–4513 (300 nM) did not significantly change mean Ihold in control DGCs (n = 6 cells / 3 animals, Fig 5B), but in 9 out of 10 epileptic DGCs (n = 8 animals, p < 0.05, KS test) it reduced the mean Ihold. The IΔ in control DGCs much less than in epileptic DGCs (0.95 ± 6.3 pA vs 20.26 ± 5.9 pA, p < 0.05, unpaired t-test). Together, these data indicate that GABAA receptors with pharmacological properties similar to that of recombinant receptors containing the α4γ2 subunits contribute to maintenance of tonic currents in epileptic DGCs.
GABA affinity of receptors mediating tonic currents
Studies of recombinant GABAA receptors containing α4γ2 subunits suggest that these receptors have lower affinity for GABA than the α4δ subunit-containing receptors. We therefore probed the availability of high affinity GABAA receptors on DGCs by directly applying a low concentration (1 µM) GABA and measuring Ihold and Irms before and after drug application.
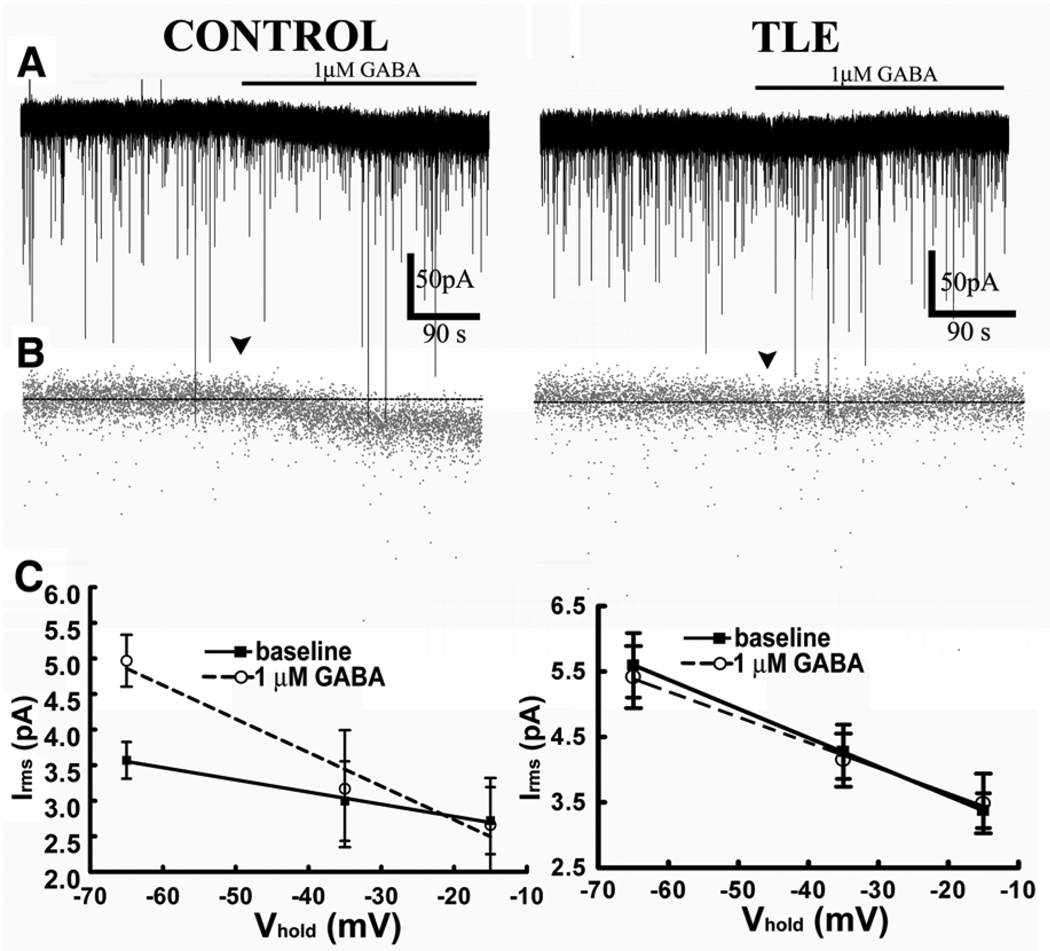
Application of 1 µM GABA revealed loss of high affinity GABAA receptors on epileptic DGCs. In epileptic DGCs, the mean Ihold was not altered by 1 µM GABA (Fig 6A, B); it was 68.3 ± 0.14 pA at baseline, and 67.3 ± 0.13 pA after application of 1 µM GABA (n = 6 cells, p > 0.05, KS test). In contrast, in control DGCs, GABA increased the mean Ihold from 48.3 ± 0.14 pA to 53.9 ± 0.18 pA (n = 6 cells, p < 0.05, KS test). These findings suggested that 1 µM GABA increased tonic GABAergic current in control but not in epileptic DGCs.
