Abstract
Within 2 yr of the arrival of the invasive container mosquito Aedes albopictus (Skuse), the previously dominant invasive mosquito Aedes aegypti (L.) disappeared from many Florida cemeteries. At some cemeteries, however, Ae. aegypti populations seem stable despite Ae. albopictus invasion. We sought to understand this variation in the outcome (exclusion, coexistence) of this invasion, given that previous experiments show that Ae. albopictus is the superior larval competitor. We tested experimentally the hypothesis that climate-dependent egg survivorship differs between exclusion and coexistence cemeteries and that differences in invasion outcome are associated with microclimate. Viability of eggs oviposited in the laboratory and suspended in vases at six cemeteries was significantly greater for Ae. aegypti than for Ae. albopictus, and greater in 2001 than in 2006. Cemeteries differed significantly in egg survivorship of Ae. albopictus, but not of Ae. aegypti, which is consistent with the hypothesis that Ae. albopictus suffers site-specific, climate-driven egg mortality that mitigates the competitive superiority of larval Ae. albopictus. Principal component (PC) analysis of microclimate records from vases during the experiments yielded three PCs accounting for >96% of the variance in both years of experiments. Multivariate analysis of variance of the three PCs revealed significant microclimate differences among the six cemeteries and between exclusion versus coexistence cemeteries. Stepwise logistic regression of egg survivorship versus microclimate PCs yielded significant fits for both species, and twice as much variance explained for Ae. albopictus as for Ae. aegypti in both years. Higher mortalities in 2006 were associated with high average daily maximum temperatures in vases, with lethal thresholds for both species at ≈40°C. From 1990 to 2007, vase occupancy by Ae. albopictus increased and that by Ae. aegypti decreased, with increasing seasonal precipitation at one well-sampled cemetery. Results support the hypothesis that locally variable climate-driven mortality of Ae. albopictus eggs contributes to patterns of exclusion of, or coexistence with, Ae. aegypti.
Keywords: coexistence, extinction, desiccation, eggs, invasive mosquitoes
Understanding the ecology of species invasions may serve both applied and basic research agendas (Sax et al. 2005, 2007). Applied and basic interests converge when invasive disease vectors threaten public health and simultaneously alter distribution and abundance of resident species, leading to new insight about roles of ecological processes, such as competition and predation (Lounibos 2002, Juliano and Lounibos 2005).
One of the most widespread invasive mosquitoes, Aedes aegypti (L.), the primary epidemic vector of dengue and yellow fever, was spread cosmotropically from Africa, probably beginning in the 15th century via shipping (Tabachnick 1991). The more recently dispersed arbovirus vector Aedes albopictus (Skuse) has a broader invasive range than its close relative Ae. aegypti because temperate populations of Ae. albopictus have egg diapause that facilitates invasion of areas with colder climates (Benedict et al. 2007). Owing to their similar life histories, including occupancy of container habitats provided by humans, Ae. aegypti and Ae. albopictus have encountered one another frequently in the tropics, with variable reported outcomes: 1) Ae. aegypti displacing Ae. albopictus in tropical Asian cities (Gilotra et al. 1967); 2) largely allopatric distributions of the two species on Madagascar (Fontenille and Rodhain 1989); and 3) apparent displacement of Ae. aegypti by Ae. albopictus in parts of the southeastern United States (O'Meara et al. 1995, Juliano 1998); and 4) coexistence in some areas of North and South America (Juliano et al. 2004; Braks et al. 2003, 2004).
Of many hypotheses proposed, interspecific larval competition is the most satisfactory mechanism to explain the rapid displacement of Ae. aegypti by Ae. albopictus in much of the southeastern United States (Juliano 1998, Juliano and Lounibos 2005). Interspecific competition is cited as the most common mechanism for population crashes of established invasive species (Simberloff and Gibbons 2004), although exotic competitors rarely cause complete extinction of residents (Davis 2003, Gurevitch and Padilla 2004). Displacement of Ae. aegypti by Ae. albopictus was more pronounced in rural and suburban habitats, which are more marginal for the former species (Juliano 1998). Where these two species currently coexist in their invasive ranges, such as in south Florida and southern Brazil, Ae. aegypti predominates in urban areas and Ae. albopictus in rural sites with more vegetation (Braks et al. 2003, Rey et al. 2006).
One of the major unanswered questions about the invasion ecology of these species is what facilitates coexistence, or the dominance of Ae. aegypti in urban habitats, despite demonstrated superiority of Ae. albopictus in larval competition (Juliano and Lounibos 2005). In the laboratory, the outcome of competition between these species is condition-specific, with Ae. albopictus superior when containers always remain wet, but Ae. aegypti superior when containers dry out regularly (Costanzo et al. 2005). The egg stage, which is characteristically laid out of water by most Aedes spp., differs among species in sensitivity to desiccation and high temperature. Eggs of Ae. aegypti tolerate desiccation and high temperature better than do eggs of Ae. albopictus (Sota and Mogi 1992a, Juliano et al. 2002).
Here, we describe results from the first field tests for differential survivorship of eggs of these species under natural conditions in south Florida. In addition, we relate differential survivorship to container-specific microclimates recorded during exposures of eggs in the field.
Cemeteries, where the immature stages of container mosquitoes may occur in water-holding vases or urns (O'Meara et al. 1992), were used as experimental sites for comparing mortalities of recently laid eggs of Ae. aegypti and Ae. albopictus. Some of these cemeteries had known histories of arrival and establishment of Ae. albopictus in south Florida (O'Meara et al. 1992, 1993) and subsequent reductions in range and abundance of Ae. aegypti (O'Meara et al. 1995). To set the stage for experiments, we first report results of mosquito surveillance since 1990 from eight cemeteries (Fig. 1) and analyze the relationship between annual patterns of monthly precipitation and incidence of the two species at one well-sampled site. Experimental sites were selected based on contrasting patterns of relative abundance of the two invasive species among cemeteries (Figs. 2 and 3), which had been used previously to test for site effects on larval competition (Juliano et al. 2004).
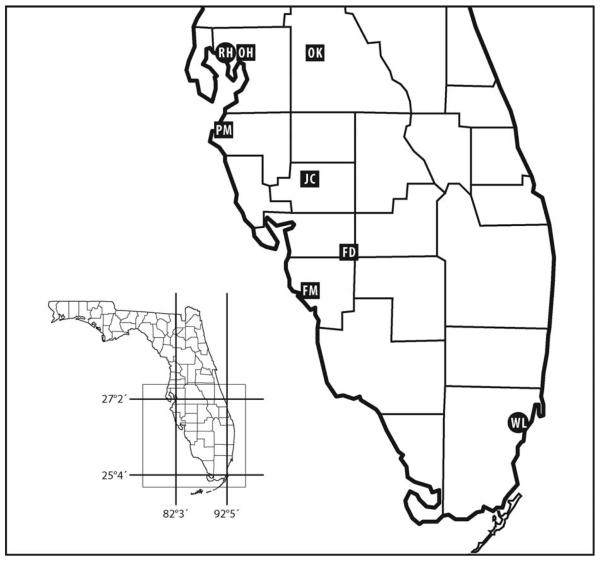
Fig. 1.
