Abstract
Dopamine deficiency associated with Parkinson’s disease (PD) results in numerous changes in striatal transmitter function and neuron morphology. Specifically, there is marked atrophy of dendrites and dendritic spines on striatal medium spiny neurons (MSN), primary targets of inputs from nigral dopamine and cortical glutamate neurons, in advanced PD and rodent models of severe dopamine depletion. Dendritic spine loss occurs via dysregulation of intraspine Cav1.3 L-type Ca2+ channels and can be prevented, in animal models, by administration of the calcium channel antagonist, nimodipine. The impact of MSN dendritic spine loss in the parkinsonian striatum on dopamine neuron graft therapy remains unexamined. Using unilaterally parkinsonian Sprague Dawley rats, we tested the hypothesis that MSN dendritic spine preservation through administration of nimodipine would result in improved therapeutic benefit and diminished graft-induced behavioral abnormalities in rats grafted with embryonic ventral midbrain cells. Analysis of rotational asymmetry and spontaneous forelimb use in the cylinder task found no significant effect of dendritic spine preservation in grafted rats. However, analyses of vibrissae-induced forelimb use, levodopa-induced dyskinesias, and graft-induced dyskinesias showed significant improvement in rats with dopamine grafts associated with preserved striatal dendritic spine density. Nimodipine treatment in this model did not impact dopamine graft survival but allowed for increased graft reinnervation of striatum. Taken together, these results demonstrate that even with grafting suboptimal numbers of cells, maintaining normal spine density on target MSNs results in overall superior behavioral efficacy of dopamine grafts.
Keywords: dyskinesia, Parkinson’s disease, nimodipine, transplantation, medium spiny neuron
Introduction
Embryonic dopamine neuron transplantation has provided symptomatic benefit for some individuals with Parkinson’s disease (PD). However, the efficacy of grafting is variable and less than would be predicted from the degree of dopamine replacement provided in many individuals (e.g. Freed et al., 2001; Olanow et al., 2003). While results from recent grafting trials for PD are disappointing, the rationale of replacing cells lost in PD remains sound and interest in this approach is re-gaining popularity. Thus, the question remains why this potentially viable therapeutic approach has not yet fully succeeded.
One factor thought to underlie this lack of success is pathology within the parkinsonian striatum, the region of graft placement. It has been shown in PD patients and animal models of the disease, that dopamine depletion is associated with a host of plastic changes in the striatum (e.g.: Brown and Gerfen, 2006, Deutch 2006; Collier et al., 2007; Meurers et al., 2009). One such change involves the primary synaptic target of afferent nigral dopaminergic neurons and descending cortical glutamate neurons, the medium spiny neuron (MSN). Normal MSNs have an abundance of dendritic spines, critical sites for synaptic integration of striatal dopamine and glutamate. In advanced PD there is a marked atrophy of dendrites and spines on these neurons (McNeill et al., 1988; Zaja-Milatovic et al., 2005; Stephens et al., 2005). Similar pathology is observed in mice and rats with severe dopamine depletion (Day et al., 2006; Neely et al., 2007). While the impact of this altered morphology on dopamine cell replacement is unclear, it would be anticipated that an absence of these critical input sites would make it difficult for grafted dopamine neurons to re-establish normal connections needed for therapeutic benefit.
It is also possible that the structural abnormalities of MSNs in the dopamine-depleted striatum could result in inappropriate graft-host contacts leading to abnormal behaviors (e.g.: graft-induced dyskinesias; GIDs). While little is know about the etiology of GIDs, we recently reported (Soderstrom et al., 2008) that in a rat model of PD aberrant synaptic features following dopamine cell grafting are associated with the expression of graft-mediated motor dysfunction. These data support the idea that abnormal synaptic reorganization within the grafted striatum contributes to the evolution of aberrant motor behaviors, however, the biological contributor(s) to aberrant graft-host connectivity remains uncertain.
The current study was designed to test the hypothesis that preventing MSN dendritic spine loss would allow for more appropriate integration of grafted neurons into the host striatum, thus resulting in increased behavioral efficacy and preventing the development of abnormal motor behaviors.
Materials and Methods
Animals and Groups
Adult male Sprague Dawley rats (225–250g at the start of the study; Harlan, Indianapolis, IN) were kept on a reverse 12-hour light-dark cycle with ad libitum food and water. The rats were housed and treated according to the rules and regulations of NIH and Institutional Guidelines on the Care and Use of Animals. These studies were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati, where they were conducted. To analyze the effects of dendritic spine preservation on dopamine grafting efficacy, parkinsonian rats were placed into 1 of 4 groups of differential MSN spine density: 1) Sham-grafted rats with vehicle pellets (severe spine atrophy, n=6), 2) Sham-grafted rats with nimodipine pellets (normal spine density, n=6) 3) Dopamine-grafted rats with vehicle pellets (severe spine atrophy, n=8), and 4) Dopamine-grafted rats with nimodipine pellets (normal spine density, n=6). An additional set of 6–8 rats was run per group and stained with a Golgi technique to confirm treatment-effect on spine density. The groups and timeline of surgeries and treatments are shown in Figure 1.
Figure 1. Experimental groups and timeline.
Rats were placed into treatment groups with differential MSN spine density: a sham-grafted group receiving cell-free media plus vehicle pellets (n=6), a sham-grafted group receiving cell-free media plus nimodipine pellets (n=6), a dopamine-grafted group receiving embryonic ventral mesencephalic cell grafts plus vehicle pellets (n=8), and a dopamine-grafted group receiving embryonic ventral mesencephalic cell grafts plus nimodipine pellets (n=6). All rats received unilateral nigrostriatal 6-OHDA lesions. All pellets were delivered 24 hours following lesion and were replaced every 20 days till the conclusion of the study. Rats received dopamine or sham grafts 4 weeks following lesion. Behavioral analyses were conducted 4 weeks following grafting and continued till the completion of the study. DA=dopamine.
Nigrostriatal Lesions
Rats were anesthetized with a chloropent solution (3 ml/kg; chloral hydrate 42.5 mg/ml, sodium pentobarbital, 8.9 mg/ml) and secured in a stereotaxic apparatus. Two μl of 6-hydroxydopamine (6-OHDA; 6μg free base/3μl 0.02% ascorbate made in sterile saline) was injected at a flow rate of 0.5μl/min using a Hamilton 26 gauge needle to 2 sites unilaterally (to the medial forebrain bundle; A/P=3.6mm, M/L=2.0mm from bregma, D/V=8.3mm from skull, and substantia nigra; A/P=4.8mm, M/L=1.7mm from bregma, D/V=8.0mm from skull).
Nimodipine Continuous Release Pellet Implantation
In a subset of dopamine-grafted rats, nimodipine treatment was used to prevent dendritic spine loss as described previously (Day et al., 2006). In these rats, anesthetized with isoflurane (administered at 3.5% with a an oxygen flow rate of 1.5L/min, total exposure time of approximately 5 minutes), 21-day continuous-release nimodipine pellets (0.8mg/kg/day; Innovative Research of America, Sarasota, FL) were implanted subcutaneously into the interscapular space 24 hours following 6-OHDA delivery and replaced throughout the experiment every 20 days. In rats receiving “vehicle pellets” inert control pellets were implanted using identical techniques.
Fetal Mesencephalic Graft Preparation
Embryonic cells of the ventral mesencephalon were obtained from embryonic day 14 (crown-rump length of 10.5 to 11.5mm) Sprague Dawley rats using micro-dissection techniques and tissue was prepared as previously described (Maries et al., 2006). Cell viability was determined by viewing a trypan blue stained sample of the cells suspension with a hemocytometer. Cells were diluted in neurobasal medium and final suspensions for transplantation were prepared to an approximate density of 66,700 cells/μl Neurobasal media (Invitrogen, Carlsbad, CA).
Fetal Mesencepahlic Allograft Injections
Four weeks following 6-OHDA lesions, rats receiving dopamine grafts were anesthetized with a chloropent solution and secured in a stereotaxic apparatus. Two injections of 1.5 μl of cell suspension were injected into the striatum at 1 site (A/P=0 mm from bregma, M/L=3.0mm from bregma) at 2 depths (D/V 1=4.3mm from skull, D/V 2=3.8mm from skull; ~100,000 cells total per site) at a flow rat of 0.5μl/min using a Hamilton 26 gauge needle for a total of 200,000 cells implanted in each rat. Sham-grafted rats received equal volumes of the cell-free suspension media. For all grafts, the needle was left in place for 3 min following deposition of tissue or vehicle.