Figure 6.
Low concentration of GABA (1 µM) enhanced tonic inhibition in control but not epileptic DGCs. A. Representative current traces recorded from control and epileptic DGCs (TLE) before and after application of 1 µM GABA in the absence of an uptake blocker or prior introduction of exogenous GABA. B. Decimated traces of (A) for better temporal resolution. C. Slope conductance of the RMS noise measured at various holding potentials before and after application of 1 µM GABA in control and epileptic DGCs. The solid line represents the regression line through observations made during baseline in control DGCs, and the dashed line represents observations made after application of GABA.
To further confirm the absence of high affinity receptors in epileptic DGCs, we measured the changes in the slope conductance of GABA-activated channels. DGCs were voltage clamped to −65, −35, and −15 mV, and the mean RMS noise was determined before and after bath application of 1 µM GABA. We found differences in the absolute levels of Irms as well as changes in the slope conductance between recordings obtained from control and epileptic DGCs (Fig 6C). Slope conductance was calculated from a linear regression of mean Irms against the holding potential. In epileptic DGCs (n = 5), 1 µM GABA did not change the slope conductance or the elevations of line intercepts (Fig 6C). By contrast, in control DGCs (n = 7), application of 1 µM GABA changed the slope conductance, as well as the elevations of the line intercepts (Fig 6C).
The changes in the absolute baseline Irms between control and epileptic DGCs suggested potential alterations in uptake mechanisms that may result in elevated extracellular GABA levels in epileptic DGCs. Elevated extracellular GABA in turn could blunt the effects of low concentration of GABA in epileptic DGCs. In order to address this concern, we performed experiments with penicillin, the noncompetitive (Macdonald and Barker, 1977), open channel (Twyman and Macdonald, 1992) GABAA receptor blocker. We have previously shown that penicillin can detect tonically open GABAA receptors (Mtchedlishvili et al., 2006). If higher baseline Irms in epileptic DGCs were due to more open GABAA receptors than in control DGCs, they would be detected by penicillin. In control DGCs (n = 6 cells / 3 animals, Fig. 7A), 300 µM penicillin inhibited Irms in each cell tested (KS test). The mean Irms was 4.845 ± 0.7 pA before and 3.899 ± 0.5 pA after application of 300 µM penicillin (p < 0.05, Fig 7C). In contrast, penicillin application did not alter the mean Irms in any epileptic DGCs (n = 5 cells / 3 animals; p > 0.05, KS test; Fig 7B). The mean Irms was 3.680 ± 0.02 pA before and 3.805 ± 0.02 pA after application of penicillin (Fig. 7D) suggesting loss of high affinity GABAA receptors on epileptic DGCs. Together, these data confirmed the loss of high affinity GABAA receptors on epileptic DGCs.
Figure 7.
Open channel blocker penicillin inhibits persistently open GABAA receptors in control DGCs but not in epileptic DGCs. In all traces Irms is shown by arrows between solid and dashed lines. A. Representative current trace recorded from control DGC before (baseline upper trace) and after (lower trace) application of 300 µM penicillin. B. Representative current traces recorded from an epileptic DGC, before (baseline top) and after (bottom) application of penicillin. C. Cumulative frequency plot of Irms during 60 30 sec epochs during before (solid line) and after (dotted line) application of penicillin from a control DGC. Note the left shift of the distribution. D. Cumulative frequency plot of Irms distribution before (solid line) and after (dotted line) application of penicillin in epileptic DGC. Note the minimal shift compared to that of control DGC.