Geographic locations in south Florida of eight cemeteries whose urns and vases were sampled from 1990 to 2007 for the aquatic stages of mosquitoes. Six of these sites, distinguished by rectangles, were used for experimental survivorship studies of eggs of Ae. aegypti and Ae. albopictus. FD, Ft. Denaud; FM, Ft. Myers; JC, Joshua Creek; OH, Orange Hill; OK, Oak Hill; PM, Palmetto; RH, Rose Hill; WL, Woodlawn.
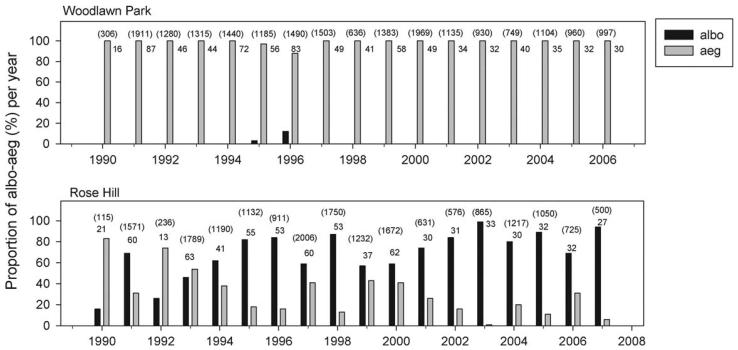
Fig. 2.
Proportional abundances of Ae. albopictus (albo) and Ae. aegypti (aeg) aquatic stages in urns and vases of two south Florida cemeteries sampled annually for 17 consecutive years. Parentheses above bars enclose the total numbers of these two species collected each year, with the numbers of positive vases indicated beneath.
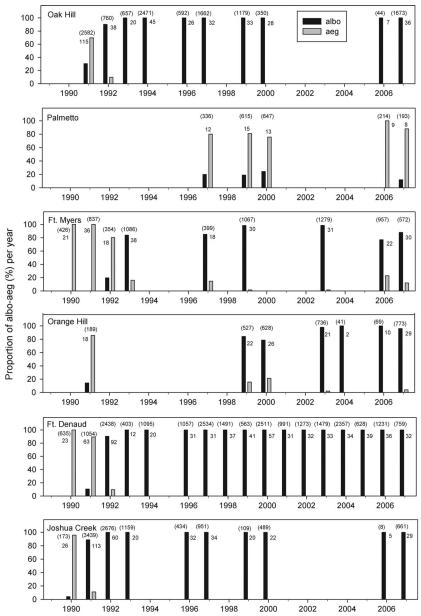
Fig. 3.
Proportional abundances of Ae. albopictus (albo) and Ae. aegypti (aeg) aquatic stages in urns and vases of six south Florida cemeteries sampled between 1990 and 2007, with one or more years absent from annual surveillance. These six, distinguished as aegypti-extinct (Ft. Denaud, Joshua Creek, and Oak Hill) and species coexistence (Ft. Myers, Orange Hill, and Palmetto) were chosen to compare cemetery types in 2001 and 2006 for differential egg survivorship. Numbers above bars as in Fig. 2.
Materials and Methods
Larval Surveillance at Eight Cemeteries
Seven cemeteries located in southwest Florida, and one in the Miami area (Fig. 1) were sampled annually, or less frequently, for the aquatic stages of mosquitoes occurring in vases or urns near gravesites. Cemeteries sampled in 1990–1992 were visited on more than one occasion each year, and results of relative abundances for those years are lumped annually (Figs. 2 and 3). Surveillance from 1993 through 2007 was based on one annual visit, the timing of which varied from year to year. In south Florida, larvae and pupae of container mosquitoes may be recovered in any month (Lounibos and Escher 2008).
The aquatic contents of any vases and urns containing water were extracted with a turkey baster or similar bulb-operated suction device. Aquatic contents >500 ml were placed directly in plastic bottles for transport in coolers to the laboratory. Contents of >500 ml were poured on site into flat plastic or enamel trays, so that larvae and pupae could be selectively removed and pipetted into a bottle with water. Each transport vessel contained mosquito immatures from exhaustive sampling of a single vase or urn.
In the laboratory, third- and fourth-instar larvae were identified to species with dichotomous keys (e.g., Darsie and Ward 2005). Younger larvae were reared in plastic trays at 27°C and a photoperiod of 14:10 (L:D) h to an identifiable stage, including adults which were allowed to eclose from pupae for identifications. Although Ae. aegypti and Ae. albopictus represented the majority of mosquitoes collected at every cemetery, eight other culicid species were identified in samples: Aedes triseriatus (Say), Aedes bahamensis Berlin, Culex nigripalpus Theobald, Culex quinquefasciatus Say, Orthopodomyia signifera (Coquillett), Toxorhynchites rutilus (Coquillett), Wyeomyia mitchellii (Theobald), and Wyeomyia vanduzeei Dyar & Knab. Undetermined Culex spp. and Anopheles spp. also were recognized. In only three cemeteries did any of the aforementioned species represent >10% of total identified Culicidae: Joshua Creek (Ae. triseriatus, 27.2%), Palmetto (Cx. quinquefasciatus, 12.4%), and Woodlawn (Cx. quinquefasciatus, 14.8%).
Sampling was conducted at Rose Hill Cemetery, Tampa, annually from 1990 to 2007, so that for this cemetery we were able to test whether monthly precipitation for each year could predict the annual frequency of vases occupied by each species. Monthly precipitation data were obtained from the South East Regional Climate Center (SERCC; http://www.sercc.com/cgi-bin/sercc/cliMAIN.pl?fl8788) for 1990–2007 and converted to metric units. For all samples within a year, regardless of when they occurred, we summed the total number of Aedes-positive vases, and the number of vases positive for each species. Note that a single vase can be positive for one or both species; hence, frequencies for the two species constitute two separate variables.
Egg Survivorship in Six Cemeteries
In springs 2001 and 2006, eggs of both Ae. albopictus and Ae. aegypti laid in the laboratory were exposed to ambient conditions for two- and four-week periods in six of the eight cemeteries (Fig. 1) in standard, green, plastic cemetery vases of 1.0-liter capacity. The April–May exposure period of 2001 was cooler and drier than the 2006 May–June exposure period at the same sites (Table 1). Long-term records show that spring is usually dry in south Florida, with the onset of rains typically occurring in June (Juliano et al. 2004).
