Abstract
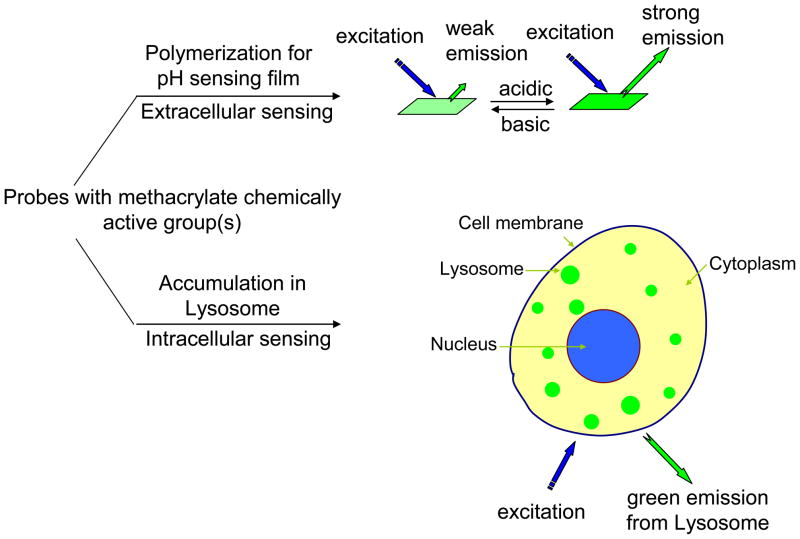
A series of new naphthalimide derivatives were synthesized and studied. Three of the materials (SM1, SM2, and SM3) possess methacrylate(s) moieties as pH sensor monomers, enabling these compounds to be polymerized with other monomers for thin film preparation for extracellular pH sensing. Herein, poly(2-hydroxyethyl methacrylate)-co-poly(acrylamide) (PHEMA-co-PAM) was chosen as the polymer matrix. Structure influences on pH responses and pKa values were studied. The film P3 composed of the sensing moiety SM3 has a pKa close to the usual biological environmental pH of ~7. It was used as an extracellular pH sensor to monitor pH change during the metabolism of prokaryotic Escherichia coli (E. coil). On the other hand, the three sensor monomers are new intracellular biomarkers to sense lysosomes of eukaryotic cells since (1) their pKa values are in a range of 5.9 to 6.8; (2) their emission intensities at acidic conditions (such as at pH 5) are much stronger than those at a neutral condition of pH 7; (3) lysosomes range in size from 0.1 to 1.2 μm in diameter with pH ranging from 4.5 to 5.0, which is much more acidic than the pH value of the cytoplasm (usually with a pH value of ~7.2); and (4) the acidity of lysosomes enables a protonation of the amino groups of the pH probes making the sensors emit brightly in acidic organelles by inhibiting the photo-induced electron transfer from the amino groups to the fluorophores. Lysosome sensing was demonstrated using live human brain glioblastoma U87MG cell line, human cervical cancer HeLa cell line, and human esophagus premalignant CP-A and CP-D cell lines by observations of small acidic spherical organelles (lysosomes) and significant colocalizations (82 ~ 95%) of the sensors with a commercially available lysosome-selective staining probe LysoTracker Red® under confocal fluorescence microscopy.
Keywords: optical sensors, pH sensor, naphthalimide derivatives, lysosome sensor, intracellular pH sensing, extracellular sensing
1. Introduction
Optical pH sensors based on changes of emission intensity and lifetime have been intensively studied as they can be non-invasive or minimally invasive, disposable, easily miniaturized (down to sub-micrometer), and simple to process (as a coating or solid layer on optical fibers and certain surfaces) for environmental analysis, medical diagnosis, and process control [1–4]. Some optical pH sensors have been used in-vivo for cell and tissue imaging [5–6] and integrated with other sensors for simultaneous monitoring of extracellular and intracellular biological (multi)-parameters [1–13]. Different types of emission-based pH sensors, including fluorescein derivatives [14–25], ruthenium and europium complexes [26–28], and rhodamines [29] have been reported. The majority of pH sensors reported so far are the fluorescein derivatives including seminaphtharhodafluor (SNARF®) [20, 21], 2′,7′-bis(carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF®) [22], fluorescein isothiocyanate-dextran® [23], fluorescein-labeled polystyrene beads [24], fluorescein-containing silica nanoparticles [25], and fluorescein labeled polymer nanoparticles [5]. Fluorescein derivatives are excellent but are known to exhibit poor photostability and small Stokes shift – a difference (in wavelength or frequency units) between positions of the band maxima of the absorption and emission spectra of the same electronic transition [29]. Ruthenium and europium complexes are suspected to be toxic and are easily interfered by other ions in aqueous solutions or in biological environments. Thus, the development of new optical pH sensors is needed and continuously growing.