Biochemical evidence for the α4γ2 subunit-containing receptors
The pharmacological studies indicated that GABAA receptors containing the α4 subunit contributed to maintenance of tonic currents in epileptic DGCs. The α4 subunit commonly associates with the δ subunit and diminished expression of the δ subunit in epileptic animals raised the possibility that the surface expression of the α4 subunit would also be diminished. We therefore studied the surface expression of the α4 subunit in the hippocampi of epileptic animals. Interestingly, in the epileptic animals, surface expression of the α4 subunit was higher than that of controls (Fig 8A). The intensity of surface signal for the α4 subunit in control animals was 29182 ± 11741 and that in epileptic animals was 39068 ± 15745 (n=6). The ratio of surface to total expression of the α4 subunit was 0.72 ± 0.13 (n=6) in control animals, and in epileptic animals, it was 1.13 ± 0.36 (n=6, an increase of 42 ± 12% p<0.05, t-test). Thus, surface expression of the α4 subunit was increased, whereas that of the δ subunit was diminished in epileptic animals. This finding suggested that in the hippocampi of epileptic animals, the α4 subunit may potentially assemble with the γ2 subunit.
Figure 8.
Surface expression of the α4 subunit increased in epileptic animals and its association with the γ2 subunit was higher. A. Surface expression of the α4 subunit was studied from surface proteins isolated from naïve (lane C) and epileptic (lane E) animals using a biotinylation assay (panel “Surface”). Total expression of the α4 subunit was also studied for normalization (panel “Total”). B. Association between the α4 and γ2 subunits was studied in control and epileptic animals. Using an anti-γ2 antibody, the γ2 subunit and associated proteins were separated from hippocampi of control (lane C) and epileptic (lane E) animals. Expression of the γ2, α4, and α1 subunits was studied by Western blotting. Expression of these subunits was also studied from total proteins for normalization (panel total). To confirm specific pull-down of the γ2 and associated proteins using anti-γ2 IgG, normal rabbit IgG was also used to pull-down proteins (lane IgG).
To confirm presence of GABAA receptors containing the α4γ2 subunits in epileptic animals, we performed co-immunoprecipitation assays. The amount of α4 subunit associated with the γ2 subunit was compared in hippocampi obtained from naïve and epileptic animals. In control animals, a weak signal for the α4 subunit was observed in the γ2 pull-down samples suggesting minor association of the α4 and γ2 subunits, a finding consistent with previous observations (Bencsits et al., 1999;Sur et al., 1999). In contrast, the signal for α4 subunit in proteins pulled-down along with the γ2 subunit from epileptic animals was stronger (Fig 8B). The amount of α1 subunit coimmunoprecipitating with the γ2 was similar in control and epileptic animals. Normal rabbit IgG failed to pull down proteins from tissue lysates, suggesting specific pull-down of the γ2 subunit and associated proteins with the anti-γ2 antibody used. Furthermore, association between the δ and the γ2 subunit (that are mutually exclusive in vivo) was also tested in pull-down samples to confirm that interaction between α4 and γ2 subunits represented an in situ interaction and not a processing artifact. In the 4 pairs of epileptic and control animals studied, expression of the δ subunit was not observed in proteins precipitated using anti γ2 subunit antibody. A representative Western blot shows absence of the δ subunit signal in immunoprecipitated samples, whereas expression of the δ subunit was observed in total proteins, lower in epileptic animals than in naïve animals as described above (Fig 8B). The amount of α4 and γ2 subunit expression was quantified using scanning densitometry in the pull-down and total protein and expressed as a ratio of the α4 subunit associated with the γ2 subunit. The ratio of the α4-γ2 co-precipitation was 0.55 ± 0.02 in the control animals and 1.12 ± 0.24 in epileptic animals. Compared to naïve animals, the α4 and γ2 co-precipitation in epileptic animals was 199 ± 33% (n=6, p<0.05, t-test) more, demonstrating a 2 fold increased association between the α4 and γ2 subunits.