Levodopa Treatment
To assess the effects of dendritic spine preservation in sham and dopamine-grafted rats on dyskinesias, rats received injections of levodopa and the peripheral decarboxylase inhibitor benserazide (12.5mg/kg levodopa; 12.5mg/kg benserazide) in sterile injection saline 1 day a week every 2 weeks beginning at 4 weeks post-grafting and continuing till the end of the study (20 weeks post-grafting). This sub-chronic paradigm of levodopa dosing was used to examine graft efficacy on levodopa-induced dyskinesia expression while minimizing any effects of levodopa itself on MSN spines. While different from daily chronic levodopa paradigms often employed to induce severe stable dyskinesias, in rats with severe dopamine-depletion, such as those used in this study, sub-chronic dosing results in levodopa-induced dyskinesia expression on first exposure (Lundblad et al., 2002).
Rotational Asymmetry Analysis
For behavioral assessment of graft efficacy, levodopa-induced rotational analyses were performed once a week every 2 weeks from week 4 to week 20 post-grafting. Amphetamine was not used in these studies as it has been shown to induce alterations to MSN dendritic spines (Robinson and Kolb, 2004). Rats received intraperitoneal injections of levodopa (12.5mg/kg levodopa; 12.5mg/kg benserazide) and rotational behavior was quantified for 1 min precisely 30 min post-injection. A final rotational asymmetry score was calculated as (contralateral rotations/total rotations ×100). Data are expressed as mean +/− SEM.
Vibrissae-induced Forelimb Placement Behavior
For behavioral assessment of lesion success and graft efficacy, rats were evaluated for vibrissae-induced forelimb response by a researcher blinded to treatment group. Rats were held with their forepaw ipsilateral to the lesion and hindpaws restrained. Their whiskers contralateral to the lesion were then brushed lightly against a raised surface. The number of times the rat responded to whisker stimulation by placing their unrestrained forepaw (contralateral to the lesion and graft) to the flat surface was calculated as a measure of striatal function (Schallert, 2006). Data are expressed as the number of successful touches per 10 trials.
Forelimb Use Cylinder Analysis
For a complex motor assessment of lesion success and graft efficacy, rats were evaluated for spontaneous forepaw use using cylinder analysis (Schallert et al., 2000). Each rat was placed in a clear Pexiglass cylinder (of dimensions described by Shallert et al., 2000) surrounded by mirrored panels to allow for evaluation of all movements and videotaped for 5 minutes or until the completion of 20 taps against the cylinder with the forepaws. The videotapes were evaluated for weight-bearing forelimb movements noting the use and disuse of each forelimb by an observer blinded to treatment group. Rats were evaluated 1 day every 2 weeks. The total number of right and left forelimb movements was totaled and a percent of use of the forelimb contralateral to the graft was obtained. Analyses were run during the rat’s dark cycle to enhance spontaneous exploratory behaviors. Data are expressed as (the number of right forelimb movements/ total number of forelimb movements) × 100.
Dyskinesia Rating
Abnormal involuntary movements or posturing associated with levodopa or graft treatment, are referred to in this text as dyskinesias. Dyskinesias observed in levodopa-treated parkinsonian rats included dystonia and hyperkinesias as described previously (Steece-Collier et al., 2003; Maries et al., 2006; Soderstrom et al., 2008). Dystonias were characterized by abnormal muscle tone, which was noted as excessive stiffness and rigidity, and/or abnormal posturing of the neck, trunk, right forepaw, and/or right hindpaw. Hyperkinesias consisted of vacuous chewing and/or tongue protrusion (orolingual), repetitive rhythmic bobbing of the head and neck (headbob) and stereotypic and/or chorea-like movements of the right forepaw (right forepaw dyskinesia). Dyskinesias were scored by an observer blinded to treatment group for 1 day every 2 weeks. Each rat was observed for 2 min precisely 30 min following levodopa delivery (a time that corresponds to peak dose dyskinesia).
A cumulative score for total dyskinesia was obtained through the summation of the frequency (0–3; with 0=no expression, 1=expression less than 50% of the time, 2=expression more than 50% of the time and 3=constant expression) and the intensity (0–3; with 0=no expression, 1=mild expression, 2=moderate expression, 3=severe expression) of the individual scores. Additionally rats were rated for novel dyskinesias that emerged with graft maturation. These included two behaviors involving orolingual and contralateral forelimb behaviors. The first was a compulsive tapping or pushing of cage litter with the forelimb contralateral to the graft (tapping dyskinesia; TPD), and a second ‘goal-directed’, stereotypic retrieving of litter with the forepaw contralateral to the graft and/or chewing of the litter (facial forelimb dyskinesia; FFD). Details of these graft-related aberrant behaviors are described elsewhere (Maries et al., 2006; Soderstrom et al., 2008). A cumulative score for total graft-induced dyskinesia was obtained with the same frequency rating as levodopa-induced dyskinesias but an intensity rating of either absent (score=0) or present (score=1).
For all behaviors observed, the intensity and frequency were quantified simultaneously. The product of the intensity and frequency scores provided a final ‘severity’ score. A detailed description of this rating scale is reported elsewhere (Steece-Collier et al., 2003; Maries et al., 2006).
Acute Nimodipine Treatment
To test whether the low dose of nimodipine (0.8 mg/kg/day) we used in the chronic release pellets to prevent dendritic spine loss would itself impact levodopa-induced dyskinesias, we examined behavior in a group of parkinsonian rats, distinct from rats used for the chronic nimodipine pellet studies. In these rats, an acute injection of nimodipine was administered in conjunction with levodopa to determine whether nimodipine had either negative or positive influences on levodopa-induced dyskinesias in our model. Rats were rendered severely parkinsonian, again without any pellet implants. All drugs were administered on the test day by intraperitoneal injection. Levodopa was administered at one of three doses: 6.0, 8.0, or 12.5 mg/kg. Doses of levodopa were varied to ensure that we were not “overwhelming” any potential “nimodipine effect” with our usual high dose of 12.5 mg/kg levodopa. Dyskinesia severity was analyzed 30 minutes post-levodopa (pre-nimodipine), which was followed by an injection of one of four test doses of nimodipine (0.08, 0.8, 8.0, or 20 mg/kg). Thirty minutes following the nimodipine injection, dyskinesias were rated a second time (post-nimodipine). A 48-hour washout was given between drug tests. Test doses of nimodipine were chosen to be 10-fold higher and lower than that used in the chronic release pellets we will use in the proposed studies (i.e.: 0.8 mg/kg). We also examined the same nimodipine dose as the pellets (0.8 mg/kg), plus a dose of 20 mg/kg, which is a higher dose, similar to that commonly employed in the literature (e.g.: Finger and Dunnett, 1989).
Golgi Staining
Rats used for dendritic spine density analysis were deeply anesthetized with 5 ml/kg penobarbital and sacrificed 20 weeks post-grafting by transcardial perfusion with room temperature 0.9% saline followed by cold 4% paraformaldehyde in 0.1 PO4 buffer at 4°C. Brains were blocked caudally approximately −3.5mm behind bregma and the forebrain block placed in a Golgi-Cox solution (1% mercury chloride, 1% potassium chromate and 1% potassium dichromate in distilled water) and allowed to develop in the dark for 14 days. Brains were then sectioned at a thickness of 100 μm on a vibrating microtome. Sections were placed on 4% gelatin subbed prepared slides and allowed to dry in a humidified chamber. The slides were then developed in ammonia hydroxide followed by Kodak Polymax fixer, and then dehydrated in a series of alcohol immersions. Finally, slides were cleared in xylene and coverslipped with DPX.
Golgi-stained Tissue Analysis
Striatal MSN spines were counted using Neurolucida software (Microbrightfield Bioscience, Williston, VT, USA ) on dendrites at 100X at 2 distances from the cell body: a proximal segment starting 50–60 μm from the cell body and a distal segment starting 120–150 μm from the cell body. Segments analyzed were approximately 30 μm in length. Spine density for each range was expressed as spines/10μm. One proximal segment and one distal segment were analyzed from a single, randomly chosen dendrite per neuron. Spine density on a total of 10 neurons per rat was determined, with group sizes ranging from 6 to 10 subjects. Thus, between 60 and 100 proximal and distal segments were analyzed for spine counts per experimental group.
