Abstract
Overweight and obesity have been prospectively associated with the risk of coronary heart disease (CHD). Less clear is the relation of excess weight to risk of CHD among men and women with comorbid conditions, and the proportion of CHD risk attributable to excess weight in the US population.
To assess the risk of CHD associated with excess weight among men and women with and without associated comorbid conditions, and determine the population attributable risk of CHD associated with excess weight.
The study population consisted of two prospective cohorts, the Health Professionals Follow-up Study (N = 42,351 men; age range at baseline, 39–75 years) and the Nurses’ Health Study (N = 76,703 women; age range at baseline, 39–65 years).
A total of 2,771 incident cases of CHD among the men and 2,359 among the women were documented over the 16 years of follow-up. Overall, the relative risk (RR) of CHD associated with BMI ≥ 30 kg/m2 compared with BMI 18.5 to 22.9 kg/m2 was 2.13 (95% CI, 1.82–2.48) among the men and 2.48 (95% CI, 2.20–2.80) among the women. The risk of CHD increased with BMI, both with and without the presence of comorbid conditions. Our estimates suggest that more than a third of all incident CHD in US men and women may be attributed to excess weight.
Excess weight is associated with increased risk of CHD among men and women, both alone and in combination with comorbid conditions, though the results require careful interpretation. A substantial proportion of incident CHD may be attributed to excess weight.
Introduction
Coronary heart disease (CHD) remains the leading cause of mortality in the United States and is projected to be the leading contributor to morbidity and mortality worldwide over the next several decades. (1) Similarly, excess weight is a major public health problem in most Western Countries and has seen an exponential rise in developing countries. (2) Despite this seemingly parallel relationship, the association between excess weight and CHD is complex. Excess weight, in particular abdominal obesity, increases the risk of developing hypercholesterolemia, hypertension, and diabetes (3–5) and each of these comorbid medical conditions is associated in turn with an increased risk of CHD. (6–7) There is also evidence to support a relationship between excess weight and risk of CHD independent of these intermediary comorbid conditions. (4) These observations have generated hypotheses that an underlying pathogenesis could explain the clustering of risk factors associated with excess weight as well as increased cardiovascular morbidity. (8–10) Regardless of whether the physiological processes which lead to obesity and these classic CHD risk factors are linked etiologically, if obesity continues to be a strong risk factor for CHD among subpopulations defined by combinations of these comorbid conditions, this could help to target patients most in need of intervention programs. Therefore, in two large ongoing cohort studies we estimated the relative risk of CHD associated with obesity across strata defined by existing comorbid conditions. With nationally-representative prevalence proportions, we further estimated the fraction of CHD morbidity attributable to excess weight and combinations of comorbid conditions in the US population.
Materials and Methods
Study population
The Health Professionals Follow-up Study (HPFS) is a prospective cohort of 51,529 male health professionals ranging in age from 39 to 75 years at enrollment in 1986. The cohort of 29,683 dentists, 3,745 optometrists, 2,218 osteopaths, 4,185 pharmacists, 1,600 podiatrists, and 10,098 veterinarians completed a baseline mailed survey of detailed information about medical history, dietary intake, lifestyle, and demographic information in 1986. Every two years subsequently, follow-up questionnaires containing information on interim medical history, and lifestyle were completed. Detailed dietary intake, assessed by a semi-quantitative food frequency questionnaire (FFQ) (11) was collected at baseline in 1986 and every four years subsequently. Principal criteria for exclusion from the analyses were 1) prevalent cancer, stroke or CHD at baseline, 2) missing height or weight, 3) diabetes onset prior to age 30 years, and 4) body mass index (BMI) < 18.5 kg/m2. After these exclusions, there were 42,351 eligible men.
The Nurses’ Health Study (NHS) cohort was formed in 1976 with the enrollment of 121,701 female nurses, aged 29 to 55 years, with the completion of a baseline questionnaire on medical history, lifestyle, and demographic information. Follow-up and exclusion criteria are similar to those described above for the HPFS. For compatibility to HPFS above we restricted analyses in the NHS to follow-up from 1986, at which time the age range was 39 to 65 years. After these exclusions, there were 76,703 eligible women.
Exposure Measurement
Excess weight was defined as a function of BMI of 23.0 kg/m2 or greater, calculated as weight (kg)/(height (m))2. Height was self reported on the 1976 (NHS) and 1988 (HPFS) questionnaires. Weight was reported on the 1986 and all subsequent follow-up questionnaires in both cohorts. In a validation study of 123 men in the HPFS and 140 women in the NHS, self-administered (by mailing), self-reported and measured weights were highly correlated, 0.97 for men and 0.97 for women. (12)
BMI was categorized using standard World Health Organization categories for healthy weight, overweight and three categories of obesity. The healthy weight range was divided into two parts, with a reference category of 18.5 to 22.9 kg/m2 and a high-normal category of 23.0 to 24.9 kg/m2. Due to the sufficient number of observations for precise estimates in the overweight range, the decision was made a priori to divide this category into two parts, while the categories of obesity were collapsed into a single category of BMI > 30.0 kg/m2 for greater precision of effect estimates. BMI was thus classified in five categories: 1. reference (BMI 18.5 to 22.9), 2. high-normal weight (BMI 23.0 to 24.9), 3. overweight I (BMI: 25.0 to 26.9), 4. overweight II (BMI: 27.0 to 29.9) and 5. obese (BMI ≥ 30.0). Participants were also classified according to comorbid conditions into five exclusive categories: 1. no comorbid conditions, 2. hypercholesterolemia only, 3. hypertension only, 4. hypercholesterolemia and hypertension only, and 5. diabetes (with or without hypertension and hypercholesterolemia). Combination of BMI and related risk factors led to a partitioning of the cohorts into 25 mutually exclusive and exhaustive categories of exposure.