Discussion
The major findings of this study were: 1) neurosteroid sensitivity of GABAA receptors mediating tonic currents was diminished in epileptic DGCs; 2) surface expression of the δ subunit of GABAA receptors was diminished and there was greater intracellular retention of the subunit; 3) tonic current was maintained in DGCs of epileptic animals but not mediated by high affinity GABAA receptors; 4) pharmacological studies demonstrated that tonic currents recorded from epileptic DGCs was not mediated by the α5γ2 subunit-containing receptors; 5) pharmacological properties of GABAA receptors contributing to tonic currents in epileptic DGCs were similar to those of α4γ2 subunit-containing GABAA receptors; and 6) surface expression of the α4 subunit increased and a larger amount of the subunit co-immunoprecipitated with the γ2 subunit in hippocampi of epileptic animals. Taken together, these findings suggest that α4γ2 subunit-containing receptors contribute to maintenance of tonic currents in the DGCs of epileptic animals. However, because of their lower affinity for GABA and neurosteroids, the presence of these receptors may contribute to seizure vulnerability.
A combination of pharmacological and biochemical studies suggested that α4γ2 subunit containing GABAA receptors contribute to tonic inhibition in epileptic DGCs. Allopregnanolone and lanthanum sensitivity of tonic currents was diminished in epileptic DGCs. Recombinant GABAA receptors composed of the α4δ subunits are blocked by lanthanum and enhanced by neurosteroids, whereas those composed of α4γ2 subunits are less sensitive to these drugs (Brown et al., 2002). Ro15–4513, which binds and modulates the αβγ2 and αβγ3 subunit-containing receptors, but not to receptors composed of the αβ, αβδ, or αβγ1 subunits (Scholze et al., 1996;Ebert et al., 1996), differentially modulated tonic currents recorded from epileptic and control DGCs.
Biochemical studies revealed that the surface expression of the α4 subunit was increased while that of its partnering δ subunit was diminished. Although, our studies were performed on whole hippocampus, the results of our study are likely to be representative of changes in the granule cell region because (1) the majority of the α4 subunit expression in the hippocampus is found in the granule layer (Sperk et al, 1997), and (2) in the epileptic animals the α4 expression in the granule cells increased whereas that in the CA1 region decreased (Tsunashima et al, 1997; Schwarzer et al, 1997b; Laurie et al, 2003). Previous electron microscopic studies from our laboratory (Sun et al, 2007) and others (Zhang et al., 2007) were suggestive of a co-assembly of the α4 and γ2 subunit. The results of the present study confirm the presence of functional α4γ2 subunit containing GABAA receptors in hippocampi of epileptic animals. A previous study reported co-assembly of the α4 and γ2 subunits as early as 24 hr after lithium-pilocarpine-induced status epilepticus (Lund et al., 2008). However, the study did not address whether the receptors were functional or if they persisted in chronically epileptic animals. Co-assembly of the α4 and γ2 subunits and increased binding of Ro15–4513 has also been observed on the removal of the δ subunit in knock out animals (Korpi et al, 2002) as well as in other conditions associated with increased seizure susceptibility such as ethanol intoxication and progesterone withdrawal (Kokka et al, 1993; Liang et al, 2006; Smith et al, 1998).
Receptors containing the α4γ2 subunit have not been reported to mediate tonic inhibition of neurons in the past. In hippocampal DGCs, the majority of tonic inhibition is maintained by receptors containing the α4δ and α5γ2 subunits. In cultured neurons, receptors composed of other subunit combinations, such as the αβ dimers and ε subunit containing GABAA receptors have been reported to contribute to tonic inhibition (Mortensen and Smart, 2006). We have previously shown increased localization of the α4 subunit at synaptic and perisynaptic locations in epileptic DGCs and evidence for functional α4γ2 subunit containing synaptic GABAA receptors (Sun et al, 2007b). These receptors may have likely contributed to the overall measures of Ihold determined in the present study.
A recent report (Zhan and Nadler, 2009) suggested that tonic inhibition of epileptic DGCs was mediated by enhanced expression of α5γ2 subunit-containing receptors, which is in contrast to our finding of a diminished component of α5γ2 subunit-containing receptors. The discrepancy between our results and those of Zhan and Nadler could result from different epilepsy models used or due to differences in the methods used for measuring tonic inhibition.