Tyrosine Hydroxylase Immunohistochemistry
Rats used for the evaluation of immunohistochemistry were deeply anesthetized with 5 ml/kg pentobarbital and sacrificed 20 weeks post-grafting by transcardial perfusion with room temperature 0.9% saline followed by cold 4% paraformaldehyde in 0.1 PO4 buffer at 4°C. The brains were removed, post-fixed in 4% paraformaldehyde for 24 hours, followed by 30% sucrose solution until saturated. All brains were then frozen on dry ice and sectioned in the coronal plane on a microtome into sections 40 μm thick. Brains were serially sectioned into 6 sets per brain and stored at −20°C in cryoprotective solution until ready for analysis.
Every sixth coronal section was stained with antisera against tyrosine hydroxylase (TH) to visualize dopamine cells and fibers (Kordower et al., 1995; Steece-Collier et al., 1995). Sections were incubated for 48 hours at 4°C in anti-TH primary antibody (1:4000; clone LNC1; Millipore-Chemicon, Temecula, CA; No. MAB318, lot No. 0509010596). This mouse monoclonal antibody was raised against purified TH protein derived from PC12 cells and recognizes an epitope on the outside of the regulatory N-terminus and detects a unique 59–61-kDa band on Western blotting with human brain tissue. Sections were then rinsed and incubated for 1 hour in 1:200 horse anti-mouse IgG rat absorbed biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) and developed using 0.05% 3,3-diaminobenzidine tetrahydrochloride (DAB) and 0.01% hydrogen peroxide.
Stereology
To quantify graft survival, TH-immunopositive (TH+) sections equally spaced at 240 μm apart were analyzed for each graft injection. Cell counts were conducted in 4 to 6 serial sections. Each section was outlined at a magnification of 4X and TH+ cells were counted at 60X with oil immersion. At this higher magnification the thickness of each section was determined in 3 separate areas and averaged to yield an average section thickness of approximately 12 μm. All cells that fell within the optical disector height of 7 μm were counted allowing for a guard zone of 2 μm from the section top and 3 μm from the section bottom. Each section was overlaid with a grid and TH+ cells with discernable nucleoli were counted in equally spaced counting frames using dedicated software (StereoInvestigator, MicroBrightField, Williston, VT). The total number of neurons was calculated using the formula: N= NV × VROI, where NV is the numerical density and VROI is the volume of the region of interest. The volume was calculated according to the procedure of Cavalieri and variability within groups was assessed via the Coefficient of Error. CEs for all analyses were < 0.10.
TH+ Fiber Density Analysis
To quantify graft innervation, TH+ sections 240 μm apart were analyzed using the Space Balls estimator program (StereoInvestigator, MicroBrightfield, Inc., Williston, VT) to obtain an unbiased estimate of TH+ neurite density in the striatum. Fiber density analyses were conducted in 4 to 6 serial sections. Contours were drawn for 3 fields of view at the lateral border of the graft at 4X and neurites that crossed the borders of the hemispheric probe were counted at 60X with oil immersion. Neurite density was calculated as neurite length/ volume. The volume was calculated according to the procedure of Cavalieri and variability within groups was assessed via the CE. CEs for all analyses were < 0.10.
Statistics
A modified bootstrapping method was used for the analysis of behaviors that had extensive temporal data. This approach involved the inclusion of re-sampled data from the following time-point groupings: “pre-graft maturation” time-point (weeks −2, 0, and 2 post-grafting), “early post-grafting” time-point (weeks 4 and 6 post-grafting) “mid post-grafting” time-point (weeks 8 and 10 post-grafting) and a “late post-grafting” time point (weeks 18 and 20 post-grafting).
A 2-way repeated measures analysis of variance (ANOVA) was performed for each behavior, to assess the effects of treatment, time and treatment by time interaction. Significant differences of main effects were determined using Bonferroni post-hoc analyses.
Differences in spine density, TH+ cell counts, and TH+ fiber densities were determined using one-way ANOVAs followed by Tukey’s post-hoc analyses.
Results
Slow-release Nimodipine Pellets Preserved Spine Density in 6-OHDA-treated rats
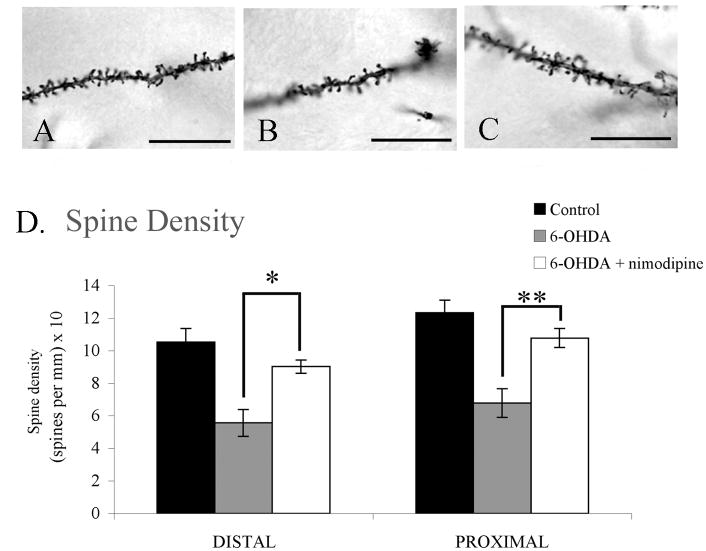
Analysis of Golgi-treated striatal tissue showed a greater than 40% reduction in spine density on dendrites both distal and proximal to the cell bodies of MSNs in the dopamine-depleted striatum compared to controls (control: distal=10.52 spines per 10 μm ±0.85, proximal=12.32 spines per 10 μm ±0.79; 6-OHDA-treated: distal=5.57 spines per 10 μm ±0.83, proximal=6.78 spines per 10 μm ±0.88). This loss was protected against in parkinsonian rats receiving nimodipine pellets at both distal and proximal sites with nimodipine-treated rats showing no significant difference from intact controls (6-OHDA + nimodipine: distal=9.02±0.41 spines per 10 μm, p=0.39; proximal=10.78 spines per 10 μm ±0.58, p=0.42) but differing significantly from parkinsonian rats receiving vehicle pellets (distal: F2,12=12.15, p=0.01; proximal: F2,12=13.54, p=0.007; Figure 2).
Figure 2. Slow-release nimodipine pellets preserve spine density in parkinsonian rats.
(AC) Photomicrographs of MSN dendrites spine density in (A) untreated controls, (B) dopamine-depleted (C) dopamine-depleted plus nimodipine treated groups. (D) Rats receiving intranigral 6-OHDA lesions showed a significant reduction in striatal spine density when compared to control rats at both distal (p=0.002) and proximal (p=0.001) dendritic sites. This loss of spine density was diminished significantly in rats receiving slow-release nimodipine pellets at both distal (*p=0.012) and proximal (**p=0.007) sites. Scale bars represent 60X.