In order to fulfill extracellular pH sensing requirement, the optical probes needed to be physically incorporated or chemically immobilized with an ion permeable polymer matrix, such as collagen, polyurethane hydrogel, or poly(2-hydroxyethyl methacrylate) [7, 8, 30]. Leaching of the physically doped sensors from the matrices is a significant problem [7] which may result in signal instability, inaccuracy of the measurement, decreased long-term applicability, and potential cytotoxicity for cells. To alleviate the leaching problems, optical sensors have been conjugated into suitable matrices either through a chemical grafting of the probes onto matrices [30] or polymerization of the probes with the precursors/monomers of the matrices [8].
For intracellular pH sensing, the sensors are required to be localized inside the cells through different mechanisms and/or approaches including pinocytosis, endocytosis, phagocytosis, or microinjections [6, 9, 11, 13] depending on materials. Current development of the intracellular pH sensors includes but not limited: (1) preparation of new optical probes to improve sensitivity and measurement accuracy as well as increase cell permeability and photostability; (2) synthesis of nanomaterials based optical probes to improve the brightness and stability for long-term cell monitoring [24] and endow the ability of targeted delivery of the optical probes [11]; and (3) investigation of the probes with specific characteristics such as the two-photon absorbing ability and near infrared (NIR) light excitable property for deep and in-vivo imaging as well as reducing the phototoxicity and photodamage caused by ultraviolet and visible light [31, 32]. One important intracellular pH sensing application is to detect acidic compartments, lysosomes, in eukaryotic cells. Lysosomes are small and spherical organelles (in the range of 0.1 – 1.2 μm in diameter) that contain digestive enzymes (acid hydrolases) [33, 34]. Lysosomes are used for the digestion of macromolecules from phagocytosis (ingestion of other dying cells or larger extracellular material, like foreign invading microbes), endocytosis (cells absorb substances such as proteins and particles with sizes less than a few hundred nanometers that cannot pass through cell plasma membrane by engulfing with cell membrane), and autophagy (microbes or old or unneeded organelles or proteins have invaded the cytoplasm) [33, 34]. Because of these functions, the pH value in the lysosomes (4.5 – 5.0) is more acidic than in cytoplasm (pH 7 – 7.3). A few lysosome probes, i.e. LysoTracker® and LysoSensors® are commercially available from Molecular Probes [35]. The LysoTracker® probes, which consist of fluorophores linked to weak bases that are only partially protonated at neutral pH, freely permeate to cell membranes and typically concentrate in spherical acidic organelles. Their mechanism of retention has not been firmly established but is likely to involve protonation and retention in the membranes of the lysosomal organelles [35]. The LysoSensor dyes are acidotropic probes that appear to accumulate in acidic organelles as the result of protonation. This protonation also relieves the fluorescence quenching of the dye by its weak base side chain, resulting in an increase in fluorescence intensity [35]. Thus, the LysoSensor reagents exhibit a pH-dependent increase in fluorescence intensity upon acidification, in contrast to the LysoTracker® probes, which exhibit fluorescence that is largely independent of pH.