Outcome Measurement
Incident CHD was defined as any case of non fatal myocardial infarction (MI) or fatal CHD outcome recorded during the 16-year follow-up period from January, 1988 through December, 2004. The World Health Organization’s criteria for MI were used for outcome coding in both cohorts: symptoms accompanied by either diagnostic changes on electrocardiogram or elevated cardiac enzymes. Ascertainment of death in the HPFS and NHS is accomplished by search of the National Death Index and by family members’ response to follow-up questionnaires. (13) In the case of a cardiovascular disease death, the subject’s medical records are reviewed, as are death certificates and autopsy results, as available.
Covariates
Information on potential confounding factors, intermediary medical conditions and lifestyle behaviors that could potentially to influence the relationship between excess weight and CHD were gathered from the 1986 baselines and updated on subsequent follow-up surveys. Family history of CHD was reported on the 1986 questionnaire, and was considered positive for myocardial infarction occurring in either parent prior to age 60 years. Cigarette smoking status was based on the baseline survey and subsequent updates, and categorized as ‘current’, ‘former’ or ‘never’. In the multivariate models, the current smokers were further classified according to three categories of daily cigarette consumption.
Information on dietary intake of alcohol, saturated fat, polyunsaturated fat, trans-fat, folate, vitamin E, and total energy were ascertained by semi-quantitative food frequency questionnaire (FFQ) (11) administered at baseline (1986) and at 4-year intervals (thus 1990, 1994, 1998) during follow-up. Intakes were categorized into quintiles and indicators coded for inclusion in the multivariate models.
Diagnoses of the intermediary comorbid conditions of hypercholesterolemia, hypertension, and diabetes were ascertained by self-report at baseline in 1986. The self-report of these comorbid conditions by health professionals has been validated. (14–16) Participants who reported onset of diabetes mellitus prior to age 30 were excluded.
Statistical Analysis
Cox proportional hazard regression was used to model the relationship between excess weight and incident CHD outcome. (17) All analyses were adjusted for age. Tests for trend in the categorical analyses were performed by fitting the median value for each category of exposure as a linear term in the model.
The primary analyses utilized baseline (1986) measures of both exposures and covariates. Additional models were also run using updated (time-varying) exposures and covariates for comparison. The intermediary medical conditions of hypercholesterolemia, hypertension and diabetes are all components of the causal pathways between excess weight and incident CHD, such that conditioning on or restricting by these conditions during follow-up might be expected to have the effect of attenuating estimates of CHD risk associated with excess weight.
The issue of bias due to reverse causation, whereby an unconsidered or unmeasured factor (e.g. undiagnosed chronic illness, early cancer, etc.) might be associated with both the outcome CHD with weight loss, was considered. (18) To address this potential source of bias, baseline (1986) values of exposures and covariates were used and the first two years of follow-up (and cases) were excluded. (18) As a result, participants were aged 41 and older at the beginning of follow-up in 1988 in these analyses. Sensitivity analyses were also conducted by restriction to never smokers to assess any influence of residual confounding by smoking.
The assumption of proportional hazards was tested formally by introducing an interaction term for follow-up time by exposure, and assessing its contribution by a likelihood ratio test.
Estimation of the population attributable risk fraction (PARF) in the general US population
The PARF measures the risk reduction in percent that would occur if exposures could be eliminated or reduced to the reference level. It is defined as:
| (1) |
where i refers to the ith of all k levels of exposure, pi to the prevalence of this level of exposure in the target population and RRi to the relative risk versus the reference group (reference BMI category, i = 1), with summations over i = 1 to k = 5 for analyses considering BMI alone, and to k = 25 for analyses considering the joint effects of BMI and comorbid conditions. PARF was estimated after adjustment for age and smoking status using the weighted sum method: (19) Unadjusted relative risks were calculated separately for each subgroup of age (less than 55, 55 to 60, 60 years of age or more) and smoking status, used in equation (1) and summed over subgroups.
The target population was the general US population aged 41 years and above, as estimated by the National Health and Nutrition Surveys (NHANES). For the purpose of this analysis, we pooled the last three NHANES surveys (NHANES 1999–2002, 2001–2002, and 2003–2004). We estimated the prevalence of each of the five BMI categories and the twenty-five joint categories of exposure separately for each subgroup of age and smoking status in NHANES enrollees aged 41 or more, after exclusion of individuals with a BMI < 18.5 or prior history of CHD, stroke, or cancer.