Maintaining Dendritic Spine Density Did Not Improve The Ability of Dopamine Grafts to Reverse Rotational Asymmetry
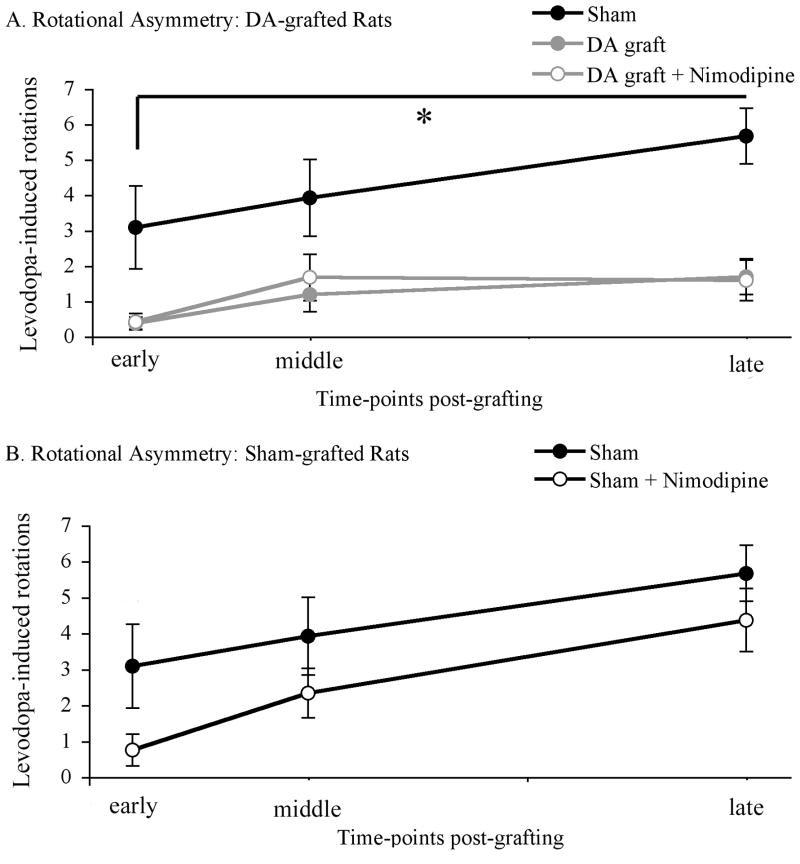
Both dopamine-grafted groups showed significantly reduced rotational behavior when compared with sham-grafted controls (early post-graft: dopamine-grafted=0.38 rotations per min ±0.18, dopamine-grafted + nimodipine=0.42 rotations per min ±0.23, sham-grafted=3.08 rotations per min ±1.17; mid post-graft: dopamine-grafted=1.19 rotations per min ±0.49, dopamine-grafted + nimodipine=1.67 rotations per min ±0.54, sham-grafted=3.92 rotations per min ±1.08; late post-graft: dopamine-grafted=1.69 rotations per min ±0.51, dopamine-grafted + nimodipine=1.58 rotations per min ±0.57, sham-grafted=5.67 rotations per min ±0.78; F2,33=22.716; p=0.001; Figure 3a). Analysis of levodopa-induced rotational behavior between dopamine-grafted rats receiving nimodipine or vehicle pellets revealed no significant difference (p=0.941) in this behavior that is easily reversed by dopamine cell replacement.
Figure 3. Rotational Asymmetry.
(A) Dopamine grafting, regardless of spine density, showed a near complete amelioration of rotational asymmetry. Both grafted groups showed a significant and near complete amelioration of levodopa-induced dyskinesias when compared to sham- grafted rats (*DA graft vs. sham graft, p=0.001; DA graft + nimodipine vs. sham graft, p=0.001). (B) Nimodipine treatment failed to amelioration levodopa-induced rotations in sham-grafted rats. DA=dopamine.
Maintaining Dendritic Spine Density Did Not Impact Rotation Asymmetry in Sham Grafted Rats
Analysis of levodopa-induced rotational behavior in sham grafted rats receiving nimodipine or vehicle pellets revealed no significant difference between groups (early post-graft: sham-grafted=3.08 rotations per min ±1.17, sham-grafted + nimodipine=0.75 rotations per min ±0.45; mid post-graft: sham-grafted=3.92 rotations per min ±1.08, sham-grafted + nimodipine=2.33 rotations per min ±0.69; late post-graft: sham-grafted=5.67 rotations per min ±0.78, sham-grafted + nimodipine=4.36 rotations per min ±0.88, F1,22=2.101; p=0.161; Figure 3b).
Maintaining Dendritic Spine Density Improved Performance on the Vibrissae-evoked Forelimb Placement Task in Dopamine-Grafted Rats
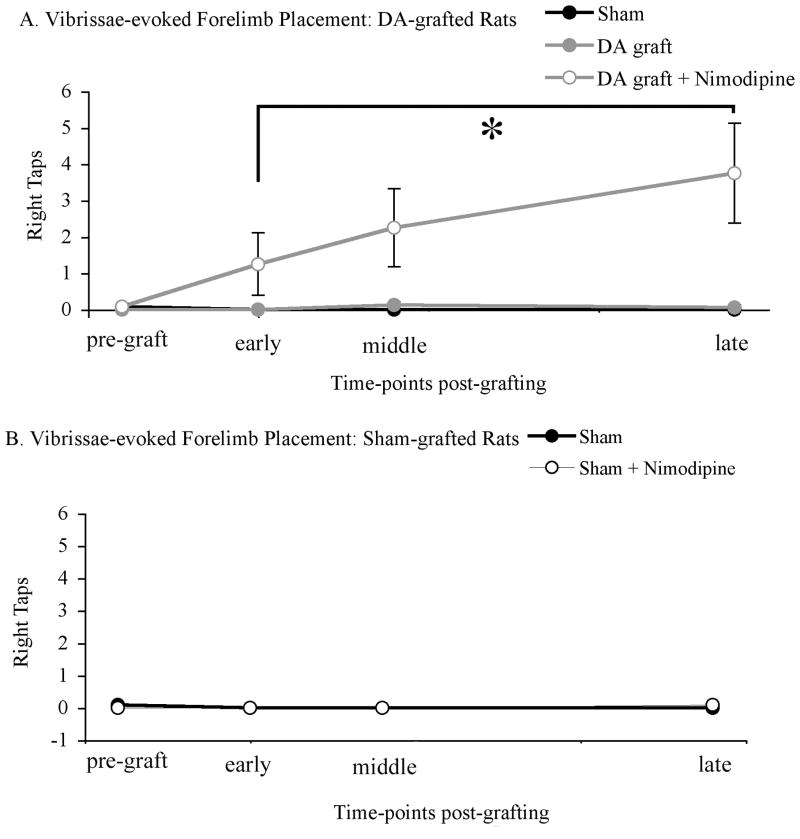
Analysis of behavior on the vibrissae-evoked forelimb placement task found a significant difference between sham-grafted, dopamine-grafted, and dopamine-grafted rats receiving nimodipine pellets (F2,75=3.937, p=0.024). While all groups showed 95% or greater impairment at an early post-graft time-point, dopamine-grafted rats receiving nimodipine pellets showed significantly greater improvement than grafted rats receiving vehicle pellets (p=0.001) and sham-grafted rats (p=0.001) at the latest time-point post-grafting (successful taps per 10 trials: sham-grafted=0±0, dopamine-grafted=0.06±0.06, dopamine-grafted + nimodipine=3.75±1.37; Figure 4a).
Figure 4. Preserved striatal spine density significantly improved vibrissae-induced forelimb placement in dopamine-grafted rats (A), but not sham-grafted rats (B).
(A) Dopamine-grafted rats receiving continuous nimodipine treatment showed significantly more vibrissae-induced forelimb placement when compared to rats receiving grafts alone (*p=0.024) and sham-grafted rats (p=0.026). (B) Nimodipine treatment had no effect on vibrissae-induced forelimb placement in sham-grafted parkinsonian rats. DA=dopamine.
Maintaining Dendritic Spine Density Did Not Impact Performance on Vibrissae-evoked Forelimb Placement Task in Sham-Grafted Parkinsonian Rats
Analysis of behavior on the vibrissae-evoked forelimb placement task found no significant difference between rats receiving nimodipine or vehicle pellets (F1,18=0.411, p=0.529) in the absence of a dopamine graft. Both groups showed no impairment prior to 6-OHDA delivery (successful taps per 10 trials: sham-grafted=10±0, sham-grafted + nimodipine=10±0), but significant stable, and equal degree of impairment at early (successful taps per 10 trials: sham-grafted=0±0, sham-grafted + nimodipine=0±0) and late time-points post-lesion (successful taps per 10 trials: sham-grafted=0±0, sham-grafted + nimodipine=0.08±0.08; Figure 4b).