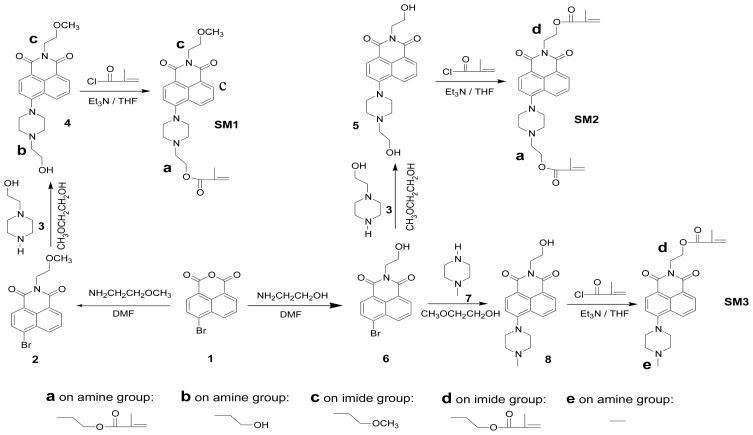
Along the line of the development of new optical pH sensors, recently, some naphthalimide derivatives were reported as a new class of optical pH sensors since they exhibited larger Stokes shifts, less susceptibility to interference by metal ions, and higher photostability than fluorescein derivatives [8, 36, 37]. However, the reported chemical structures of the naphthalimide derivatives as pH sensors are limited and almost all of the studies are about extracellular pH sensing. This study is directed toward the synthesis, characterization, and bio-application of a series of naphthalimide derivatives (SM1, SM2, and SM3 shown in Scheme 1) functionalizing as sensors and monomers (called sensor monomers). The sensor monomers possess reactive functional methacrylate moieties, so that they are able to be chemically immobilized into an ion permeable matrix [herein, we use poly(2-hydroxyethyl methacrylate)-co-polyacrylamide as the matrix, for short, PHEMA-co-PAM] for extracellular pH sensing (Figure 1). SM3 was first reported by other scientists [38, 39]. Previously, we chemically immobilized SM3 into the PHEMA-co-PAM matrix and studied the responses to pH in different aqueous media with different ion strengths and at various temperatures [40]. But it has not been reported as an intracellular nor extracellular sensor in biological environments. Herein, structure influences of the sensor monomers, as well as the PHEMA-co-PAM thin film influences on pH responses and pKa values were studied. The three sensor monomers (SM1, SM2 and SM3) were demonstrated to be new intracellular biomarkers for lysosome sensing using human brain glioblastoma U87MG cells, human cervical cancer HeLa cells, and human esophagus premalignant CP-A and CP-D cells. Extracellular pH sensing ability was studied through a measurement of environmental pH change during the growth of Escherichia coli (E. coli) using a PHEMA-co-PAM film composed of the sensing moiety of SM3.
Scheme 1.
Synthesis of the naphthalimide derivatives.
Figure 1.
Schematic drawing of the application of the methacrylate functional moiety-containing naphthalimide derivatives for intracellular and extracellular pH sensing.
2. Experimental Sections
2.1 Materials and reagents
All chemicals and solvents were of analytical grade and were used without further purifications. Methoxyethylene glycol, triethyl amine, tetrahydrofuran (THF), 4-bromo-1,8-naphthalic anhydride (compound 1), N-(2-hydroxyethyl)piperazine (compound 3), methacryloyl chloride, N,N′-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), trimethylsilylpropyl acrylate (TMSPA), 2-hydroxyethyl methacrylate (HEMA), azobisisobutyronitrile (AIBN), and acrylamide (AM) were commercially available from Aldrich and used without further purification. N-(2-Methoxyethyl)-4-bromo-1,8,-naphthalimide (compounds 2), N-(2-hydroxyethyl)-4-bromo-1,8,-naphthalimide (compound 6), N-(2-hydroxyethyl)-4-(4-methylpiperazine-1-yl)-1,8,-naphthalimide (compound 8), and N-(2-methacryloyloxyethyl)-4-(4-methylpiperazine-1-yl)-1,8,-naphthalimide (SM3) were prepared according to known procedures [38, 40]. Ethyloxylate trimethylolpropane triacrylate (SR454®) is a commercial product of Sartomer Company (Exton, PA). Doubly distilled water was used for the preparation of the buffer solutions. The pH values were determined with a digital pH meter (Thermo Electron Corporation, Beverly, MA) calibrated at room temperature (23 ± 2 °C) with standard buffers of pH 10.01, 7.00, and 4.01. Britton-Robinson (B-R) buffers composed of acetic acid, boric acid, phosphoric acid, and sodium hydroxide were used for tuning pH values. For fluorescence measurements, quartz glass from University Wafer (South Boston, MA) was cut into squares of 1.31 cm × 1.31 cm using a dicing saw (Microautomation, Billerica, MA).
Hoechst 33342, LysoTracker Red®, Dulbecco’s Modified Eagle Medium (DMEM), and Keratinocyte medium were purchased from Invitrogen (Carlsbad, CA). DMEM was used for U87MG and HeLa cell culture. Keratinocyte medium was used for CP-A and CP-D cells culture. Lysogeny Broth (LB) medium made of 10 g tryptone, 5 g yeast extract and 10 g NaCl in 1 L distilled water was used for E. coli cultures. The pH of the LB medium for titration of the sensing films was adjusted using sodium hydroxide and hydrochloric acid.