The question of whether tonic inhibition of DGCs is preserved or compromised in epileptic animals largely depends on the GABA concentration in the extrasynaptic space. In contrast to competitive antagonists, Penicillin G is a non-competitive antagonist of the GABAA receptor (Macdonald and Barker, 1977) which inhibits GABAA receptors by blocking the chloride channel in all open states (Twyman and Macdonald, 1992). Since tonic inhibition is mediated by persistently open channels, an open channel blocker is likely to preferentially block GABAA receptors that mediate tonic inhibition. A previous study demonstrated that penicillin preferentially blocked persistently open GABAA receptors whereas transiently activated synaptic currents were partially inhibited by penicillin (Mtchedlishvili and Kapur, 2006). The current study demonstrated a loss of penicillin-sensitive persistently open conductance in epileptic DGCs. It therefore appears that in epileptic DGCs, tonic inhibition is preserved under conditions where low affinity GABAA receptors could be activated by elevating extracellular GABA concentration by applying 3 µM GABA with concomitant blockade of GABA transporters. Further, tonic inhibition in epileptic DGCs may not be mediated by high affinity, slowly desensitizing GABAA receptors that can be activated by a low (1 µM) concentration of GABA in control DGCs. In these experiments we found that the baseline Irms in control DGCs were lower than in epileptic DGCs. These differences may be due to differences in the absolute predrug baseline noise levels during recordings or due to altered physiology such that enhanced GABA rendered the receptors insensitive to 1 µM GABA.
Although spillover from synapses may be a source of GABA in the extracellular space, the concentration of extracellular GABA is largely regulated by the equilibrium potential of GABA transporters such as GAT-1(Hamann et al., 2002;Wu et al., 2007;Wu et al., 2006). The intra- and extracellular concentration of GABA, Na+, and Cl−, and the membrane potential determine the equilibrium potential of GAT-1. Under physiological conditions, it is estimated that GAT −1 maintains the extracellular concentration of GABA in the 0.1 – 0.6 µM range, which is supported by direct measurements of extracellular GABA (Glykys and Mody, 2007). Increasing intracellular GABA, depolarization of neurons, and intracellular accumulation of Na+ are predicted to elevate extracellular GABA. It is currently unclear whether conditions in epileptic DGCs are sufficient to elevate extracellular GABA above 1 µM, which is necessary to activate low affinity receptors mediating tonic inhibition in epileptic DGCs. A previous study in the pilocarpine model of epilepsy found that the GAT-1 transporter-mediated GABA uptake or clearance remained unaffected in both DGCs and CA1 neurons (Frahm et al., 2003). Further, a recent study revealed that in patients with intractable TLE localized to the hippocampus, the levels of extracellular GABA seldom exceeded 1 µM (Pan et al., 2008). Thus it is possible that the extracellular concentration of GABA in epileptic dentate gyrus may be insufficient to activate α4γ2 subunit-containing GABAA receptors. Further studies with realistic estimates of extracellular GABA concentration are necessary to confirm whether tonic inhibition of DGCs is preserved or compromised in epileptic animals.
Neurosteroid modulation of tonic inhibition in epileptic DGCs was diminished. Neurosteroids are metabolic products derived from the circulating steroid hormones, progesterone, testosterone, and cortisol (Compagnone and Mellon, 2000). Neurosteroids exert an anticonvulsant action, largely by potentiation of GABAA receptor currents (Kokate et al., 1996; Lambert et al., 1996). The current study demonstrates that diminished neurosteroid modulation of tonic inhibition in epileptic DGCs is due in part to the loss of extrasynaptic δ subunit-containing receptors. We have also previously shown that while the response to the lower concentrations of allopregnanolone is diminished in epileptic DGCs, a higher concentration of allopregnanolone (100 nM) that has direct actions on the GABAA receptor (Callachan et al., 1987; Shu et al., 2004) is similarly effective in epileptic DGCs (Mtchedlishvili et al., 2001; Sun et al., 2007). Reduced neurosteroid modulation is likely to diminish the gating function of DGCs and therefore increase the susceptibility to seizures. In addition, neurosteroid levels in the hippocampus fluctuate and allow neurons to respond to a changing hormonal milieu. A recent study demonstrated that neurosteroids controlled seizures in epileptic animals (Lawrence et al, 2010). Preventing metabolism of progesterone to allopregnanolone using finasteride markedly enhanced the frequency of spontaneous seizures in female epileptic rats that had either basal or artificially elevated progesterone levels suggesting that diminished neurosteroid sensitivity enhanced seizure susceptibility.