Results
During the 16 years of follow-up from 1988 through 2004, a total of 2,771 cases of incident CHD were confirmed among the 42,351 men and 2,359 cases were confirmed among the 76,703 women. The distributions of selected CHD risk factors by BMI category at baseline are shown in Table 1.
Table 1.
Baseline characteristics, by category of body mass index in 1986, 42,351 men in the Health Professionals Follow-up Study and 76,890 women in the Nurses’ Health Study
| BMI (kg/m2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||||
| 18.5–22.9 (n=7,669) | 23.0–24.9 (n=12,104) | 25.0–26.9 (n=11,466) | 27.0–29.9 (n=7,712) | 30.0+ (n=3,400) | 18.5–22.9 (n=28,038) | 18.5–24.9 (n=16,498) | 25.0–26.9 (n=11,140) | 27.0–29.9 (n=10,391) | 30.0+ (n=10,636) | |
| Age (years) | 53.0 | 53.4 | 54.0 | 54.1 | 53.5 | 53.0 | 54.2 | 54.7 | 54.8 | 54.3 |
| Hypercholesterolemia (%) | 8.9 | 10.2 | 10.6 | 11.6 | 12.1 | 5.0 | 6.2 | 7.4 | 8.8 | 8.1 |
| Hypertension (%) | 12.9 | 16.1 | 19.8 | 26.2 | 35.4 | 7.0 | 10.3 | 14.2 | 19.5 | 27.4 |
| Diabetes (%) | 1.8 | 1.9 | 2.0 | 2.5 | 4.4 | 0.5 | 0.8 | 1.7 | 2.5 | 6.5 |
| Smoking Status | ||||||||||
| Never Smoker (%) | 54.2 | 50.1 | 45.6 | 44.7 | 42.9 | 42.8 | 45.0 | 45.6 | 47.4 | 49.5 |
| Former Smoker (%) | 35.3 | 40.7 | 44.3 | 45.1 | 47.4 | 33.1 | 34.4 | 34.7 | 34.7 | 35.9 |
| Current Smoker (%) | 10.5 | 9.2 | 10.1 | 10.2 | 9.7 | 24.1 | 20.6 | 19.7 | 17.9 | 14.6 |
Compared to men with BMI 18.5 to 22.9 kg/m2, men with a BMI 23.0 to 24.9 kg/m2 had an age-adjusted relative risk (RR) of CHD of 1.11 (95 percent confidence interval (CI) 0.97–1.26) (Table 2). The age-adjusted RR increased further with excess weight, to 2.28 (95% CI 1.96–2.66) for BMI of ≥ 30.0 kg/m2. After adjustment for potential confounding factors, the multivariate adjusted RRs were only modestly attenuated.
Table 2.
Relative Risk of CHD, by category of body mass index among men, the Health Professionals Follow-up Study*, and women, the Nurses’ Health Study#
| BMI | Cases | Person- Years | Rate (105 P-Y)−1 | Age adjusted RR (95% CI) | Multivariate RR* (95% CI) | Cases | Person- Years | Rate (105 P-Y) −1 | Age adjusted RR (95% CI) | Multivariate RR# (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||||
| 18.5–22.9 | 349 | 104,289 | 335 | 1.00 (Ref) | 1.00 (Ref) | 588 | 431,982 | 136 | 1.00 (Ref) | 1.00 (Ref) |
| 23.0–24.9 | 625 | 165,202 | 378 | 1.11 (0.97–1.26) | 1.13 (0.99–1.29) | 426 | 252,844 | 168 | 1.10 (0.97–1.25) | 1.14 (1.00–1.29) |
| 25.0–26.9 | 821 | 155,919 | 527 | 1.50 (1.32–1.70) | 1.47 (1.30–1.67) | 362 | 169,351 | 214 | 1.36 (1.19–1.55) | 1.41 (1.24–1.61) |
| 27.0–29.9 | 643 | 103,836 | 619 | 1.80 (1.58–2.05) | 1.75 (1.53–2.00) | 413 | 156,534 | 264 | 1.68 (1.47–1.90) | 1.71 (1.51–1.95) |
| 30.0+ | 333 | 45,571 | 731 | 2.28 (1.96–2.66) | 2.13 (1.82–2.48) | 570 | 158,618 | 359 | 2.44 (2.17–2.74) | 2.48 (2.20–2.80) |
Adjusted for age, family history of myocardial infarction, smoking, height, marital status, profession, intake of alcohol, saturated fat, polyunsaturated fat, trans fat, folate, vitamin E, and total energy.
Adjusted for age, family history of myocardial infarction, smoking, height, marital status, hormone replacement therapy, intake of alcohol, saturated fat, polyunsaturated fat, trans fat, folate, vitamin E, and total energy.