Table 1.
Means of average daily temperatures and total precipitation at three airports during periods of egg exposure at nearby cemeteries
| 2001: 12 April–17 May |
2006: 1 May–2 June |
|||
|---|---|---|---|---|
| Mean T (°C) (SE) | Rainfall (cm) | Mean T (°C) (SE) | Rainfall (cm) | |
| Airport (lat., long.) | ||||
| Page Field, Tampa (26° 35′ N, 81° 52′ W) | 22.4 (0.3) | 0.36 | 26.3 (0.3) | 5.44 |
| Linder Regional, Lakeland (27° 59′ N, 82° 01′ W) | 23.0 (0.4) | 0.00 | 24.3 (0.4) | 8.53 |
| Vandenburg, Tampa (28° 01′ N, 82° 21′ W) | 21.9 (0.5) | 1.09 | 24.9 (0.5) | 3.38 |
Recent colonies (F2–F3) of the two species, derived from eggs and larvae field-collected in south Florida, were blood-fed on restrained chickens in cages (University of Florida IACUC approval VB-17). Four days later, female mosquitoes were offered oviposition paddles of either wooden tongue depressors (2001) or Masonite pieces of similar size (2006) partially suspended in tap water in black cups. Depending on the density of eggs laid, paddles were removed from cages 24 or 48 h after exposure, so that the maximal possible age difference of eggs on a paddle was 2 d. Eggs were allowed to embryonate for3din humidified chambers at 27°C before transport to the field. Mean numbers of eggs exposed in the field ranged from 12 to 146 per species per vase in 2001 and 40–517 per species per vase in 2006 (Supplemental Table S1 [online only]).
Rectangular grids were overlaid on aerial images of each cemetery, and numbers were assigned to each rectangle on the grid. Locations for setting each experimental vase were determined by random draws of these numbers, without replacement. Twenty-eight vases were set in each cemetery in 2001 and 24 in 2006, placed, where possible, in the shade of trees or grave stones. Each labeled vase had a drainage hole at its bottom, covered with screen to prevent entry of vagile insects, and insecticide (Permanone) was applied to the vase stem to deter entry of ants. Plastic screen netting (1.0-mm mesh), secured with a UV-resistant cable-tie (3M Brand), was used to cover each vase and to prevent wild mosquitoes from ovipositing.
In 2001, four experimental vases were randomly chosen after placement in the field to harbor a Hobo (model H08-032-08, Onset Corp., Bourne, MA) data logger, set to record temperature and humidity once every 10 min; owing to the size of each Hobo, no space remained in these vases to hold paddles with eggs. In 2006, climatic data were recorded in four vases, randomly selected per cemetery, with Ibuttons (model 1923, Maxim Corp., Sunnyvale CA) which, owing to their smaller size, were glued inside each vase, leaving room for co-occupancy with paddles containing eggs.
One week after vases had been set, eggs on paddles were suspended within by means of spring clips attached to vase walls. One paddle of Ae. aegypti eggs was suspended directly opposite one paddle of Ae. albopictus eggs so that the two species would experience the same vase microclimate. Immediately after paddles were introduced, vases were covered securely with netting, so that only rainfall or fine particulate matter could enter. As it was logistically possible to set only two cemeteries per day with paddles or depressors, the timing of exposure of eggs differed from 0 to 4 d between cemeteries. However, data loggers at each cemetery provided microclimate records precisely coincident with egg exposure periods.
At 2- and 4-wk intervals of egg exposure, 12 vases were randomly selected for removal of their eggs from each cemetery. At times of vase collection, microclimatic data were downloaded on site from each data logger to a laptop computer. Paddles were removed from vases and placed in humidified containers for transport in coolers (≈15°C) to the laboratory. Hoboes and Ibuttons were maintained in situ in their vases for the entire four week durations of experiments.
In the laboratory, each paddle was inspected under a dissecting microscope to remove previously hatched eggs and to count unhatched eggs. (Some premature hatching occurred, particularly in 2006, because the thick Masonite paddles retained sufficient moisture from the laboratory to stimulate hatch shortly after embryonation.) Subsequently, individual paddles were submersed for 24 h in 300 ml of deionized water containing 50 mg of larval food (1:1, yeast:lactalbumin) to stimulate hatching. After 24 h, paddles were removed and slowly dried for three days, then reimmersed in 300 ml of fresh, deionized water with larval food. The sum of larvae produced by these two immersions divided by the total number of embryonated eggs was used to calculate proportion hatching. Embryonated eggs were distinguished from infertile eggs by the bleaching method of Trpis (1970).
Data Analyses
Six microclimate variables were derived from records downloaded from each data logger: mean daily maximum (tmax), minimum (tmin), and average (tmean) temperatures (°C), and mean daily maximum (rhmax), minimum (rhmin), and average (rhmean) relative humidities. To reduce the number of correlated variables, these six microclimate means were subjected to principal components (PCs) analyses with PROC FACTOR of (SAS Institute 1990). PC scores that explained the most variance for both years were used as dependent variables in a multivariate analysis of variance (MANOVA) to test for differences in microclimate between years and cemetery types (n = 2, extinction versus coexistence of Ae aegypti) and among cemeteries (n = 6). The relative importance of the first three PCs for explaining intercemetery microclimate differences was quantified with standardized canonical coefficients (SSCs Scheiner 2001).
Proportions of eggs of the two species that hatched were analyzed by MANOVA using PROC GLM of SAS (SAS Institute 1990) with type, cemetery (type), weeks of exposure (two versus four), and year (2001 versus 2006) as independent variables. Cemeteries in our design do not represent a typical random effect, in the sense that we did not choose them at random from all possible coexistence or exclusion cemeteries. We instead chose them based on previous knowledge of these sites. Thus, we treated cemeteries within types as fixed effects in a MANOVA with the proportion of hatching eggs of each species as separate, dependent variables, and then compared egg survivorship for the different cemeteries.
Interspecific differences in egg survivorship were analyzed by paired t-tests (pairing within vases; PROC TTEST of SAS Institute 1990), for the 2- and 4-wk data (arcsine-transformed proportions) across all cemeteries in both years. Stepwise logistic regression (PROC LOGISTIC, SAS Institute 1990) was used to determine whether microclimate variables, summarized by PC scores, could explain egg survivorship patterns in the six cemeteries. Separate logistic regressions were performed for the 2001 and 2006 data sets.
For the 1990–2007 Rose Hill vase surveillance data, we ran multiple stepwise logistic regression (PROC LOGISTIC, SAS Institute 1990) of frequency of positive vases for each species in each year versus monthly precipitation for January through October of each year. Threshold for a variable to enter or leave the model was P = 0.05. Because precipitation for November and December was missing in some years, and nearly all collections took place before November, we omitted November and December precipitation from this analysis. Running regressions including November and December, but omitting years with missing precipitation data, yielded similar conclusions.