Maintaining Dendritic Spine Density Reduced Levodopa-induced Dyskinesia Expression in Both Dopamine and Sham-grafted Rats
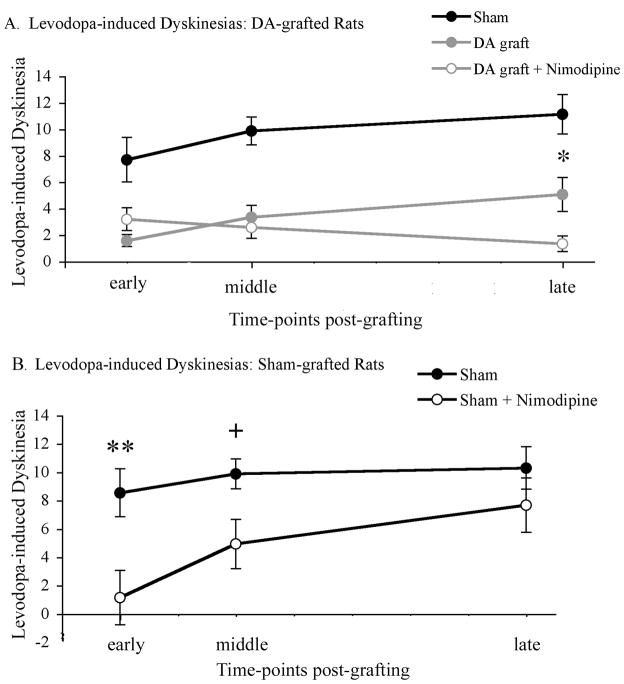
Analysis of levodopa-induced dyskinesias found that while there was a small and gradual sensitization of dyskinesia in sham-grafted rats there was a significant blunting of dyskinesia in both dopamine-grafted groups (Figure 5a). There was a significant difference between groups (F2,33=33.012, p=0.001) with both dopamine-grafted groups differing significantly from sham-grafted rats at all time-points examined (p=0.001). Interestingly, the anti-dyskinetic effect of grafted dopamine neurons was most notable in the severely parkinsonian rats with maintained striatal dendritic spine density. Indeed, dopamine-grafted rats receiving slow-release nimodipine pellets showed significantly less severe levodopa-induced dyskinesias at the latest time-point examined when compared with rats receiving dopamine grafts plus vehicle pellets (dyskinesia severity scores: dopamine-grafted=5.06±1.29, dopamine-grafted + nimodipine=1.33±0.59; p=0.02; Figure 5a).
Figure 5. Preserving spine density significantly reduced the occurrence of levodopa-induced dyskinesias in dopamine-grafted rats (A) and sham-grafted rats (B).
(A) Both dopamine-grafted groups showed a significant amelioration of levodopa-induced dyskinesias (p=0.001). However, dopamine grafts placed into parkinsonian rats with intact spine density (DA graft + nimodipine) showed a significant further reduction in dyskinesias at later post-graft time-points compared with parkinsonian rats grafted with dopamine neurons and experiencing dendritic spine loss (DA graft + vehicle, *p=0.02). (B) Preserving spine density also resulted in an amelioration of levodopa-induced dyskinesias in parkinsonian rats receiving sham grafts at early (**p=0.005) and middle (+p=0.013) time-points post-grafting, but not by the conclusion of the experiment (p=0.176). DA=dopamine.
In sham-grafted parkinsonian rats, maintaining dendritic spine density with nimodipine pellets resulted in significantly lower levels of levodopa-induced dyskinesias compared to sham-grafted rats receiving vehicle pellets at early (dyskinesia severity scores: sham-grafted=8.54±1.58, sham-grafted + nimodipine=1.15±0.22; p=0.005) and middle (sham-grafted=9,88±1.05, sham-grafted + nimodipine=4.93±1.44; p=0.013) time-points post-grafting (Figure 5b). However, unlike dopamine-grafted rats where dyskinesia prevention was maintained in rats with preserved spine density, repeated dosing of levodopa in the absence of a graft resulted in a loss of this “buffering” capacity over time (late time-point dyskinesia severity scores:: sham-grafted=10.29±1.41, sham-grafted + nimodipine=7.68±1.67; p=0.176).
Acute Nimodipine Treatment Did Not Impact Dyskinesias
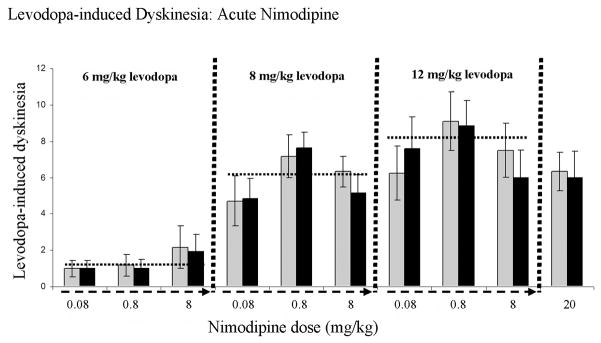
Analysis of levodopa-induced dyskinesias in non-pelleted, acutely drug-tested rats found that there is no apparent nimodipine-levodopa drug-drug interaction that impacts behavioral indices used in the current study. Indeed none of the nimodipine doses tested, ranging from 10-fold less to approximately 30-fold higher than the dose employed in the slow-release pellets, directly interfered with, or potentiated levodopa-induced dyskinesias (6mg/kg levodopa: 0.08mg/kg nimodipine p=1.0, 0.8 mg/kg nimodipine p=0.836, 8mg/kg nimodipine p=0.871; 8mg/kg levodopa: 0.08mg/kg nimodipine p=0.944, 0.8 mg/kg nimodipine p=0.761, 8mg/kg nimodipine p=0.382; 12.5mg/kg levodopa: 0.08mg/kg nimodipine p=0.574, 0.8 mg/kg nimodipine p=0.908, 8mg/kg nimodipine p=0.492, 20mg/kg nimodipine p=0.856; Figure 6).
Figure 6. Acute nimodipine treatment did not impact levodopa-induced dyskinesias.
A group of parkinsonian rats, distinct from rats used for the chronic nimodipine pellet studies, were employed. Dyskinesia severity was analyzed at 30 minutes after levodopa, but prior to nimodipine (light gray bars). Immediately following this behavioral evaluation, dyskinetic rats were injected with 1 of 4 doses of nimodipine (0.08, 0.8, 8.0, or 20 mg/kg) and dyskinetic behaviors rated again, 30 minutes after nimodipine (black bars). Dashed arrows on x-axis indicate escalating doses of nimodipine tested in combination with 1 of 3 doses of levodopa (6.0, 8.0, or 12.5 mg/kg). The horizontal dashed lines indicate the statistical dyskinesia average for each dose of the 3 doses of levodopa used.
Maintaining Dendritic Spine Density in the Presence of a Dopamine Graft Did Not Improve Behavior in the Forelimb Use Cylinder Test
Neither nimodipine treatment nor dopamine grafting appeared to have any overall significant effect on performance in the cylinder task (F2,11=1.843, p=0.204) with the moderate number of dopamine neurons grafted in this study. At all time-points examined, no significant effect of dopamine grafting plus vehicle pellets was observed, with sham-grafted and dopamine-grafted rats showing no difference in forelimb use to one another (p=0.978). While dopamine-grafted rats receiving nimodipine pellets trended towards improved performance, this was not statistically significant at any of the time-points observed (p=0.203; Figure 7).
Figure 7. Preserving spine density did not significantly improve performance on the cylinder test.
No difference was seen in right forelimb use in the cylinder task between groups (p=0.204). DA=dopamine.
Maintaining Dendritic Spine Density Transiently Reduced the Expression of Graft-induced Dyskinesias
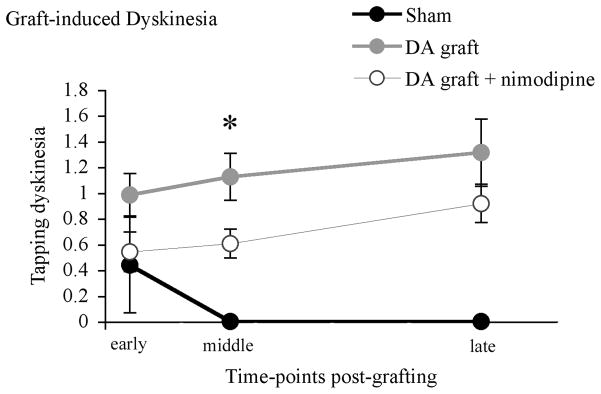
As we have reported previously, sham-grafted rats showed little-to-no expression of the graft-induced forepaw tapping dyskinesia (TPD). In contrast by the middle time-point examined, both dopamine-grafted groups showed an increasing level of TPD with dopamine-grafted rats receiving nimodipine pellets showing a significant reduction in TPD expression when compared with dopamine-grafted rats receiving vehicle pellets (TPD severity scores: dopamine-grafted=1.13±0.29, dopamine-grafted + nimodipine=0.60±0.19; p=0.04). However, this benefit was lost over time and there was no significant difference between the two dopamine-grafted groups by the conclusion of the experiment (TPD severity scores: dopamine-grafted=1.31±0.46, dopamine-grafted + nimodipine=0.92±0.26; F2,33=1,739, p=0.191; Figure 8).
Figure 8. Spine density preservation resulted in a transient improvement in the expression of the graft-induced dyskinesia, tapping.
Continuous nimodipine treatment resulted in a significant decrease in the severity score of tapping dyskinesia in dopamine-grafted rats (DA graft + nimodipine) when compared with rats receiving dopamine-grafts alone (DA graft, *p=0.04) at a middle time-point post-grafting; however this effect was lost at later post-graft time-points (p=0.191). DA=dopamine.