Among women, we used the same BMI category cutoffs and referent group. Women with a BMI 23.0 to 24.9 kg/m2 had an age-adjusted RR of CHD of 1.10 (95% CI 0.97–1.25). The age-adjusted RR increased with excess weight to 2.44 (95% CI 2.17–2.74) with BMI of ≥ 30.0 kg/m2. After adjustment for potential confounding factors, multivariate adjusted RRs were slightly stronger.
In order to assess the effect of updating BMI and covariates on the effect estimates, we re-ran the multivariate models in each cohort using BMI updated with each (2 year) period of follow-up, though still lagged by 2 years. Compared to the estimates derived from our primary approach (in Table 2), the RRs in men were slightly attenuated, with the RR associated with BMI 23.0 to 24.9 of 1.04 (95% CI 0.91–1.18), BMI 25.0 to 26.9 of 1.37 (95% CI 1.21–1.55), with BMI 27.0 to 29.9 kg/m2 RR of 1.56 (95% CI 1.37–1.77) and with BMI of ≥ 30.0 kg/m2 RR of 1.77 (95% CI 1.53–2.05). A similar pattern of modest attenuation was found in women, in models containing updated BMI and covariates, with RR associated with BMI 23.0 to 24.9 of 1.14 (95% CI 0.99–1.30), with BMI 25.0 to 26.9 of 1.34 (95% CI 1.16–1.54), with BMI 27.0 to 29.9 kg/m2 RR of 1.53 (95% CI 1.34–1.75) and with BMI of ≥ 30.0 kg/m2 RR of 2.09 (95% CI 1.85–2.37).
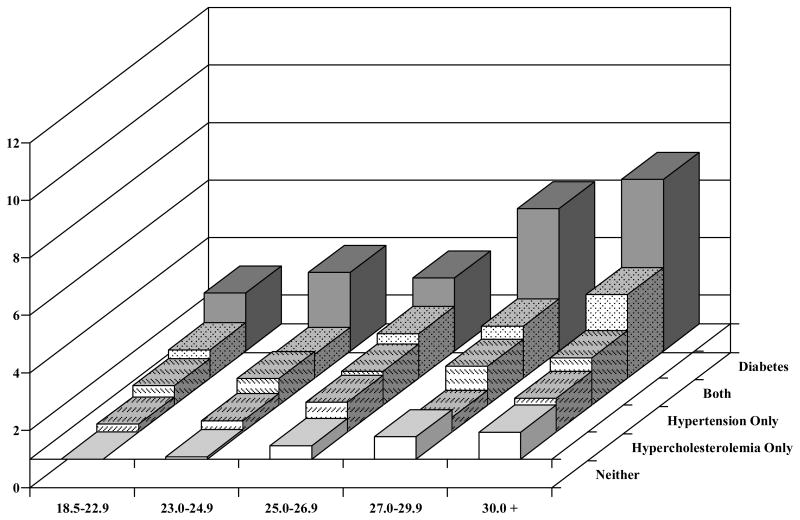
Figure 1 shows the RRs of CHD by BMI category across comorbid conditions, adjusted for potential confounding factors, among the men. The common reference category for all comparisons is men with a BMI of 18.5 to 22.9 kg/m2 and no associated comorbid conditions. Among men without either hypercholesterolemia, hypertension, or diabetes, we found a RR of CHD of 1.08 (95%CI 0.92–1.28) among those with a BMI of 23.0 to 24.9 kg/m2 and a RR 1.94 (95% CI 1.57–2.40) among those with BMI 30 kg/m2 and above. For men with hypercholesterolemia only, the RR was 1.28 (95% CI 0.85–1.91) among those with a BMI of 23.0 to 24.9 kg/m2 and 2.17 (95% CI 1.23–3.83) among those with BMI 30 kg/m2 and above.
Figure 1.
Relative Risk of CHD by BMI, hypercholesterolemia, hypertension, both, or diabetes, the Health Professionals Follow-up Study
* Adjusted for age, family history of MI, smoking, height, marital status, profession, intake of alcohol, saturated fat, polyunsaturated fat, trans fat, folate, vitamin E, and total energy.