Results
Patterns of Establishment and Exclusion
Of the five cemeteries sampled in 1990 for mosquito immatures, Ae. albopictus was detected, at low relative abundances, only in two northerly locations, RH and JC (Figs. 1-3). In three cemeteries in which Ae. albopictus was first detected in 1991, FD, OH, and OK, container surveillance that year revealed low abundances of this species relative to Ae. aegypti (Fig. 3). In the most southerly cemetery studied, WL, Ae. albopictus was not detected until 1995 (Fig. 2). For the five cemeteries in which surveillance commenced in 1990, there was a significant negative correlation between latitude and year of first detection of Ae. albopictus (r = −0.94, P = 0.019).
In the five cemeteries where Ae. albopictus was first detected in 1990–1992 and which were sampled the following year, a dramatic shift in relative abundances, favoring Ae. albopictus over Ae. aegypti, was observed within one year after establishment (Figs. 2 and 3). In three of these five sites, OK, FD, and JC, Ae. aegypti disappeared from vase samples after 1992 and was never detected again in the subsequent 15 yr (Fig. 3). In three other cemeteries (OH, FM, and RH), Ae. aegypti persisted through 2007 but at low abundances relative to Ae. albopictus, and compared with its numerical dominance in the early 1990s at these same sites (Figs. 2 and 3).
At PM, which was sampled less regularly and only since 1997, Ae. aegypti coexisted in greater abundance relative to Ae. albopictus (Fig. 3), and at WL, an apparent establishment of Ae. albopictus was transient, lasting only 2 yr (Fig. 2).
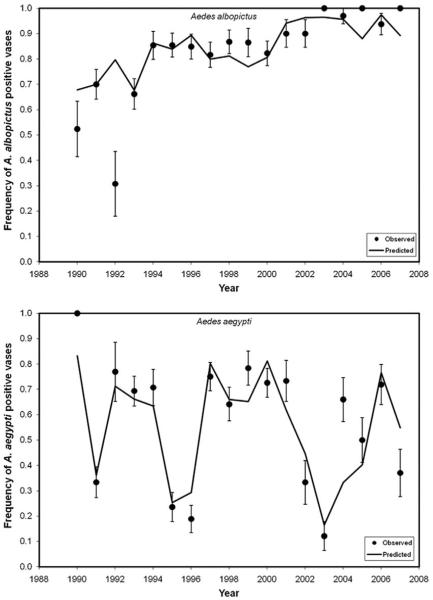
For both species, regressions of frequency of vases occupied at Rose Hill versus annual monthly precipitation for that year were significant and yielded relatively high R2 (Table 2). For both species, precipitation in June was the first variable entered into the model and yielded the strongest individual prediction based on a chi-square test (Table 2). The sign of the regression coefficient for June was opposite for the two species, indicating Ae. albopictus vase occupancy increased, and Ae. aegypti vase occupancy decreased with June precipitation (Table 2). Two of the three months that were retained in the final model for Ae. albopictus had positive coefficients, whereas 5 of 6 mo that were retained in the model for Ae. aegypti had negative coefficients (Table 2), indicating that, in general, Ae. albopictus increased but Ae. aegypti decreased in vase occupancy with increasing precipitation. Ae. albopictus vase occupancy depended most on precipitation in summer and early autumn, whereas vase occupancy of Ae. aegypti depended most on precipitation in summer, spring, and late winter (Table 2). Frequency of Ae. albopictus vases increased over time and was high in most years (Fig. 4). In contrast, frequency of Ae. aegypti showed frequent and precipitous drops and recoveries that were generally well predicted by precipitation data for that year (Fig. 4).
Table 2.
Multiple logistic regression for frequency of vases occupiedby each speciesatRose Hillinayear (1990–2007) versus annual monthly mean precipitation for that yeara
| Precipitation variable |
df | Regression parameter |
SE | χ2 | P |
|---|---|---|---|---|---|
| Ae. albopictus: R2 = 0.7731 | |||||
| Intercept | 1 | −0.5594 | 0.3981 | 1.9747 | 0.1600 |
| June | 1 | 0.00973 | 0.00164 | 35.3396 | <0.0001 |
| Sept. | 1 | 0.00671 | 0.00153 | 19.1546 | <0.0001 |
| Oct. | 1 | −0.00570 | 0.00259 | 4.8401 | 0.0278 |
| Ae. aegypti: R2 = 0.9779 | |||||
| Intercept | 1 | 3.2240 | 0.3252 | 98.2676 | <0.0001 |
| June | 1 | −0.00636 | 0.00112 | 32.4146 | <0.0001 |
| May | 1 | −0.00622 | 0.00210 | 8.7993 | 0.0030 |
| Jan. | 1 | −0.00635 | 0.00252 | 6.3565 | 0.0117 |
| Aug. | 1 | −0.00467 | 0.00131 | 12.7440 | 0.0004 |
| March | 1 | −0.00708 | 0.00216 | 10.7040 | 0.0011 |
| Feb. | 1 | 0.00238 | 0.00114 | 4.3421 | 0.0372 |
Predictor variables listed in order of entry to the stepwise model.
Fig. 4.
Plots of annual proportions of vases positive for Ae. albopictus and for Ae. aegypti from Rose Hill Cemetery, Tampa. Points show observed proportion of vases positive (± SE, estimated based on binomial distribution, Sokal and Rohlf 1995). Numbers of Aedes-positive vases sampled given in Fig. 2. Predicted curve is the result of stepwise multiple logistic regressions (statistics in Table 3) of annual vase occupancy for a species versus monthly precipitation (data from the SERCC).
Microclimates and Coexistence Versus Extinction Cemeteries
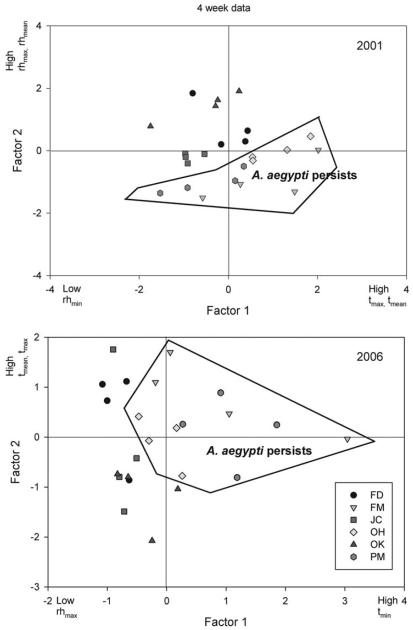
The first three PCs accounted for 96.8–97.8% of the variance in microclimate variables in the separate 2001 and 2006 and combined data sets (Table 3). However, rotated factor scores indicated that different variables had strong loadings on the important PCs in the 2 yr, tmax, tmin, and rhmin contributing most heavily to PC-1 in 2001 and tmin and rhmax contributing most to PC-1 in 2006 (Table 3). PC-1 was positively related to hot, low humidity microclimates, as reflected by the strong positive loadings on tmax, tmean and negative on rhmin in 2001 and the combined data from both years. On average, aegypti-coexistence cemeteries in 2001 had positive scores on PC-1 and negative scores on PC-2, and in 2006 positive scores on both PC-1 and PC-2 (Fig. 5). These aegypti-coexistence cemeteries tended in 2001 to have higher tmax and tmean and lower rhmin and in 2006 higher tmin and lower rhmax (Table 3).