Maintaining Dendritic Spine Density Impacted Graft Innervation
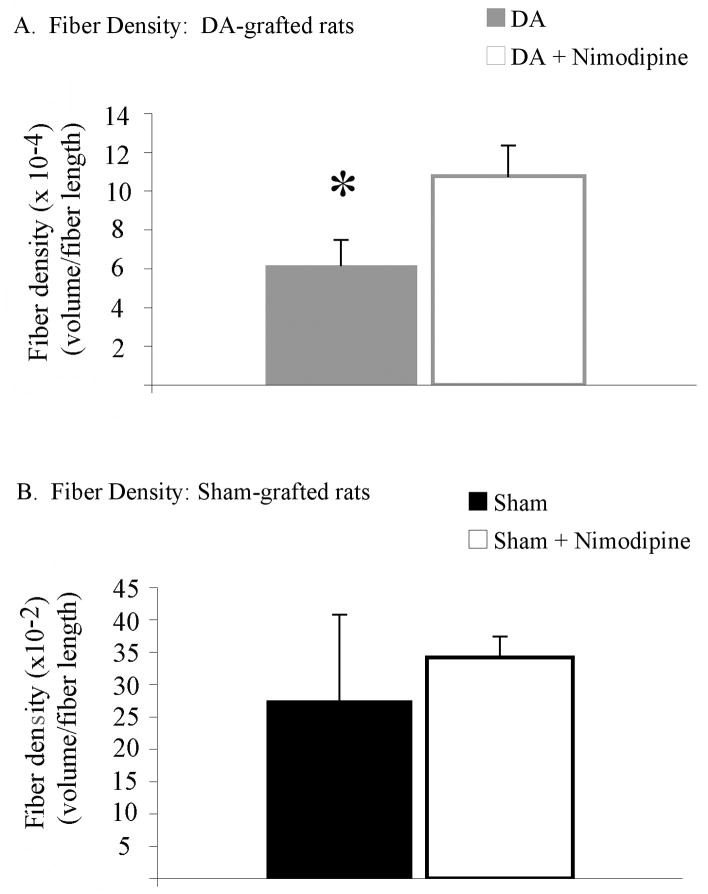
Fiber density analysis revealed a significant effect of spine density preservation through nimodipine treatment on graft neurite outgrowth between dopamine-grafted groups (t1,2=−2.200, p=0.050, Figure 9). Despite comparable graft survival (below), dopamine-grafted rats receiving nimodipine pellets showed a 17% increase in graft-derived fiber innervation compared with dopamine-grafted rats receiving vehicle pellets (graft volume (μm3)/fiber length (μm): dopamine-grafted=0.006±0.001, dopamine-grafted + nimodipine=0.011±0.001).
Figure 9. Spine density preservation did result in an increase in graft outgrowth.
(A) Despite having no effect on graft survival, continuous nimodipine treatment did result in an increase in TH+ fiber density in dopamine-grafted rats compared with rats receiving grafts alone (* p=0.05). (B) Preserving spine density had no effect on fiber density in sham-grafted rats. DA=dopamine.
Continual Nimodipine Treatment Did Not Impact Graft Survival
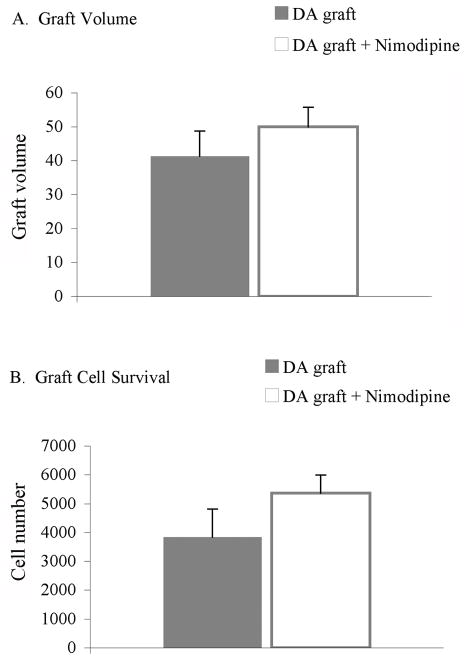
The enhanced behavioral response of dopamine-grafted rats receiving nimodipine pellets compared with dopamine-grafted rats receiving vehicle pellets occurred despite no significant difference in graft volume (dopamine-grafted=41.29 μm3±7.42, dopamine-grafted + nimodipine=50.0 μm3±5.72; t1,2=−0.930, p=0.001; Figure 10a) or the number of surviving TH+ grafted cells (dopamine-grafted=3836.85 TH+ cells±971.65, dopamine-grafted + nimodipine=5368.94 TH+ cells±620.25; t1,2=1.302, p=0.219; Figure 10b).
Figure 10. Nimodipine pellets did not effect the survival of dopamine grafts.
Continuous nimodipine treatment did not result in a difference in graft volume (p=0.371) or graft survival (p=0.219) between grafted groups. DA=dopamine.
Discussion
We report here the first evidence to suggest that MSN dendritic spine loss noted in advanced PD may contribute to the decreased efficacy of dopamine graft therapy. Data from the present study demonstrates that when the same number of embryonic ventral mesencephalic cells are grafted into two distinct cohorts of severely parkinsonian rats, those with normal striatal MSN dendritic spine density show superior prevention of the development and escalation of dyskinesias, and amelioration of sensorimotor deficits measured with the vibrissae motor test when compared to parkinsonian rats with dendritic spine loss. This finding provides a mechanism that may explain why patients with less severe disease progression (Olanow et al., 2003) and rats with less severe dopamine depletion (Kirik et al., 2001) respond more favorably to dopamine cell replacement therapy.
Striatal Neuron Pathology in PD
It has long been known that striatal dopamine loss results in distinct morphological alterations to MSNs in postmortem PD brains including significant regression of dendrite length and loss of dendritic spines with advanced disease (McNeill et al, 1988; Zaja-Milatovic et al, 2005; Stephens et al, 2005). The loss of dendritic spines following dopamine depletion has recently been linked to dysregulation of Cav 1.3 Ca2+ channels on MSN (Day et al., 2006). Identification of this mechanism has provided a means to examine the effects of this spine loss on symptomatic PD therapies in animal models.
Medium spiny neurons account for approximately 95% of the neurons within the striatum and their spines are the anatomical substrates that receive input from the cortex and substantia nigra. Typically cortical glutamate afferents synapse onto the head of a dendritic spine while nigral dopamine afferents synapse onto the neck of the same spine. The excitatory glutamate input is modulated within the spine by the nigral dopamine input. Due to unique properties of the striatum, both dopamine and glutamate are necessary for the synaptic plasticity required for normal motor function and memory storage. It can be imagined that loss of these critical dendritic structures with progressive loss of dopamine in PD would impact symptomatic therapies, including dopamine neuron grafting; however, this idea has not been investigated.
The Potential Impact of Dendritic Spine Preservation On Dopamine Cell Replacement Therapy
It has long been appreciated that newly formed TH+ endings in the grafted striatum have atypical modes of termination (Freund et al., 1985; Mahalik et al., 1985; Leranth et al., 1998), indicating that the synaptic circuitry of the dopamine-depleted, grafted striatum varies from the normal circuitry. The consequences of such remodeling may underlie the lack of full efficacy and/or development of therapy mediated side-effects seen in the grafted, parkinsonian brain. We recently reported that in the same rat model of PD used in the current study, specific aberrant synaptic features in the grafted striatum, including a decrease in the proportion of appropriate axo-spinous connections between grafted and host cells, are associated with the expression of graft-mediated motor dysfunction (Soderstrom et al., 2008). It is reasonable to suggest that MSN pathology, particularly the loss of normal dendritic spines and accompanying alterations of corticostriatal afferents, are critical elements that predispose this abnormal structure/function relationship.
While much research has focused on attempting to improve graft cell survival and/or identifying viable regenerative factors for host dopamine terminals, overcoming these obstacles may still fail to produce effective therapies if changes in the parkinsonian striatum exist that prevent establishment of normal physiological synapses between the new dopamine terminals and striatal neurons. We would predict, based in part on the current study and in part on the known physiology of the striatum, that therapeutic benefit of striatal dopamine axon terminal replacement, regardless of the approach (e.g.: primary neuron grafts, stem cell grafts, neurotrophic factor-induced sprouting) will be limited if normal structural input sites such as dendritic spines are reduced.