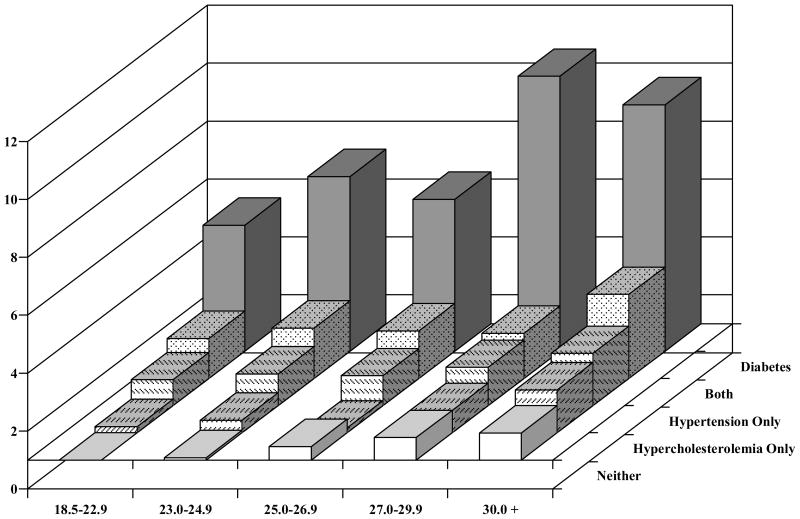
Figure 2 shows the corresponding relative risks among the women. For those without either hypercholesterolemia, hypertension, or diabetes, we found a RR of 1.08 (95% CI 0.93–1.26) for women with a BMI 23.0 to 24.9 kg/m2, and among those with BMI 30 kg/m2 and above, a RR of 2.12 (95% CI 1.81–2.47). Among those women with both hypercholesterolemia and hypertension, but not diabetes, the RR was 2.37 (95% CI 1.43–3.94) for BMI 23.0 to 24.9 kg/m2 and 3.90 (95% CI 2.66–5.71) for BMI of ≥ 30.0 kg/m2. Among women with diabetes, the RRs were 5.35 (95% CI 3.25–8.80) for BMI 23.0 to 24.9 kg/m2 and 9.51 (95% CI 7.72–11.72) for BMI of ≥ 30.0 kg/m2.
Figure 2.
Relative Risk of CHD by BMI, hypercholesterolemia, hypertension, both, or diabetes, the Nurses Health Study
* Adjusted for age, family history of MI, smoking, height, marital status, profession, intake of alcohol, saturated fat, polyunsaturated fat, trans fat, folate, vitamin E, and total energy.
To evaluate the incremental risk of CHD associated with each level of excess weight within the subgroups of men and women with comorbid health conditions we ran multivariate models in each subgroup separately, with results presented in Table 3 (men) and Table 4 (women). We found a positive trend associated with increasing BMI among the men without these health conditions as well as among men with hypertension alone, with both hypercholesterolemia and hypertension, and with diabetes. The tests for trend were not significant for men with hypercholesterolemia only. Among the women, we found a significant positive trend of increasing CHD risk with increasing BMI in women without comorbid conditions, with hypercholesterolemia alone and with hypertension alone, as shown in Table 4. Trend tests were not significant in subgroups of women with both hypercholesterolemia and hypertension in combination and in women with diabetes.
Table 3.
Relative Risk of CHD, by category of body mass index and associated comorbid conditions among men, the Health Professionals Follow-up Study*
| BMI | None (Cases = 1571) | Hypercholesterolemia Only (Cases = 186) | Hypertension Only (Cases = 643) | Hypercholesterolemia and Hypertension (Cases = 168) | Diabetes (Cases = 203) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate RR* (95% CI) | ||||||||||
| 18.5–22.9 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| 23.0–24.9 | 1.07 (0.90–1.26) | 0.99 (0.55–1.79) | 1.28 (0.92–1.77) | 0.79 (0.32–1.96) | 1.08 (0.49–2.41) | |||||
| 25.0–26.9 | 1.44 (1.22–1.70) | 1.61 (0.89–2.91) | 1.40 (1.02–1.92) | 0.76 (0.33–1.78) | 0.83 (0.38–1.86) | |||||
| 27.0–29.9 | 1.76 (1.48–2.10) | 1.04 (0.52–2.06) | 1.58 (1.14–2.17) | 0.96 (0.40–2.30) | 1.72 (0.75–3.92) | |||||
| 30.0+ | 1.95 (1.57–2.42) | 1.84 (0.79–4.26) | 1.60 (1.12–2.28) | 1.86 (0.74–4.71) | 2.75 (1.23–6.14) | |||||
| P Trend | <0.0001 | 0.16 | 0.0049 | 0.045 | 0.0021 | |||||
Adjusted for age, family history of myocardial infarction, smoking, height, marital status, profession, intake of alcohol, saturated fat, polyunsaturated fat, trans fat, folate, vitamin E, and total energy.
Table 4.
Relative Risk of CHD, by category of body mass index and associated comorbid conditions among women, the Nurses’ Health Study#
| BMI | None (Cases = 1507) | Hypercholesterolemia Only (Cases = 109) | Hypertension Only (Cases = 397) | Hypercholesterolemia and Hypertension (Cases = 104) | Diabetes (Cases = 242) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate RR* (95% CI) | ||||||||||
| 18.5–22.9 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |||||
| 23.0–24.9 | 1.08 (0.93–1.26) | 1.13 (0.60–2.14) | 1.10 (0.78–1.57) | 0.83 (0.34–1.99) | 1.38 (0.61–3.12) | |||||
| 25.0–26.9 | 1.41 (1.21–1.65) | 0.69 (0.34–1.40) | 1.10 (0.76–1.59) | 1.26 (0.52–3.07) | 1.10 (0.51–2.39) | |||||
| 27.0–29.9 | 1.54 (1.31–1.81) | 1.34 (0.68–2.63) | 1.24 (0.88–1.75) | 0.83 (0.36–1.92) | 1.74 (0.88–3.44) | |||||
| 30.0+ | 2.14 (1.83–2.50) | 2.09 (1.05–4.15) | 1.48 (1.08–2.05) | 1.02 (0.45–2.30) | 1.42 (0.75–2.69) | |||||
| P Trend | <0.0001 | 0.044 | 0.008 | 0.97 | 0.44 | |||||
Adjusted for age, family history of myocardial infarction, smoking, height, marital status, hormone replacement therapy, intake of alcohol, saturated fat, polyunsaturated fat, trans fat, folate, vitamin E, and total energy.