Reduced affinity for GABA and neurosteroids in receptors mediating tonic inhibition of DGCs likely contribute to seizure susceptibility of the hippocampus. Persistently open GABAA receptors contribute significantly to inhibition of DGCs, and absence of this conductance is likely to contribute to the disinhibition of these neurons. In addition, because the α4γ2 subunit-containing receptors desensitize faster and extensively compared to even those receptors containing the α1γ2 subunits during prolonged exposure to GABA (Lagrange et al., 2007), it is likely that the contribution of such receptors to maintenance of tonic inhibition is limited. It may also be likely that receptors composed of other subunits such as αβ that are found in hippocampal pyramidal neurons (Mortensen and Smart, 2006) or those containing the neurosteroid insensitive ε subunit (Belelli et al., 2002) could participate in the tonic inhibition of granule cells and compensate for the loss of the δ subunit containing receptors in the epileptic DGCs. Loss of neurosteroid modulation is likely to further diminish inhibition of DGCs. Other studies suggest that loss of specific subsets of GABAergic interneurons can modestly reduce inhibition of DGCs (Sloviter, 1987;Obenaus et al., 1993;Kobayashi and Buckmaster, 2003;Sun et al., 2007). A combination of reduced tonic inhibition, reduced neurosteroid sensitivity of tonic inhibition, and diminished synaptic inhibition is likely to diminish the gating function of DGCs.
The α4bγ2 receptors represent unique therapeutic target for antiepileptic drugs. The pharmacological properties of α4βxγ2 receptors are distinct from the α1βxγ2 receptors (Wafford et al, 1996; Sieghart, 2006). Although these receptors are mostly benzodiazepine insensitive, selective benzodiazepines such as flumazenil and Ro15–4530 act as partial agonists. Hence a benzodiazepine with selective action against the α4γ2 subunit-containing receptors may have minimal sedative effects but powerful anticonvulsant properties and can be used as therapeutic tool.
Research Highlight
-
➢
GABAA receptor-mediated tonic currents recorded from hippocampal granule cells are enhanced by neurosteroids in health.
-
➢
Neurosteroid sensitivity of tonic currents recorded from granule cells of experimental animals with temporal lobe epilepsy is diminished.
-
➢
Diminished surface expression of the δ subunit-containing GABAA receptors and the assembly of novel α4γ2 subunit-containing receptors could explain diminished neurosteroid sensitivity observed in epilepsy.
Acknowledgement
This study was supported by NIH grants RO1 NS 040337 and RO1 NS RO1 NS044370
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Araujo F, Ruano D, Vitorica J. Absence of association between [delta] and [gamma]2 subunits in native GABAA receptors from rat brain. Eur J of Pharmacol. 1998;347:347–353. doi: 10.1016/s0014-2999(98)00122-8. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native GABAA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Williamson JM, Cornett JF, Spradlin S, Chen ZF. Design and construction of a long-term continuous video-EEG monitoring unit for simultaneous recording of multiple small animals. Brain Res Brain Research Protocols. 1997;2:85–97. doi: 10.1016/s1385-299x(97)00033-0. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. 755–773. [DOI] [PubMed] [Google Scholar]
- Ebert V, Scholze P, Sieghart W. Extensive heterogeneity of recombinant GABAA receptors expressed in α4β3γ2-transfected human embryonic kidney 293 cells. Neuropharmacology. 1996;35:1323–1330. doi: 10.1016/s0028-3908(96)00062-7. [DOI] [PubMed] [Google Scholar]
- Frahm C, Stief F, Zuschratter W, Draguhn A. Unaltered control of extracellular GABA-concentration through GAT-1 in the hippocampus of rats after pilocarpine-induced status epilepticus. Epilepsy Res. 2003;52:243–252. doi: 10.1016/s0920-1211(02)00233-4. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-Specific Trafficking of GABAA receptors during Status Epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Herd MB, Belelli D, Lambert JJ. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with GABA-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Korpi ER, Mihalek RM, Sinkkonen ST, Hauer B, Hevers W, Homanics GE, Sieghart W, Luddens H. Altered receptor subtypes in the forebrain of GABAA receptor δ subunit deficient mice: recruitment of the γ2 subunits. Neuroscience. 2002;109:733–743. doi: 10.1016/s0306-4522(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J. Physiol. 2007;578:655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Callachan H, Peters JA. Neurosteroid modulation of native and recombinant GABAA receptors. Cell Mol Neurobiol. 1996;16:155–174. doi: 10.1007/BF02088174. [DOI] [PubMed] [Google Scholar]
- Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol. 2010;67:689–693. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Pitkanen A, Nissinen J, Soini SL, Korpi ER, Holopainen IE. Selective changes in GABAA receptor subunits in the hippocampus in spontaneously seizing rats with chronic temporal lobe epilepsy. Neuroscience Letters. 2003;349:58–62. doi: 10.1016/s0304-3940(03)00735-3. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by 'continuous' hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Kapur J, Stringer JL. Recurrent spontaneous hippocampal seizures in the rat as a chronic sequela to limbic status epilepticus. Epilepsy Res. 1990;6:110–118. doi: 10.1016/0920-1211(90)90085-a. [DOI] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Barker JL. Pentylenetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurones. Nature (Lond) 1977;5613:720–721. doi: 10.1038/267720a0. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in GABAA receptor δ subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J. Diminished allopregnanolone enhancement of GABAA receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol. 2001;537:453–465. doi: 10.1111/j.1469-7793.2001.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Schwarzer C, Gasser E, Kato N, Vezzani A, Sperk G. Altered expression of GABAA and GABAB receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neuroscience. 2005;134:691–704. doi: 10.1016/j.neuroscience.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Cavus I, Kim J, Hetherington HP, Spencer DD. Hippocampal extracellular GABA correlates with metabolism in human epilepsy. Metab Brain Dis. 2008;23:457–468. doi: 10.1007/s11011-008-9106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptor: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rajasekaran K, Sun C, Bertram EH. Altered pharmacology and GABAA receptor subunit expression in dorsal midline thalamic neurons in limbic epilepsy. Neurobiol Dis. 2009;33:119–132. doi: 10.1016/j.nbd.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O'Malley BW, Rogawski MA. Anticonvulsant Activity of Progesterone and Neurosteroids in Progesterone Receptor Knockout Mice. J Pharmacol Exptl Therap. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Scholze P, Ebert V, Sieghart W. Affinity of various ligands for GABAA receptors containing α4β3γ2, α4γ2, or α1β3γ2 subunits. Eur J Pharmacol. 1996;304:155–162. doi: 10.1016/0014-2999(96)00088-x. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Marksteiner J, Kroesen S, Kohl C, Sperk G, Winkler H. Secretoneurin: a market in rat hippocampal pathways. Journal of Comparative Neurology. 1997;377:29–40. [PubMed] [Google Scholar]
- Schwarzer C, Tsunashima K, Wanzenbock C, Fuchs K, Sieghart W, Sperk G. GABAA receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience. 1997;80:1001–1017. doi: 10.1016/s0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007a;500:876–893. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of epilepsy. J Neurosci. 2007b;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of α1, α4, γ2, and δ subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the GABAA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. 541–563. [DOI] [PubMed] [Google Scholar]
- Tsunashima K, Schwarzer C, Kirchmair E, Sieghart W, Sperk G. GABAA receptor subunits in the rat hippocampus III: altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience. 1997;80:1019–1032. doi: 10.1016/s0306-4522(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic-properties of mouse spinal-cord neurons in culture. J Physiol (Lond) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB. The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. J Neurophysiol. 2006;96:2425–2436. doi: 10.1152/jn.00545.2006. [DOI] [PubMed] [Google Scholar]
- Zhan RZ, Nadler JV. Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol. 2009;102:670–681. doi: 10.1152/jn.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered Localization of GABAA receptor Subunits on Dentate Granule Cell Dendrites Influences Tonic and Phasic Inhibition in a Mouse Model of Epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Buckmaster PS. Dysfunction of the dentate basket cell circuit in a rat model of temporal lobe epilepsy. J Neurosci. 2009;29:7846–7856. doi: 10.1523/JNEUROSCI.6199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]