Table 3.
Principal components analyses, showing first three PCs and their rotated factor scores for six microclimate variables in cemetery vasesa
| Principal components analyses |
||||||||
|---|---|---|---|---|---|---|---|---|
| Eigenvalue | Proportion | tmin | tmax | tmean | rhmin | rhmax | rhmean | |
| 2001 | ||||||||
| 1 | 3.296 | 0.549 | 0.096 | 0.975 | 0.929 | −0.942 | 0.200 | −0.476 |
| 2 | 1.951 | 0.325 | −0.321 | 0.012 | −0.038 | 0.262 | 0.846 | 0.860 |
| 3 | 0.603 | 0.101 | 0.937 | −0.009 | 0.322 | 0.136 | −0.473 | −0.140 |
| 2006 | ||||||||
| 1 | 3.373 | 0.562 | 0.975 | −0.485 | 0.067 | 0.574 | −0.859 | −0.125 |
| 2 | 2.022 | 0.337 | −0.091 | 0.822 | 0.953 | 0.646 | 0.091 | 0.503 |
| 3 | 0.414 | 0.069 | 0.077 | −0.213 | −0.238 | 0.466 | 0.473 | 0.845 |
| Yr combined | ||||||||
| 1 | 2.900 | 0.483 | 0.389 | 0.973 | 0.922 | −0.845 | 0.208 | −0.441 |
| 2 | 1.579 | 0.263 | 0.259 | 0.153 | 0.242 | 0.284 | 0.754 | 0.884 |
| 3 | 1.391 | 0.232 | 0.874 | −0.067 | 0.259 | 0.421 | −0.610 | 0.070 |
Values denoted in bold represent strong factor contributions for these PCs.
Fig. 5.
Plots of the first two PCs, which accounted for 87–90% of variances, for the 4-wk microclimate records from 24 vases in six cemeteries in 2001 and 2006. Records from cemeteries in which Ae. aegypti persists are enclosed by lines. Microclimate variables with high factor loadings are indicated on axes for each principal component.
A MANOVA using scores from the first three PCs as independent variables detected significant differences in microclimates among cemeteries and between cemetery types and years (Table 4). SCCs indicated that PC-2 contributed the most to all three significant relationships. Although the interaction between cemetery and year was significant, the interaction between type and year was not, indicating a consistency between years in comparative microcli-mates of coexistence and exclusion cemeteries.
Table 4.
MANOVA results for thefirst three microclimate PCsin six cemeteries, distinguished bytype (coexistence versus extinction)a
| Effect | df (num, den) | Pillai's trace | F | P | PC1 | PC2 | PC3 |
|---|---|---|---|---|---|---|---|
| Cemetery (type) | 12, 108 | 1.084 | 5.09 | <0.001 | 1.61 | 4.61 | 1.36 |
| Type | 3, 34 | 0.772 | 38.33 | <0.001 | 1.86 | 4.64 | 1.23 |
| Yr | 3, 34 | 0.967 | 335.85 | <0.001 | 0.10 | 2.74 | 1.33 |
| Type × yr | 3, 34 | 0.124 | 1.60 | 0.2069 | |||
| Cemetery × yr | 12, 108 | 0.939 | 4.10 | <0.001 |
Most important PCs for each significant effect are denoted by SCCs in bold.
Egg Survivorship
Mean egg survivorship was consistently significantly higher for Ae. aegypti in the same vases with Ae. albopictus (Table 5). MANOVA on the proportions (arcsine-transformed) of Ae. aegypti and Ae. albopictus eggs surviving detected significant effects of weeks, year, cemetery (type), and a marginally significant effect of type (Table 6). Two-way interactions were significant, except for type × year (Table 6), indicating that trends in survivorship between cemetery types were consistent between years of experiments.
Table 5.
Mean ± SE survivorship of Aedes spp. eggs paired in vases and exposed to ambient conditions in six cemeteries in different years
| 2-wk exposure |
4-wk exposure |
|||||
|---|---|---|---|---|---|---|
| Ae. aegypti | Ae. albopictus | ta | Ae. aegypti | Ae. albopictus | ta | |
| 2001 | 0.809 (0.037) | 0.489 (0.042) | 8.27 | 0.720 (0.040) | 0.279 (0.043) | 9.72 |
| 2006 | 0.490 (0.055) | 0.289 (0.048) | 5.23 | 0.310 (0.054) | 0.199 (0.041) | 3.16 |
All paired t-tests significant at P < 0.001 except 4-wk exposure in 2006, for which P = 0.002; df for each test were 69, 70, or 71.
Paired t-tests examine the significance of mean differences between the two species for each week and year condition.
Table 6.