To address possible residual confounding by cigarette smoking, we conducted sub-analyses restricted to those men and women who had never smoked. Among men, with respect to reference BMI of 18.5 to 22.9 kg/m2, the RRs were for each category very similar to those of the full cohort, with a RR of 1.21 (95% CI 0.98–1.49 for BMI 23.0 to 24.9 kg/m2, 1.51 (95% CI 1.23–1.86) for BMI 25.0 to 26.9 kg/m2, 2.01 (95% CI 1.62–2.49) for BMI 27.0 to 29.9 kg/m2, and 2.33 (95% CI 1.81–3.01) for BMI > 30 kg/m2. In analyses restricted to women who had never smoked, the RRs for each BMI category were somewhat stronger than those derived from the full cohort, 1.48 (95% CI 1.16–1.88) for BMI 23.0 to 24.9 kg/m2, 1.80 (95% CI 1.41–2.31) for BMI 25.0 to 26.9 kg/m2, 2.30 (95%CI 1.81–2.90) for BMI 27.0 to 29.9 kg/m2, and 3.26 (95% CI 2.60–4.07) for BMI > 30 kg/m2.
The estimates of PARF for participants with diabetes necessarily included any additional effects of hypercholesterolemia and/or hypertension as well, since individuals with diabetes were all categorized as such, regardless of the presence or absences of these other comorbid conditions. In sub-analyses where the category of diabetes was split between those with or without an associated comorbid condition, we did not find meaningful modification of the BMI-CHD effect estimates (data not shown).
Simpler, more parsimonious models of CHD risk by BMI category and comorbid conditions were developed, with covariate terms adjusting for age and smoking only. This step was necessitated by the lack of directly comparable covariate information in the NHANES data. Comparisons of estimates from these models to those from the full multivariate models showed acceptably minimal (<10%) changes in RRs in all categories in both men and women. Accordingly, the RRs derived from these simpler models in the HPFS and NHS were applied to prevalence estimates of the joint distributions of the same categories of age, smoking, BMI, and comorbid conditions, in the US population to produce PARF estimates.
In PARF analyses based on BMI alone (without consideration of presence or absence of hypercholesterolemia, hypertension, or diabetes), adjusting for age and smoking, the reference group was participants in the BMI category 18.5 to 22.9 kg/m2. Among men, the PARF associated with higher BMI categories using this reference was 38.7%. Among women, the PARF was 43.5%.
PARF analyses were also conducted including the full affects of the associated comorbid conditions of hypercholesterolemia, hypertension, and diabetes, with the reference group in this case being participants in the BMI category of 18.5 to 22.9 kg/m2 and without any of the associated comorbidities. The PARF for men in these joint effects analyses was 60.4%, while that for women was 64.7%.
Discussion
These analyses provide further evidence, to be interpreted with care, that the risk of CHD is strongly associated with excess weight, both alone and in combination with comormidities. Our relative risk estimates per BMI increment are generally consistent or slightly higher than those previously published. (20,21) We observed a significant risk increase with mild overweight (BMI between 25.0 and 26.9 kg/m2) in both men and women. In the traditionally considered healthy range BMI category of 23.0 and 24.9 kg/m2, we found a trend towards increased CHD risk in men and a significantly increased CHD risk in women. The results are also quite clinically relevant, as the differences in risk between normal weight and excess weight fall in the range of the differences observed between intervention and control groups in clinical trials of established therapies for primary prevention of CHD. (22,23)
We report the risk of incident CHD associated with excess weight, both alone and in combination with comorbid conditions, in men and women, using data from two large, prospective cohorts of men and women. The present study has several major strengths, which include its prospective design, the large size of the study population, the large number of incident cases of fatal CHD and non-fatal MI among men and women without known CHD at baseline, the long term follow-up, and validated repeated measurement of important exposures and covariates. The HPFS and NHS cohort designs are particularly well suited for addressing concerns surrounding possible reverse causation (18) -- the onset of preclinical or non-cardiac disease (that increases near term risk of CHD event) which may in turn affect BMI. By lagging exposure and covariates at baseline and excluding incident cases in the first two years of follow-up, the impact on effect estimates of sub-clinical disease associated with weight change should be minimized. In addition, the relatively homogenous nature of these cohorts of health professionals reduces potential confounding factors, particularly those commonly associated with socioeconomic status or access to medical care.