2.2 Syntheses
2.2.1 Synthesis of N-(2-methoxyethyl)-4-(4-(2-hydroxyethyl)-piperazine-1-yl)-1,8,-naphthalimide (compound 4)
Compound 2 (1.8 g, 5.4 mmol) and compound 3 (1.5 g, 11.6 mmol) were mixed in methoxylethylene glycol (30 mL) and heated under reflux overnight. After the solvent was removed under reduced pressure, the residue was purified by column chromatography using methylene chloride/methanol (20:1 by volume) as the eluent. 1.31 g of yellow product was obtained. Yield: 63%. 1H NMR (CDCl3, δ, ppm): 8.58 (d, 1H), 8.51 (d, 1H), 8.37 (d, 1H), 7.69 (m, 1H), 7.26 (d, 1H), 4.42 (t, 2H), 4.16 (br, 2H), 3.92 (m, 4H), 3.71 (t, 2H), 3.58 (m, 2H), 3.36 (s, 3H), 3.35 (m, 4H). 13C NMR (CDCl3, δ, ppm): 164.53, 164.03, 155.78, 132.64, 131.22, 130.22, 129.91, 126.12, 125.65, 123.15, 117.72, 114.93, 69.66, 59.54, 58.76, 57.81, 53.02, 52.96, 39.06. HRMS (APCI+): m/e calculated for C21H26N3O4 (M + H) 384.1923; found. 384.1935.
2.2.2 Synthesis of N-(2-methoxyethyl)-4-(4-(2-methacryloyloxyethyl)-piperazine-1-yl)-1,8,-naphthalimide (SM1)
Compound 4 (1.3 g, 3.39 mmol) and triethyl amine (700 mg, 6.93 mmol) were dissolved in 60 mL dry THF. To this solution, 500 mg of methacryloyl chloride (4.81 mmol) in 5 mL THF was added slowly at 0–5 °C. After the mixture was stirred at room temperature overnight, the mixture was poured into water. Organic materials were extracted in methylene chloride. After the methylene chloride was dried over Na2SO4, the solvent was removed and the residue was purified by column chromatography using methylene chloride/methanol (20:1 by volume) as the eluent. 0.82 g of a yellow solid was obtained. Yield: 54 %. 1H NMR (CDCl3, δ, ppm): 8.58 (d, 1H), 8.51 (d, 1H), 8.35 (d, 1H), 7.69 (m, 1H), 7.23 (d, 1H), 6.14 (s, 1H), 5.61 (s, 1H), 4.42 (m, 4H), 3.71 (t, 2H), 3.36 (s, 3H), 3.35 (m, 4H), 2.98 (m, 6H), 1.96 (s, 3H). 13C NMR (CDCl3, δ, ppm): 167.27, 164.54, 164.04, 155.90, 136.22, 132.65, 131.19, 130.27, 129.91, 126.11, 125.67, 125.60, 123.13, 116.61, 114.90, 69.66, 62.24, 58.75, 56.56, 53.37, 53.00, 39.05, 18.35. HRMS (APCI+): m/e calculated for C25H30N3O5 (M + H) 452.2185; found. 452.2177.
2.2.3 Synthesis of N-(2-hydroxyethyl)-4-(4-(2-hydroxyethyl)-piperazine-1-yl)-1,8,-napththalimide (compound 5)
The synthesis of compound 5 was similar to the preparative procedure of compound 4. Yield: 85 %. 1H NMR (CD3OD, δ, ppm): 8.53 (d, 1H), 8.49 (d, 1H), 8.47 (m, 1H), 7.75 (m, 1H), 7.31 (m, 1H), 4.30 (t, 2H), 3.77 (m, 6H), 3.52 (t, 1H), 3.45 (t, 1H), 2.89 (m, 4H), 2.70 (m, 2H), 2.54 (m, 2H). HRMS (APCI+): m/e calculated for C20H24N3O4 (M + H) 370.1767; found. 370.1756.
2.2.4 Synthesis of N-(2-methacryloyloxyethyl)-4-(4-(2-methacryloyloxyethyl)-piperazine-1-yl)-1,8,-naphthalimide (SM2)
The synthesis of SM2 was similar to the preparative procedure of SM1. Yield: 67 %. 1H NMR (CDCl3, δ, ppm): 8.56 (d, 1H), 8.52 (d, 1H), 8.38 (d, 1H), 7.68 (m, 1H), 7.24 (m, 1H), 6.13 (s, 1H), 6.03 (s, 1H), 5.98 (s, 1H), 5.49 (s, 1H), 4.53 (t, 2H), 4.47 (t, 2H), 4.19 (br, 2H), 3.31 (br, 4H), 2.87 (br, 6H), 1.96 (s, 3H), 1.85 (s, 3H). ). 13C NMR (CDCl3, δ, ppm): 167.25, 167.15, 164.36, 163.84, 155.99, 136.21, 136.02, 132.63, 131.14, 130.39, 129.89, 126.11, 125.74, 125.67, 125.62, 122.97, 116.40, 114.92, 62.22, 62.01, 56.55, 53.53, 52.94, 38.66, 18.35, 18.23. HRMS (APCI+): m/e calculated for C28H32N3O6 (M + H) 506.2291; found. 506.2266.