The Potential Impact of Dendritic Spine Preservation On Levodopa Therapy In Sham-Grafted Rats
While the precise mechanism by which dopamine depletion contributes to the development of levodopa-induced dyskinesias remains unclear, it is known that increasing severity of dopamine denervation appears to increase the likelihood of dyskinesia development (Mones et al, 1971; Langston and Ballard, 1984; Caligiuri and Lohr, 1993; Fahn 2000). Accordingly, it has been hypothesized that dendritic spine loss secondary to increasing striatal dopamine depletion creates an environment where levodopa catalyzes synaptopathology that results in expression of levodopa-induced dyskinesias.
Two control groups in the current study allowed examination of dyskinetic behavior in non-dopamine grafted parkinsonian rats with and without normal dendritic spine density. We observed, in these non-grafted groups, that preventing the loss of striatal dendritic spines allowed for significant buffering against dyskinesia development in severely parkinsonian rats. This finding is similar to that reported recently by Schuster and colleagues (2009) who found that striatal spine preservation (with the calcium channel blocker isradipine) protected against particular aspects of levodopa-induced dyskinesia development using a low dose of levodopa (6 mg/kg).
Importantly, the acute pharmacologic studies reported here, demonstrate that there is no inhibitory or enhancing interaction of acute calcium channel blockade with nimodipine on levodopa-induced dyskinesias. This suggests that behavioral findings with low dose calcium channel blockade are more likely related to the integrity of dendritic spines on MSNs associated with the chronic nimodipine (or isradipine) regimen rather than calcium channel blockade per se.
While spine preservation delayed the onset of levodopa-induced dyskinesias in this model, this was lost with repeated high dose levodopa in the non-dopamine grafted rats. Pathology of MSN, particularly the loss of normal dendritic spines and accompanying alterations of corticostriatal afferents, appears to be an important element that predisposes the development of levodopa-induced dyskinesias in animal models of PD. However, it remains unclear how spine loss impacts glutamate-dependent synaptic plasticity, contributes to levodopa-induced dyskinesia development, and whether aspects of this mechanism may be valuable for improving levodopa therapy in PD patients.
Was the Improved Graft Efficacy Observed in this Study Related to Nimodipine Treatment or Spine Preservation?
It is not possible to answer this question unequivocally. However, our finding that the dose of nimodipine employed in our study did not impact graft volume or survival of grafted TH+ cells, suggests that the enhanced behavioral impact of grafting in the nimodipine-treated rats was not due to a pharmacological enhancement of dopamine graft cell number as has been reported under different grafting conditions with larger doses of this drug (Finger et al., 1989; Brundin et al., 2000). It is interesting that rats with nimodipine pellets in this study showed a significantly greater degree of TH+ fiber density within the grafted striatum compared to rats with vehicle pellets. It is possible that the increase in normal structural contact sites within the striatum of the nimodipine-treated rats promoted the outgrowth and/or stability of TH+ terminals from grafted dopamine neurons.
In addition to a lack of impact of nimodipine on graft cell survival or volume, acute administration of doses of nimodipine up to approximately 30-fold higher or 10-fold lower than that found in the pellets used in the current study showed no impact on the motor behaviors quantified. These findings strongly support that the impact of nimodipine in this paradigm is through mechanisms other than those discussed above. We hypothesize the mechanism to be related to normalized spine density, allowing for increase in physiological input sights for TH+ fiber reinnervation, and normalized synaptic inputs from grafted cells. Even if nimodipine was improving graft function via a pharmacological mechanism not detected here, this drug is readily employed in humans and not contraindicated for use with clinical grafting. Our hypothesis that nimodipine-treated rats show superior graft-derived benefit due to the preservation of critical neuron structure (i.e.: spines) within the striatum, remains to be systematically investigated with ultrastructural analyses and is the subject of future studies in our laboratory.
Will Spine Preservation Alone Be Enough?
While dendritic spine preservation may allow for enhanced efficacy (e.g.: prevention of levodopa-induced dyskinesias; reversal of motor impairment) and diminished side-effects (e.g.: prevention of GIDs) of dopamine graft therapy, several attributes of spine preservation and innate plasticity within the striatum warrant further consideration. Specifically, while the current study found enhanced graft-derived benefit in parkinsonian subjects with preserved dendritic spine density, the impact was relatively small. While significant, especially given the small number of cells grafted into severely parkinsonian subjects in this study, it might have been anticipated that a larger impact could have been achieved if structural integrity of striatal MSNs was entirely normal. However, despite the fact that it is possible to maintain a normal number of dendritic spines by inhibiting aberrant Ca2+ signaling within these structures, other pathological issues may still exist in the parkinsonian striatum. For example, it is possible that synaptic sites on the rescued, de-nuded spines could have acquired new inputs in the interim between the nigral lesion and grafting. Indeed, structural preservation of dendritic spines in the absence of normal dopamine synapses could result in the establishment of ectopic, non-dopamine synapses, an idea supported by Meredith and colleagues (2000). In such a scenario, despite normal spine density, newly formed dopamine terminals from tissue grafting would be compromised in their ability to establish appropriate synaptic contact.
The Role of Dendritic Spine Preservation in Graft-induced Dyskinesia Expression
Our finding that rats with preserved dendritic spine density showed an initial prevention of GID-like behaviors suggests a role for dendritic spine loss in the development of GID. Indeed, our previous findings (Soderstrom et al., 2008) have shown that a decrease in the proportion of appropriate axo-spinous synapses between grafted and host cells to more atypical axo-dendritic or axo-somatic endings is associated with an increase in the expression of aberrant behavior following grafting.
Interestingly in the current study we found a gradual reversal of GID attenuation despite maintained spine preservation and increased graft re-innervation in nimodipine-treated grafted rats. While the mechanism(s) responsible for the gradual re-emergence of GID in this study is unknown, our previous work has shown that additional synaptic changes independent of the state of MSN spine integrity observed in the grafted striatum may be playing a role. Specifically an increase in the proportion of asymmetrical dopaminergic synapses and perforated non-dopaminergic synapses were also found to impact the occurrence of GID. We found the prevalence of these atypical features correlated strongly with the immune response observed in allografted rats, a factor that would also exist in the allografting protocol (grafting between outbred Sprague Dawley rats) employed in the current study.
It is possible that in the current study initially appropriate synaptic contacts are made onto appropriate targets (due to the maintenance of spine density) resulting in the prevention of GID development. However, as time passes and the synapses are increasingly exposed to an environment full of immunogenic signals, they may (while remaining on appropriate targets) begin to show atypical synaptic features of increased excitability (i.e. increased asymmetry and perforation) and lead to GID expression. Analyses of the ultrastructural profiles and immunological statuses of the subjects used in the current study are underway to help determine the role of MSN spine preservation on the development of GIDs.
Understanding the Complexities of Striatal Pathology Can Guide Therapeutics for PD
Based on the initial findings reported here, it could be predicted that normalizing dendrite morphology would allow for near complete normalization of complex behaviors affected in PD following grafting, dependent on the extent of dopamine cell replacement. Alternatively, it is possible that normalizing a single pathological factor (e.g. spine density) within the severely dopamine-depleted parkinsonian brain will be “too little, too late”. In reality, regardless of the morphological integrity of striatal MSNs, dendrites/spines are highly plastic structures and if maintained devoid of normal dopamine input will likely acquire ectopic synaptic input (Guerra et al., 1997; Maeda et al., 2005). Further, it is known that there are alterations in receptor trafficking and regulation in the severely dopamine-depleted striatum (Dunah et al., 2004; Picconi et al., 2008, etc.). Thus, it is becoming more and more apparent that: 1) manipulating a single factor will likely be unable to maximize (graft-mediated) therapy in PD, and 2) that complex changes associated with moderate to severe PD may present challenges that preclude optimal symptomatic therapy. As clinical grafting trials for PD begin to re-emerge, consideration of dopamine terminal replacement via grafted cells might best be considered in individuals with less severe pathology. Earlier intervention remains controversial and widely debated, however, a large body of evidence, from both preclinical and clinical studies, demonstrates that therapies such as dopamine neuron grafting are not, and may never be effective in subjects with severe dopamine depletion (e.g.: Breysse et al, 2007; Linazasoro, 2009; Truong and Wolters, 2009; Winkler et al., 2005). Intervention, such as preserving dendritic spine morphology, together with dopamine terminal replacement earlier in disease offers therapeutic promise that does not seem probable in advanced PD.