MANOVA results for egg survivorship (proportions of Ae. aegypti and Ae. albopictus hatching) with independent variables type, week, year, and interactions, with cemetery (type) a fixed effect
| Effect | Pillai's trace |
F | df (num, den) |
P |
|---|---|---|---|---|
| Type | 0.02 | 2.92 | 2, 258 | 0.056 |
| Cemetery (type) | 0.17 | 6.03 | 8, 518 | <0.001 |
| Yr | 0.21 | 33.95 | 2, 258 | <0.001 |
| Type × yr | 0.02 | 2.04 | 2, 258 | 0.1320 |
| Cemetery × yr | 0.06 | 2.08 | 8, 518 | 0.036 |
| Week | 0.06 | 8.26 | 2, 258 | <0.001 |
| Type × wk | 0.05 | 6.82 | 2, 258 | 0.001 |
| Cemetery × wk | 0.06 | 2.02 | 8, 518 | 0.042 |
| Yr × wk | 0.03 | 4.64 | 2, 258 | 0.011 |
| Type × yr × wk | 0.01 | 1.02 | 2, 258 | 0.363 |
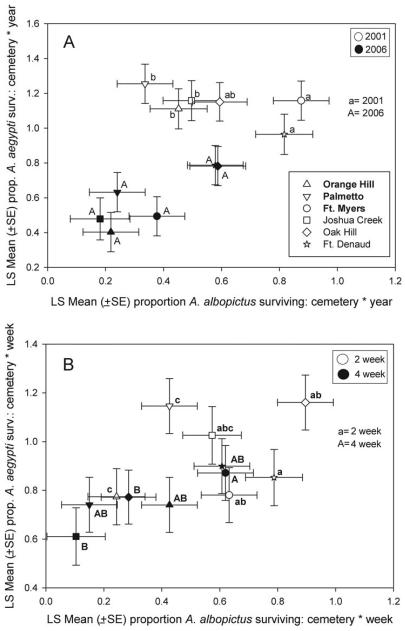
For Ae. aegypti there were no significant differences among cemeteries in mean proportions surviving (pairwise Tukey–Kramer tests, all P > 0.43) (Table 7). For Ae. albopictus, although extinction (lsmean[SE] = 0.54[0.11]) and coexistence (lsmean[SE] = 0.42[0.10]) cemeteries did not differ significantly (P = 0.45), several pairs of sites differed significantly in mean proportions surviving (Table 7). Specifically, at two extinction sites, OK and FD, egg survival of Ae. albopictus was significantly higher than at PM, a coexistence site, and FD had higher survivorship than OH, another coexistence site (Table 7). Bivariate means comparisons derived from the significant interactions of cemetery × year and cemetery × week yielded similar trends. In particular, significant differences between bivariate means in 2001 (Fig. 6A) and for 2- and 4-wk exposure periods (Fig. 6B) were more strongly influenced by intercemetery differences in Ae. albopictus survivorship than that of Ae. aegypti.
Table 7.
Least-square (LS) means of egg survivorship by cemetery (type) for the 2001 and 2006 experimental exposures
| Cemetery | Type | LS meana (arcsine-transformed) proportion surviving |
|
|---|---|---|---|
| Ae. albopictus (SE) | Ae. aegypti (SE) | ||
| Orange Hill | Coexistence | 0.335bc (0.069) | 0.757a (0.081) |
| Palmetto | Coexistence | 0.289b (0.068) | 0.943a (0.080) |
| Ft. Myers | Coexistence | 0.626a (0.068) | 0.826a (0.080) |
| Joshua Creek | Extinction | 0.339bc (0.071) | 0.818a (0.083) |
| Oak Hill | Extinction | 0.590ac (0.067) | 0.966a (0.079) |
| Ft. Denaud | Extinction | 0.698a (0.069) | 0.876a (0.081) |
Significant differences (P < 0.05) in post hoc comparisons by Tukey's tests denoted by absence of a common letter in a column of means.
Fig. 6.
Least-square bivariate means of egg survivorship of Ae. aegypti versus Ae. albopictus for the interactions cemetery × year (A) and cemetery × week (B). Coexistence cemeteries are noted in bold in the legend. Absence of a common letter indicates significant differences (P < 0.05) detected between bivariate means with Bonferroni-type adjustments for number of tests (k = 16 for comparisons by year in [A] and by weeks in [B]).
Linking Microclimate and Egg Survivorship
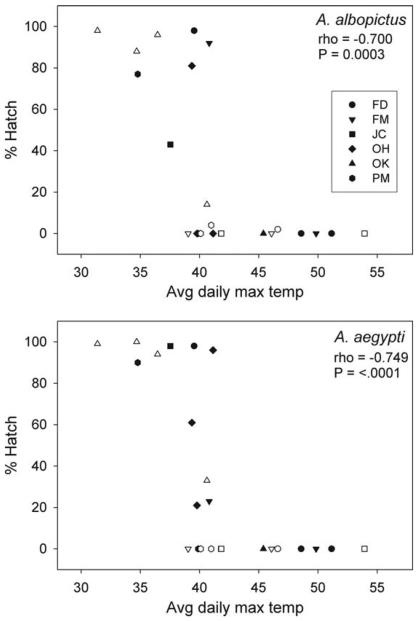
The higher mortality of eggs on paddles in 2006 was associated with higher regional temperatures recorded in that year (Table 1). Owing to the fact that 24 vases with experimental eggs also contained Ibutton data loggers, it was possible to associate egg mortality with microclimates within the same vase. Strong negative correlations were observed for both species between average daily maximum temperatures and egg hatch-ability (arcsine-transformed) (Fig. 7). For both species, high mean tmax values were lethal, killing all eggs on exposed paddles. Eggs of both species suffered complete, or nearly so, mortality in vases maintained for four weeks, all of which recorded daily mean tmax values of >39°C (Fig. 7).
Fig. 7.
Plots of egg survivorship versus mean daily maximum temperatures from Ibutton data loggers located in the same vases as experimental paddles containing mosquito eggs. Open symbols represent two-week and filled symbols are four-week exposures. There are no 4-wk records from OH because all paddles, chosen randomly for removal, in Ibutton vases at this cemetery were removed after 2 wk. Rho is Spearman's rank-order coefficient from correlations performed on arcsine-transformed proportions hatching.
Stepwise logistic regressions were performed, separately for each species, to relate two-week egg survivorship with microclimate variables, condensed by separate PCA analyses for 2001 and 2006. In 2001, all three PCs contributed to the model, although their order of entry differed between Ae. albopictus and Ae. aegypti (Table 8). The model for Ae. aegypti explained >12% of the variance in egg survivorship, but the model for Ae. albopictus explained >20% (Table 8).
Table 8.
Stepwise logistic regression results for 2-wk egg survivorship versus microclimate PCs from six cemeteries in south Florida
| Source | Step | Estimate (SE) | χ2 | P | Adjusted R2 |
|---|---|---|---|---|---|
| 2001: Ae. albopictus (n = 69) | |||||
| Intercept | 0.21 (0.03) | 703.78 | <0.0001 | ||
| PC2 | 1 | 1.11 (0.05) | 274.57 | <0.0001 | 0.079 |
| PC3 | 2 | 1.45 (0.07) | 201.97 | <0.0001 | 0.136 |
| PC1 | 3 | −1.04 (0.07) | 245.81 | <0.001 | 0.201 |
| 2001: Ae. aegypti (n = 68) | |||||
| Intercept | 2.24 (0.05) | 491.52 | <0.0001 | ||
| PC1 | 1 | −1.41 (0.07) | 305.13 | <0.0001 | 0.078 |
| PC3 | 2 | 1.18 (0.11) | 131.42 | <0.0001 | 0.117 |
| PC2 | 3 | 0.13 (0.05) | 6.78 | 0.009 | 0.119 |
| 2006: Ae. albopictus (n = 71) | |||||
| Intercept | −0.55 (0.02) | 870.69 | <0.0001 | ||
| PC2 | 1 | 0.54 (0.02) | 592.53 | <0.0001 | 0.041 |
| PC3 | 2 | 0.32 (0.02) | 257.06 | <0.0001 | 0.058 |
| 2006: Ae. aegypti (n = 70) | |||||
| Intercept | 628.63 | <0.0001 | |||
| PC3 | 1 | 0.44 (0.02) | 439.39 | <0.0001 | 0.023 |
| PC2 | 2 | 0.24 (0.02) | 178.55 | <0.0001 | 0.033 |
| PC1 | 3 | −0.15 (0.03) | 24.42 | <0.001 | 0.035 |
As expected, the significant stepwise regression models for 2006 explained less variance than those for 2001, and one PC did not contribute significantly to the model for Ae. albopictus (Table 8). As observed for 2001, microclimate variables for 2006 explained more variance in egg hatch of Ae. albopictus than Ae. aegypti (Table 8).