2.3 Polymerization – the preparation of PHEMA-co-PAM copolymer sensor thin films for extracellular pH sensing
The thin films were prepared according to our published protocol [40, 41]. Briefly, 1 mg of the sensor monomer SM1, SM2 or SM3, HEMA (800 mg), AM (150 mg), SR454 (50 mg), and AIBN (10 mg) were dissolved in 1 mL DMF as stock solutions. 10 μL of the stock solutions were added onto the surface of the TMSPA-modified quartz glass and covered with a clean but untreated cover slip to make a sandwich structure. Using TMSPA to modify the quartz glass is to enable the sensors and matrices to be chemically grafted onto a quartz substrate [41]. To produce the polymer thin film with good mechanical stability, SR454 was used as a crosslinker. To further increase the water and ion permeability, AM was added as a second monomer for the thin film formation [8, 36]. The thickness was controlled using 25-μm Kapton tape (DuPont, Wilmington, DE). The sandwich set-up was placed into a vacuum oven, which was then evacuated and refilled with nitrogen three times. Polymerization was carried out under nitrogen at 80 °C for 1.5 hours in the oven. The quartz glasses with polymer membranes were removed from the oven, with Kapton tape and non-surface modified glass being removed from the polymerized membrane surface. The polymer membranes on the quartz glasses were washed three times using methanol to remove any remaining non-polymerized monomers and residual DMF. The films were dried and stored in the dark at room temperature.
2.4 Instruments and characterization
A Varian liquid-state NMR operated at 400 MHz for 1H NMR and 100 MHz for 13C NMR was used for NMR spectra measurements. High resolution mass spectrometry (HRMS) was performed by the ASU Mass Spectrometry Laboratory. An oxygen plasma cleaner (Harrick Plasma, Ithaca, NY) was used for quartz glass surface activation. A Shimadzu UV-3600 UV-VIS-NIR spectrophotometer (Shimadzu Scientific Instruments, Columbia, MD) was used for absorbance measurements. A Shimadzu RF-5301 spectrofluorophotometer was used for fluorescence measurements. For easy measurement of the films in liquid solutions, quartz glass was cut with a dicing saw into squares of 1.31 cm × 1.31 cm, which can fit diagonally into a quartz fluorescence cuvette to enable the sensing membrane be positioned at an angle of 45° to the excitation light.
2.5 Culture of U87MG, HeLa, CP-A and CP-D cells for intracellular sensing and bioimaging
U87MG and HeLa cells (American Type Culture Collection, ATCC, Manassas, VA) were cultured in DMEM supplemented with 10% fetal bovine serum, 5% penicillin, 2 mM L-glutamine (Sigma), and incubated at 37 °C in a 5% CO2 atmosphere. CP-A and CP-D cells (kindly provided by Dr. Brian J. Reid at Fred Hutchison Cancer Research Center, Seattle, WA) were cultured in Keratinocyte-serum free medium (Invitrogen, Carlsbad, CA) supplemented with Bovine Pituitary Extract (BPE) and human recombinant Epidermal Growth Factor (rEGF, Invitrogen) at 37 °C in a 5% CO2 atmosphere. Cells were seeded onto 96-well plates at 10,000 cells per well, and incubated for 1 day. Sensors dissolved in DMSO were added to the medium to make the sensor concentrations in a range of 100 nM to 5 μM. 10 minutes of internalization was found to be sufficient for achieving satisfied images. To confirm that the sensors became localized in lysosomes, LysoTracker Red® was added to co-stain lysosomes following the 10 minutes of cell internalization of sensors. Cells were incubated for additional 30 minutes for observation of colocalization of the sensors and the LyoTracker Red®. The medium was removed and the cells were washed once with cold phosphate buffered saline (PBS). Hoechst 33342 dissolved in fresh medium was then added into the medium to stain cell nuclei for 30 minutes. Concentrations of LysoTracker Red® and Hoechst 33342 were 100 nM and 1 μM, respectively, unless specific indicated. Under Nikon Eclipse TE2000E confocal fluorescence microscope (Melville, NY), Hoechst 33342 was excited at 402 nm and its blue emission was collected using a 450/35 nm filter set; sensors (4, SM1, SM2, and SM3) were excited at 445 nm and their green emissions were collected using a 515/30 nm filter set; LysoTracker Red® was excited at 561 nm and its red emission was collected using a 605/75 nm filter set.