Acknowledgments
The authors would like to thank Dr. Ariel Deutch of Vanderbilt University for his valuable guidance on nimodipine pellet formation and use. We would also like to thank Dr. Timothy Schallert of University of Texas at Austin for his expert guidance on behavioral test paradigms. Further we would like to acknowledge the outstanding technical assistance of Jennifer Stancati and Brian Daley. This work is supported in part by R01NS045132, P50NS058830, The Udall Center of Excellence at the University of Cincinnati, and the Michael J. Fox Foundation.
Abbreviations
- ANOVA
Analysis of Variance
- DA
Dopamine
- FFD
Facial Forelimb Dyskinesia
- GID
Graft-induced Dyskinesia
- MSN
Medium spiny neuron
- PD
Parkinson’s disease
- TH
Tyrosine hydroxylase
- TPD
Tapping dyskinesia
Bibliography
- Breysse N, Carlsson T, Winkler C, Björklund A, Kirik D. The functional impact of the intrastriatal dopamine neuron grafts in parkinsonian rats is reduced with advancing disease. 2007;27:5849–56. doi: 10.1523/JNEUROSCI.0626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Gerfen CR. Plasticity within striatal direct pathway neurons after neonatal dopamine depletion is mediated through a novel functional coupling of serotonin 5-HT2 receptors to the ERK 1/2 map kinase pathway. J Comp Neurol. 2006;498:415–30. doi: 10.1002/cne.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Karlsson J, Emgard M, Schierle GS, Hansson O, Petersen A, Castilho RF. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 2000;9:179–95. doi: 10.1177/096368970000900205. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Lohr JB. Worsening of postural tremor in patients with levodopa- induced dyskinesia: a quantitative analysis. Clin Neuropharmacol. 1993;16:244–50. doi: 10.1097/00002826-199306000-00008. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, Bakay RA, Sladek JR, Jr, Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–9. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Deutch AY. Striatal plasticity in parkinsonism: dystrophic changes in medium spiny neurons and progression in Parkinson's disease. J Neural Transm Suppl. 2006;70:67–70. doi: 10.1007/978-3-211-45295-0_12. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol Pharmacol. 2004;65:121–9. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Fahn S. The spectrum of levodopa-induced dyskinesias. Ann Neurol. 2000;47:S2–9. [PubMed] [Google Scholar]
- Finger S, Dunnett SB. Nimodipine enhances growth and vascularization of neural grafts. Exp Neurol. 1989;104:1–9. doi: 10.1016/0014-4886(89)90001-0. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. N Engl J Med. 2001;344:710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Freund TF, Bolam JP, Bjorkland A, Stenevi U, Dunnett SB, Powell JF, Smith AD. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: a tyrosine hydroxylase immunocytochemical study. 1985;5:603–16. doi: 10.1523/JNEUROSCI.05-03-00603.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra MJ, Liste I, Labandeira-Garcia JL. Effects of lesions of the nigrostriatal pathway and of nigral grafts on striatal serotonergic innervation in adult rats. Neuroreport. 1997;8:3485–8. doi: 10.1097/00001756-199711100-00014. [DOI] [PubMed] [Google Scholar]
- Kirik D, Winkler C, Bjorklund A. Growth and functional efficacy of intrastriatal nigral transplants depend on the extent of nigrostriatal degeneration. J Neurosci. 2001;21:2889–96. doi: 10.1523/JNEUROSCI.21-08-02889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR, Hauser RA, Smith DA, Nauert GM, Perl DP, Olanow W. Neuropathological evidence of graft survival and striatal reinnervation after transplantation of fetal mesencephalic tissue in a patient with Parkinson’s disease. N Engl J Med. 1995;332:1118–24. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P. Parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): implications for treatment and pathogenesis of Parkinson’s disease. Can J Neurol Sci. 1984;11:160–5. doi: 10.1017/s0317167100046333. [DOI] [PubMed] [Google Scholar]
- Leranth C, Sladek JR, Jr, Roth RH, Redmond DE., Jr Efferent synaptic connections of dopaminergic neurons grafted into the caudate nucleus of experimentally induced parkinsonian monkeys are different from those of control animals. Exp Brain Res. 1998;123:323–33. doi: 10.1007/s002210050575. [DOI] [PubMed] [Google Scholar]
- Linazasoro G. A global view of Parkinson's disease pathogenesis: implications for natural history and neuroprotection. Parkinsonism Relat Disord. 2009;15:401–5. doi: 10.1016/j.parkreldis.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioral measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15:120–32. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Mahalik TJ, Finger TE, Stromberg I, Olson L. Substantia nigra transplants into denervated striatum of the rat: ultrastructure of graft and host interconnections. J Comp Neurol. 1985;240:60–70. doi: 10.1002/cne.902400105. [DOI] [PubMed] [Google Scholar]
- Maeda T, Nagata K, Yoshida Y, Kannari K. Serotonergic hyperinnervation into the dopaminergic denervated striatum compensates for dopamine conversion from exogenously administered l-DOPA. Brain Res. 2005;1046:230–3. doi: 10.1016/j.brainres.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, Shannon K, Steece-Collier K. Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol Dis. 2006;21:165–80. doi: 10.1016/j.nbd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res. 1988;455:148–52. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- Meredith GE, De Souza IE, Hyde TM, Tipper G, Wong ML, Egan MF. Persistent alterations in dendrites, spines, and dynorphinergic synapses in the nucleus accumbens shell of rats with neuroleptic-induced dyskinesias. J Neurosci. 2000;20:7798–806. doi: 10.1523/JNEUROSCI.20-20-07798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurers BH, Dziewczapolski G, Shi T, Bittner A, Kamme F, Shults CW. Dopamine depletion induces distinct compensatory gene expression changes in DARPP-32 signal transduction cascades of striatonigral and striatopallidal neurons. J Neurosci. 2009;29:6828–39. doi: 10.1523/JNEUROSCI.5310-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mones RJ. Levodopa-induced dyskinesia in the normal rhesus monkey. Mt Sinai J Med. 1971;39:197–201. [PubMed] [Google Scholar]
- Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neurosci. 2007;149:457–64. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–14. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Picconi B, Ghiglieri V, Bagetta V, Barone I, Sgobio C, Calabresi P. Striatal synaptic changes in experimental parkinsonism: role of NMDA receptor trafficking in PSD. Parkinsonism Relat Disord. 2008;14(Suppl 2):S145–9. doi: 10.1016/j.parkreldis.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster S, Doudnikoff E, Rylander D, Berthet A, Aubert I, Ittrich C, Block B, Cenci MA, Surmeier DJ, Hengerer B, Bezard E. Antagonizing L-type Ca2+ channel reduceds development of abnormal involuntary movement in the rat model of L-3,4- dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry. 2009;65:518–26. doi: 10.1016/j.biopsych.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Soderstrom KE, Meredith G, Freeman TB, McGuire SO, Collier TJ, Sortwell CE, Wu Q, Steece-Collier K. The synaptic impact of the host immune response in a parkinsonian allograft rat model: Influence on graft-derived aberrant behaviors. Neurobiol Dis. 2008;32:229–42. doi: 10.1016/j.nbd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steece-Collier K, Yurek DM, Collier TJ, Junn FS, Sladek JR., Jr The detrimental effect of levodopa on behavioral efficacy of fetal dopamine grafts in rats is irreversible following prolonged withdrawal of chronic dosing. Brain Res. 1995;676:404–8. doi: 10.1016/0006-8993(95)00149-k. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Collier TJ, Danielson PD, Kurlan R, Yurek DM, Sladek JR., Jr Embryonic mesencephalic grafts increase levodopa-induced forelimb hyperkinesias in parkinsonian rats. Mov Disord. 2003;18:1442–54. doi: 10.1002/mds.10588. [DOI] [PubMed] [Google Scholar]
- Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, Bell JE, Kilford L, Kingsbury AE, Daniel SE, Ingham CA. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience. 2005;132:741–54. doi: 10.1016/j.neuroscience.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Truong D, Wolters E. Recognition and management of Parkinson's disease during the premotor (prodromal) phase. Expert Rev Neurother. 2009;9:847–57. doi: 10.1586/ern.09.50. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Björklund A. Cell transplantation in Parkinson's disease: how can we make it work? Trends Neurosci. 2005;28:86–92. doi: 10.1016/j.tins.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson’s disease. Neurology. 2005;64:545–7. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]