We found that excess weight in combination with hypercholesterolemia, hypertension, both hypercholesterolemia and hypertension, or diabetes was strongly and independently associated with an increased risk of incident CHD. Despite the known impact of body weight on intermediary comorbid conditions, we also found that men and women free of these conditions at baseline had higher risk of CHD with increased body weight. These results suggest that the effect of higher body weight on CHD may be very complex and involve biological mechanisms other than blood pressure, cholesterol, and diabetes. Trends towards increased risk in both men and women without intermediary conditions were found with a BMI as low as 23.0 to 24.9 kg/m2.
Estimation of the PARFs is a way to quantify the burden associated with the obesity epidemic in this country. (24) We estimated that more than one third (38.7% in men, 42.7% in women) of the incident CHD occurring in U.S. residents aged 41 years and above can be attributed to BMI of 23.0 and above. Interpretation of these PARF results requires an assumption of causality and is in reference to the counterfactual population in which all individuals have BMI of 18.5 to 22.9 kg/m2. This PARF estimation might be expected to underestimate the PARF, due to any misclassification of excess weight within BMI categories. In the joint effects PARF analyses, we estimated that well over half (60.9% in men, 64.5% in women) can be attributed to BMI of 23.0 and above in combination with associated comorbid conditions of hypercholesterolemia, hypertension, and/or diabetes. Interpretation of these joint effects PARF estimates is in reference to BMI 18.5 to 22.9 kg/m2 and no associated comorbid conditions. With this reference, the PARF estimation might be expected to overestimate the PARF, since it reflects the combined effects of excess weight and each of the associated comorbidities.
Our findings of increased risk associated with excess weight and comorbid conditions are of some relevance to components of the metabolic syndrome or cardio-metabolic risk (25) which combines excess weight, in particular abdominal obesity, and diabetes (or impaired glucose metabolism), though we did not study metabolic syndrome itself. The substantial CHD morbidity associated with excess weight in both men and women is consistent with published data. (26) The findings need to be interpreted cautiously as the definitions for excess weight (BMI categorization) are arbitrary, and the way in which intermediate comorbid conditions are considered in the analyses determines appropriate interpretation.
While several previous prospective studies have reported an association between excess weight and incident CHD, few have had the large numbers of study subjects and incident cases across the range of BMI and comorbid medical conditions, well validated measurement of excess weight and important covariates, and the long term follow-up of the Health Professionals Follow-up Study and the Nurses’ Health Study. These features allow the current study to report risk estimates of excess weight, alone and in combination with associated intermediary comorbid conditions in both men and women, with satisfactory precision.
There is evidence (4,6,7) that the effect of excess weight on risk of CHD may be partly mediated through the comorbid conditions examined in these analyses. Indeed, when the subgroup analyses were restricted to those men or women with one of the intermediary conditions, the association between BMI and CHD was modestly attenuated, compared with the results from the full cohorts, consistent with the effect of obesity being at least partly mediated through these intermediary conditions. Hypertension, hypercholesterolemia, and diabetes may mark steps in pathways by which excess weight influences CHD risk. The restriction of analyses to participants with these comorbid conditions has the effect of conditioning on potential intermediate factors in a prospective relationship between excess weight and risk of CHD. Despite this expected attenuation and the limited number of cases in some strata, tests for positive trend were significant in many of the subgroups. Obesity has been found to be a strong independent risk factor for hypertension, (5) hypercholesterolemia, (27) and diabetes, (16) and each is associated with development of CHD. (6,7) While the basic cellular mechanisms linking obesity to CHD have not been fully elucidated, there is some evidence to suggest that cytokines associated with adipocytes and a chronic inflammatory state may play roles. (28,29) Abdominal obesity, especially when measured by computed tomography scan or other more technical measures, is associated with dyslipidemia, hypertension, hypercoaguable states, hyperglycemia and now also adipokine related insulin resistance, (28,30) all factors which left untreated increase cardiovascular disease risk to some extent at all ages.
One might speculate that some of our results suggest that the increased risk of CHD associated with excess weight is modest or absent among those with comorbid conditions. We would caution against drawing such a conclusion, however, as the findings must be carefully considered. As previously discussed, these comorbid conditions may be intermediates, so that analyses restricted to those participants with those intermediates would be expected to show attenuation of main effect estimates. Variability in the severity of each condition may exist across the range of BMI. The relative contributions of excess weight and each comorbid condition to the risk of CHD cannot be easily teased apart. The primary focus of these analyses is in estimating the joint effects of excess weight and these comorbid conditions.
Some potential limitations should be considered with respect to the results of the present study. First, height and body weight were self-reported, as were baseline and updated covariates. Validation studies (30) have documented the accuracy of self-reported exposure measurements in these two cohorts. Second, there remains the possibility of unmeasured or residual confounding. With validated measures of important covariates, and careful consideration of these covariates as potential confounding factors in the analyses, residual confounding should be minimized.