2.6 Culture of E. coli for extracellular pH sensing
E. coli were cultured overnight in LB medium from a single colony. The concentration of E. coli in culture was estimated by measuring the optical density at 600 nm (OD600). OD600 value of 1 indicated a concentration of 5.0 × 108 E. coli/mL [43]. After the E. coli concentration of the overnight culture was determined, appropriate dilutions were made using fresh LB medium to achieve the desired initial E. coli concentrations for experiments.
2.7 Viable cell counts using Trypan Blue
Using the typical staining procedure of Trypan Blue [44], to the cell culture medium (100 μL) with eukaryotic cells in a 96 well microplate with 10,000 cells/well that had internalized the sensors for 30 minutes, 10 μL of 0.4% Trypan Blue stain was added and mixed thoroughly with the medium. After standing for 5 minutes at room temperature (22 °C), the cells were imaged using an optical microscope in bright field mode. Dead cells appeared blue as they were stained, whereas, healthy cells appeared as transparent because of the resistance to being stained. Cells were counted and the ratio of dead cells to live cells was calculated. Each experiment was repeated 3 times.
2.8 Image Analysis for colocalization calculation
Colocalization analysis was quantitatively performed using Matlab software (Natick, MA). Multiple colocalization metrics, including Pearson’s sample correlation factor (Rr) and Manders’ overlap coefficient (R), were computed from the acquired confocal image datasets [42, 45].
Rr is a well defined and commonly accepted means for describing the extent of overlap between image pairs. It is calculated according to the following equation (1):
| (1) |
where S1i is signal intensity of pixels in the red channel from LysoTracker Red® and S2i is signal intensity of pixels in the green channel from the pH probes of SM1, SM2, SM3 or 4, S1aver and S2aver are average intensities of the red and green channels respectively.
Rr is a value computed to be between −1 and 1, with −1 being absolutely no overlap between images and 1 being perfect image registration. Rr takes into account only the similarity of shapes between images and does not take into account image intensity.
As Rr does some averaging of pixel information and can return negative values, another method, the Manders’ overlap coefficient, R, is simultaneously used to describe the overlap. The calculation formula was shown in equation 2.
| (2) |
The calculation of R does not perform any pixel averaging functions, so correlations are returned as values between 0 and 1. A value of zero means that there are no overlapping pixels, a value of 1 means that the overlapping of the green and red pixels is 100%. This method is not sensitive to intensity variations in the image analysis.
For analysis, three sets of red-green images were used for the computation of each coefficient from which the mean and the variance values were computed.
3. Results and discussions
3.1 Synthesis
Following Scheme 1, three sensor monomers (SM1, SM2 and SM3) and their intermediates were prepared from starting material 1. The new compounds (4, 5, SM1 and SM2) were characterized using 1H NMR, 13C NMR, and high resolution mass spectroscopy (HRMS).
3.2 Chemical conjugation of SM1, SM2, and SM3 with poly(2-hydroxyethyl methacrylate)-co-polyacrylamide (PHEMA-co-PAM)
The sensing films grafted on the quartz glasses were prepared using a thermal polymerization approach under nitrogen using AIBN as an initiator. Three polymer thin films (thickness: ~25 μm), P1, P2, and P3, which contained sensing moieties of SM1, SM2, and SM3, respectively, were prepared on quartz substrates.