Discussion
The significant negative correlation between latitude and date of first detection of Ae. albopictus corroborates the north to south spread of this invasive species throughout Florida (O'Meara et al. 1993). After the initial impacts of Ae. albopictus during the acute stage (Strayer et al. 2006) of its invasions, the eight cemeteries surveyed retained consistent patterns of mosquito relative abundance in subsequent years (Figs. 2 and 3). Although it may seem that proportional abundances of Ae. aegypti and Ae. albopictus are currently at equilibrium at these sites, long-term studies of invasive species effects indicate that responses of resident fauna may change after many years (Strayer et al. 2006). During the current chronic phase of the Ae. albopictus invasion in the southeast, ecological and evolutionary processes that could modulate its effects include changes in 1) the abiotic environment (e.g., warming), 2) the biological community (e.g., addition of other invasive species into the container community), or 3) the invader itself (e.g., through natural selection). Climate-change induced hotter and drier conditions in south Florida could, based on the current and other studies (Juliano et al. 2002, Costanzo et al. 2005), favor a recrudescence of Ae. aegypti relative to Ae. albopictus.
We previously demonstrated that the impact of larval competition, believed to account for the reductions in range and abundance of Ae. aegypti during the acute phase of the Ae. albopictus invasion of the southeast (Juliano 1998), was consistent across cemeteries (Juliano et al. 2004) and, therefore, variation in the aquatic environments is unlikely to account for variation of outcome of invasion among cemeteries (Figs. 2 and 3). The rapidity and apparent permanence of the asymmetric effects of larval competition may be related to the relative simplicity of the aquatic community in cemetery vases and urns, where predators and other macrofauna, beside these two Aedes spp., are uncommon. In the more diverse aquatic communities of shaded tires or treeholes occupied by Ae. albopictus, the superior competitive ability of this invasive species is mitigated by selective predation that favors its coexistence with an inferior competitor, the native treehole mosquito Ae. triseriatus (Griswold and Lounibos 2005, 2006; Juliano and Lounibos 2005).
Previous geographic surveys (Fontenille and Rodhain 1989, Leisnham and Juliano 2009) and laboratory experiments (Sota and Mogi 1992a, Juliano et al. 2002, Costanzo et al. 2005) have suggested an important role of climate in determining distribution and abundance where the ranges of the two species overlap. Ours is the first field test of differential viability of a nonaquatic stage of the two species exposed to natural environments in their invasive range. Although superior survivorship of the Ae. aegypti egg was clearly evident, cemetery-specific effects did not unequivocally support our hypothesis, in part because of low statistical power to demonstrate an effect of cemetery type (coexistence versus extinction). Significant differences were demonstrated in Ae. albopictus mortality among cemeteries, albeit not consistently in the hypothesized direction of coexistence > extinction. The lack of differences in Ae. aegypti survival among cemeteries, and the relatively small amount of variance explained by microclimate in logistic regression models (Table 8) are consistent with previous observations indicating that the egg stage of this earlier invader is much less sensitive to climate.
Annual differences in monthly precipitation provided strong prediction of frequencies of the two species, and supported the expectation that wetter conditions favor Ae. albopictus (regression coefficients primarily >0) and drier conditions favor Ae. aegypti (regression coefficients primarily <0). This result is consistent with the hypothesis that differential death by desiccation of eggs plays a prominent role in determining interyear, within-site variation in abundances of these species. This result coupled with mechanistic studies of desiccation tolerance of eggs (Sota and Mogi 1992a, Juliano et al. 2002), laboratory competition experiments manipulating drying regimes (Costanzo et al. 2005), previous investigations of distribution and abundance (Fontinelle and Rodhain 1989, Juliano et al. 2002) and differences in humidity and temperature among sites (this study), provide convincing circumstantial evidence that drying of containers is a prominent determinant of the distribution and abundance of these species, and that the different responses of the two species to this environmental variable may contribute to stable coexistence of these competitors in a fluctuating environment (Chesson 2000).
Drought resistance of the Ae. aegypti egg has been long known (Christophers 1960) and was probably selected for in the course of domestication of the anthropophilic subspecies that may have acquired such traits in North Africa before its widespread dissemination, chiefly by shipping (Tabachnick 1991). Desiccation resistance has been shown in the laboratory to be a heritable trait in eggs of Ae. albopictus, leaving open the potential of this species also to adapt to drier environments (Sota 1993).
When exposed to high temperatures, eggs of both species were vulnerable to the high average daily maxima observed in some vases during the 2006 experiment (Fig. 7). Thermal death at ≈40°C confirms old laboratory studies that showed the survivability transition of Ae. aegypti eggs was between 39 and 42°C (Bacot 1916). These unexpectedly high average daily maximum temperatures (Fig. 7) observed in randomly placed vases suggest that there should be strong selection on ovipositing females to lay eggs in cool and shady sites.
It should be noted that the exposed eggs used in this study were not in diapause, which is unknown in Ae. aegypti (Christophers 1960) but confers enhanced survivorship on temperate Ae. albopictus eggs exposed to humidity stress (Sota and Mogi 1992b). Although egg diapause has been identified in south Florida populations of Ae. albopictus (Lounibos et al. 2003), this dormant stage is observed in nature only during December and January in south Florida (M.H.R., unpublished) and would not be naturally observed during the springtime periods of our experiments.
It was probably an oversimplification of our initial hypothesis to lump the three coexistence cemeteries as one “type.” In two of the three, FM and OH, the abundance of Ae. aegypti relative to Ae. albopictus has been persistently low since the acute stage of the Ae. albopictus invasion (Fig. 3). Although Ae. aegypti is also relatively rare at RH, near OH (Figs. 1 and 2), our analysis also shows that frequencies of both species fluctuate from year to year, and are strongly related to precipitation in different months (Table 2; Fig. 4.). By contrast, PM perennially had far more Ae. aegypti than Ae. albopictus (Fig. 3), but was lumped into the same coexistence category as FM and OH.