In summary, our results indicate that excess weight carries substantially increased risk of CHD, both alone and in combination with hypercholesterolemia, hypertension, or diabetes, in both men and women, results which should be interpreted carefully. The findings support efforts aimed at prevention of excess weight, particularly prior to the development of associated intermediary conditions. Our results also indicate that a substantial proportion of the incident CHD in both men and women may be attributed to excess weight, though the application of these findings from observational data to estimate causal relationships must be carefully considered. These findings have particular public health importance in light of recent and continuing trends towards greater prevalence of overweight and obesity in the US population as a whole.
Acknowledgments
We thank Stephanie Chiuve for assistance in programming.
Funding/Support
This work was funded by NIH grants HL35464 and CA55075, and by Sanofi-Aventis (AF, HC, ER).
Footnotes
Access to Data
Dr. Eric Rimm had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
There are no conflicts of interest to disclose.
References
- 1.Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S, Bazzarre T. Cardiovascular health in childhood: A statement for health professionals from the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2002;106(1):143–60. doi: 10.1161/01.cir.0000019555.61092.9e. [DOI] [PubMed] [Google Scholar]
- 2.US Dept Health and Human Services. The Surgeon General’s Call to Action to Prevent and Decrease Overweight and Obesity. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [Google Scholar]
- 3.Cassano PA, Rosner B, Vokonas PS, et al. Obesity and body fat distribution in relation to the incidence of non-insulin-dependent diabetes mellitus. Am J Epidemiol. 1992;136:1474–86. doi: 10.1093/oxfordjournals.aje.a116468. [DOI] [PubMed] [Google Scholar]
- 4.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 5.Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P. Relationship of physical activity and body mass index to the risk of hypertension: a prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 6.Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 7.Khot UNKM, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, Ellis SG, Lincoff AM, Topol EJ. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health. Third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): executive summary. Bethesda, MD: National Institutes of Health; 2001. (NIH publ. no. 01-3670) [Google Scholar]
- 10.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American Journal of Epidemiology. 1992;135(10):1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 12.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:4555–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 13.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. American Journal of Epidemiology. 1984;119(5):837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 14.Colditz G, Martin P, Stampfer M. Validation of questionnaire information on risk factors and disease outcomes in a prospective study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 15.Cho E, Manson JE, Stampfer MJ, Solomon CG, Colditz GA, Speizer FE, Willett WC, Hu FB. A prospective study of obesity and risk of coronary heart disease among diabetic women. Diabetes Care. 2002 Jul;25(7):1142–8. doi: 10.2337/diacare.25.7.1142. [DOI] [PubMed] [Google Scholar]
- 16.Manson J, Colditz G, Stampfer M. A prospective study of maturity-onset diabtetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991;151:1141–1147. [PubMed] [Google Scholar]
- 17.Hosmer D, Lemeshow S. Applied Survival Analysis. John Wiley & Sons, Inc; New York: 1999. [Google Scholar]
- 18.Willett WC, Hu FB, Colditz GA, Manson JE. Underweight, overweight, obesity, and excess deaths. JAMA. 2005;294:551. doi: 10.1001/jama.294.5.551-a. [DOI] [PubMed] [Google Scholar]
- 19.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10:195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Wilson PWF, Nam BH, D’Agostino RB. stratification of obesity as a coronary risk factor. Am J Cardiol. 2002 Oct 1;90(7):697–701. doi: 10.1016/s0002-9149(02)02592-4. [DOI] [PubMed] [Google Scholar]
- 21.Hedblad B, Jonsson S, Nilsson P, Engstrom G, Berglund G, Janzon L. Obesity and myocardial infarction--vulnerability related to occupational level and marital status. A 23-year follow-up of an urban male Swedish population. J Intern Med. 2002 Dec;252(6):542–50. doi: 10.1046/j.1365-2796.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- 22.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,356 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 23.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, Graubard BI, Williamson DF. Methods of calculating deaths attributable to obesity. Am J Epidemiol. 2004;160:331–338. doi: 10.1093/aje/kwh222. [DOI] [PubMed] [Google Scholar]
- 25.Aronne LJ. Cardiovascular Risk Reduction: Focus on Managing Cardiometabolic Risk Factors. Obesity (Silver Spring) 2006 Jun;14(suppl_3):119S–120S. [Google Scholar]
- 26.Wilson PWF, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002 Sep 9;162(16):1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 27.Gostynski M, Gutzwiller F, Kuulasmaa K, Doring A, Ferrario M, Grafnetter D, Pajak A. Analysis of the relationship between total cholesterol, age, body mass index among males and females in the WHO MONICA Project. Int J Obes Relat Metab Disord. 2004;28:1082–1090. doi: 10.1038/sj.ijo.0802714. [DOI] [PubMed] [Google Scholar]
- 28.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 29.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–46. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 30.Sundell J. Obesity and diabetes as risk factors for coronary artery disease: from the epidemiological aspect to the initial vascular mechanisms. Diabetes Obes Metab. 2005 Jan;7:9–20. doi: 10.1111/j.1463-1326.2004.00375.x. [DOI] [PubMed] [Google Scholar]