3.3 pH responses of 4, SM1, SM2 and SM3
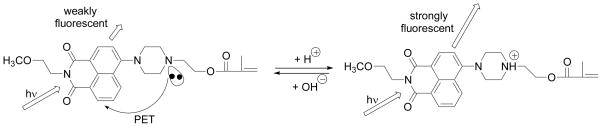
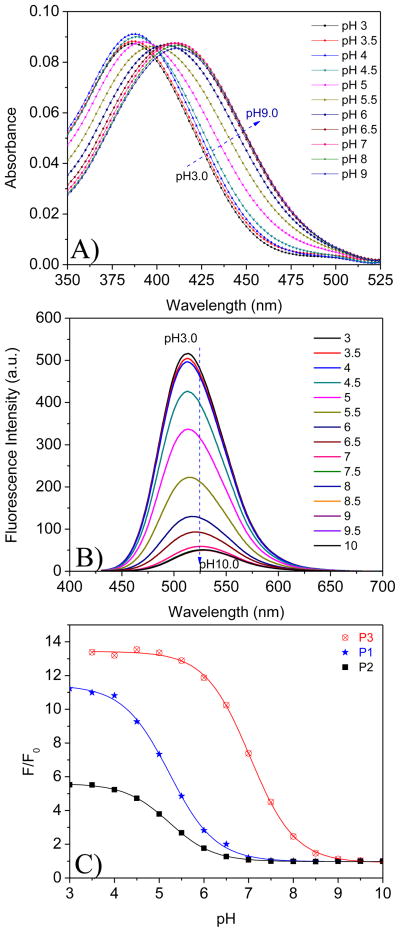
The pH responses of SM1, SM2, SM3 and one of their precursors 4 was studied. Clear solutions were obtained after dispersing their DMSO solutions into the B-R buffers and used for absorption and emission measurements. Typical UV-Vis and emission spectral changes of SM1 in conditions of varied pH were given in Figures 2A and 2B. Bathochromic shifted absorption spectra were observed from acidic to basic conditions. These were similar to other amino-naphthalimide-containing pH sensors [8, 36, 38, 39]. Almost no fluorescence was observed at neutral and basic conditions of pH > 7.5. Conversely, emission from the solution increased as the pH was decreased. When the pH value was lower than 4.5, the fluorescence intensity reached its maximum. This fluorescence intensity change is attributed to photo-induced electron transfer (PET) in the pH sensor being suppressed by a protonation of the amino group (Figure 3). When a fluorophore is attached to an electron quencher (usually one or more nitrogen-containing functional groups which are non-conjugated to the fluorophore), PET occurs between them [8, 36, 46–48]. In the piperazinyl group of SM1, the nitrogen atom with a methacryloyloxyethyl (substituent a, Scheme 1) is not directly connected to the naphthalimide fluorophore, of which the amine moiety is a strong electron donor. PET occurs from the lone electron pair of the amine group to the acceptor naphthalimide fluorophore, making the sensor weakly fluorescent. At a lower pH, however, the protonation of the amino group diminishes the PET effect and, in turn, leads to restoration of the fluorescence originating from the fluorophore, amino-1,8-naphthalimide. Hence, a remarked increase in emission intensity was observed at low pH. The typical dynamic range of pH response for SM1 was from 7.5 to 4.5. The fluorescence intensity changes are described well by a sigmoidal function (Boltzmann fitting) as shown in equation 3.
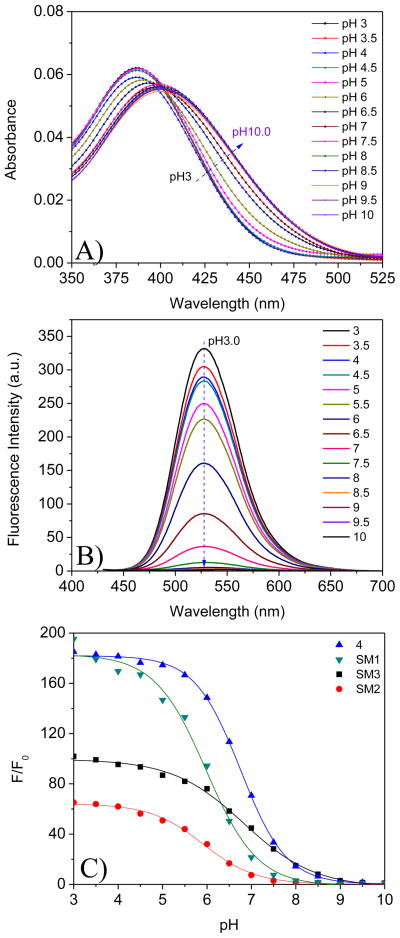
Figure 2.
UV-Vis spectral change (A) and emission change (B) of SM1 in B-R buffer at different pH values. Figure 2C shows fluorescence intensity (at 517 nm) ratios of SM1, SM2, SM3, and 4 from pH 3 to pH 10. F0 is the fluorescence intensity at pH 10. Excitation wavelength for emission spectra was 405 nm. Concentrations of the sensors were 5 μM.
Figure 3.