Previous studies on oviposition by these two species in south Florida showed that Ae. albopictus was positively associated with vegetation and Ae. aegypti with urban landscape features, such as pavement (Rey et al. 2006). Although landscape features at our cemeteries have not been quantified, sites where Ae. albopictus excluded Ae. aegypti (FD, OK, and JC) are more rural and vegetated, whereas those where Ae. aegypti persists (FM, OH, and RH) or dominates (WL) are suburban or urban, with less vegetation and more pavement. These latter cemeteries also place Ae. aegypti in closer proximity to humans, known as the preferred bloodmeal host of many populations of its domestic subspecies (Christophers 1960). Adult Ae aegypti also survive longer than adult Ae albopictus in dry conditions (Mogi et al. 1996, Reiskind and Lounibos 2009). Thus, superior survival in both the adult and egg stages probably contributes to the coexistence of Ae aegypti with Ae albopictus in the hotter, drier cemeteries.
Supplementary Material
Acknowledgments
We thank K. Damal, L. Evans, E. G. Murrell, R. Raban, and S. Woods for field collections, counting and identifying mosquitoes, and setting and monitoring field experiments. We are grateful to D. Yee for helpful comments on an earlier draft of the manuscript. This research was supported by National Institutes of Health grant R01-AI44793.
References Cited
- Bacot AW. Report of the entomological investigation taken for the Commission for the year 1914–15. Rep. Yellow Fever Commum. 1916;3:1–191. [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lourenço-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida, USA. J. Med. Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourenço-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and A. albopictus (Diptera: Culicidae) in Brazil. Ann. Entomol. Soc. Am. 2004;97:130–139. [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000;31:343–366. [Google Scholar]
- Christophers SR. Aëdes aegypti (L.) the yellow fever mosquito: its life history, bionomics and structure. Cambridge University Press; Cambridge, United Kingdom: 1960. [Google Scholar]
- Costanzo KS, Kesavaraju B, Juliano SA. Condition-specific competition in container mosquitoes: the role of non-competing life-history stages. Ecology. 2005;86:3289–3295. doi: 10.1890/05-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. 2nd ed University Press of Florida; Gainesville, FL: 2005. [Google Scholar]
- Davis MA. Does competition from new species threaten long-term residents with extinction? Bioscience. 2003;53:481–489. [Google Scholar]
- Fontenille D, Rodhain F. Biology and distribution of Aedes albopictus and Aedes aegypti in Madagascar. J. Am. Mosq. Control Assoc. 1989;5:219–225. [PubMed] [Google Scholar]
- Gilotra SK, Rozeboom LE, Bhattacharya NC. Observations on possible competitive displacement between populations of Aedes aegypti Linnaeus and Aedes albopictus Skuse in Calcutta. Bull. WHO. 1967;37:437–446. [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol. Entomol. 2005;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Predator identity and additive effects in a treehole community. Ecology. 2006;87:987–995. doi: 10.1890/0012-9658(2006)87[987:piaaei]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitch J, Padilla DK. Are invasive species a major cause of extinctions? Trends Ecol. Evol. 2004;19:470–474. doi: 10.1016/j.tree.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol. Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O'Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O'Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in south Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisnham PT, Juliano SA. Spatial and temporal patterns of coexistence between competing Aedes mosquitoes in urban Florida. Oecologia. 2009;160:343–352. doi: 10.1007/s00442-009-1305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annu. Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL. Sex ratios of mosquitoes from long-term censuses of Florida tree holes. J. Am. Mosq. Control Assoc. 2008;24:11–15. doi: 10.2987/5656.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL, Lourenço-de-Oliveira R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 2003;96:512–518. [Google Scholar]
- Mogi M, Miyagi I, Abadi K, Syafruddin Inter- and intraspecific variation in resistance to desiccation by adult Aedes (Stegomyia) spp. (Diptera: Culicidae) from Indonesia. J. Med. Entomol. 1996;33:53–57. doi: 10.1093/jmedent/33.1.53. [DOI] [PubMed] [Google Scholar]
- O'Meara GF, Gettman AD, Evans LF, Scheel FD. Invasion of cemeteries in Florida by Aedes albopictus. J. Am. Mosq. Control Assoc. 1992;8:1–10. [PubMed] [Google Scholar]
- O'Meara GF, Gettman AD, Evans LF, Curtis GA. The spread of Aedes albopictus in Florida. Am. Entomol. 1993;39:163–172. [Google Scholar]
- O'Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Lounibos LP. Intraspecific competition and adult survival in Aedes aegypti and Aedes albopictus. Med. Vet. Entomol. 2009;23:62–68. doi: 10.1111/j.1365-2915.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey JR, Nishimura N, Wagner B, Braks MAH, O'Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in south Florida. J. Med. Entomol. 2006;43:1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute . SAS/STAT users guide, version 6. 4th ed. SAS Institute; Cary, NC: 1990. [Google Scholar]
- Sax DF, Stachowicz JJ, Gaines SD. Where do we go from here? In: Sax DF, Stachowicz JJ, Gaines SD, editors. Species invasions: insights into ecology, evolution, and biogeography. Sinauer; Sunderland, MA: 2005. pp. 467–480. [Google Scholar]
- Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Gosberg RK, Hastings A, Holt RD, Mayfield MM, et al. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007;22:465–471. doi: 10.1016/j.tree.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevtich J, editors. Design and analysis of ecological experiments. 2nd ed. Oxford University Press; Oxford, United Kingdom: 2001. pp. 99–115. [Google Scholar]
- Simberloff D, Gibbons L. Now you see them, now you don't—population crashes of established introduced species. Biol. Invasions. 2004;6:161–172. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd ed. W. H. Freeman; New York: 1995. [Google Scholar]
- Sota T. Response to selection for desiccation resistance in Aedes albopictus eggs (Diptera: Culicidae) Appl. Entomol. Zool. 1993;28:161–168. [Google Scholar]
- Sota T, Mogi M. Interspecific variation in desiccation survival time of Aedes (Stegomyia) mosquitoes eggs is correlated with habitat and egg size. Oecologia. 1992a;90:353–358. doi: 10.1007/BF00317691. [DOI] [PubMed] [Google Scholar]
- Sota T, Mogi M. Survival time and resistance to desiccation of diapause and non-diapause eggs of temperate Aedes (Stegomyia) mosquitoes. Entomol. Exp. Appl. 1992b;63:155–161. [Google Scholar]
- Strayer DL, Eviner VT, Jeschke JM, Pace ML. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 2006;21:645–651. doi: 10.1016/j.tree.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Tabachnick WJ. Evolutionary genetics and arthropod-borne disease: the yellow fever mosquito. Am. Entomol. 1991;37:14–24. [Google Scholar]
- Trpis M. A new bleaching and decalcifying method for general use in zoology. Can. J. Zool. 1970;48:892–893. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.