Schematic drawing for photo-induced electron transfer (PET) using SM1 as an example.
| (3) |
I is the fluorescence intensity measured at varying pH values. I0 is that at the highest pH value (pH 10) used during the titration, respectively. m1, m2, pKa′, and p are empirical parameters describing the initial value (m1), the final value (m2), the point of inflection (pKa′), and the width (p) of the sigmoidal curve. The fluorescence intensity changes and their curve fittings are shown in Figure 2C. The apparent pKa value (pKa′) was calculated to be 5.98 for SM1 in B-R buffers. The fitting was highly reliable with a correlation coefficient (R2) of 0.995.
Similar to the photophysical properties of SM1, fluorescence enhancement by acidity was also observed in the solutions of 4, SM2 and SM3. Their intensity changes and Boltzmann fittings are also given in Figure 2C. The fluorescence intensity ratios of pH 3 to pH 10 follow an order of 4 ~ SM1 > SM3 > SM2. pKa values follow an order of SM3 (6.83) ~ 4 (6.76) > SM1 (5.98) ~ SM2 (5.90). These differences indicated the influence of structures on pH sensitivity (e.g., I/I0) and pKa values. SM1 and 4 have the same substituent c (Scheme 1) on the imide side, but different substituents a and b on their pH sensitive amino groups. Groups a and b (Scheme 1) did not affect the pH sensitivities for SM1 and 4, but influenced the pKa values of SM1 and 4 significantly, showing that the substituents on the pH sensitive amino groups play a critical role for pKa. The smaller substituent on the amine group enables a higher pKa as it is more easily protonated. This observation was further confirmed in SM3, which has the smallest substituent (a methyl group, e, shown in SM3, Scheme 1) on the pH sensitive amino group and shows the highest pKa. Because SM1 and SM2 have the same substituent a on their pH sensitive amino moieties, the pKa values of the two compounds are quite similar. When the substituents on the imides become more hydrophobic, the sensitivity of their corresponding compounds to pH decreases. The substituent d on SM2 is more hydrophobic than c on SM1, making the compound SM2 less sensitive than SM1. Although SM3 has the same substituent d as that of SM2, the substituent e of SM3 is much smaller than the segment a on SM2, making the sensitivity of SM3 to pH larger than that of SM2.
3.4 pH response of thin films P1, P2, and P3
The sensor thin films were immersed diagonally into cuvettes and were titrated using B-R buffers. Typical absorbance and fluorescence changes of P1 at different pH values are given in Figures 4A and 4B. Similar to its corresponding sensor monomer SM1, bathochromic shifted absorption spectra from acidic to basic conditions and increased fluorescence intensity with a decrease in pH value were observed for P1. Emission intensity changes and Boltzmann fittings of P1, P2 and P3 are given in Figure 4C. Results showed that pKa values of P1, P2 and P3 were 5.23, 5.22 and 7.01, respectively. Fluorescence intensity ratios from pH 3 to pH 10 followed an order of P3 > P1 > P2. In the PHEMA-co-PAM thin films, the monomers, SM1, SM2 and SM3, were polymerized into their matrices. The movements of the sensing moieties in the polymer thin films are not completely free, being restricted by the polymer chains. This restriction of the movement of the sensing moieties in P1, P2 and P3 makes the pH sensitivities of the thin films much smaller than those of their corresponding monomers, SM1, SM2 and SM3. P3 possesses the sensing moiety SM3, which has one methacrylate substituent d on the imide group. In the thin film, the pH sensing moiety (e) in P3 is far from the polymer main-chain so that the interaction of the amino group with a proton is much easier than those with substituent a in P1 and P2. Thus the sensitivity to pH value of P3 is higher than P1 and P2. SM2 has two methacrylate groups, which, after polymerization, most restrict the movements of the sensing moieties in P2 among the three polymers, resulting in the lowest sensitivity to pH. Similar to the pKa trends of SM3, SM1 and SM2, the smallest substituent e on the amino group of SM3 enables P3 to have the highest pKa. Thus the structure influence on the pH responses was studied and evaluated. It was also found that the polymerization has a significant effect on the pKa values and sensitivities, which differ from molecule to molecule. Accordingly, some interesting phenomena were observed: (1) the sensitivity of SM1 is better than SM3, whereas the sensitivity of P3 is better than P1; and (2) P1 is shifted to a lower pH value compared to SM1, whereas P3 is shifted to a higher pH value range compared to SM3. These studies may provide new insights for the design of sensors and evaluation of structure and polymerization influence on pH optical probes’ sensitivities and pKa values.
Figure 4.
UV-vis spectral change (A) and emission change (B) of P1 in B-R buffer at different pH values. Figure 4C shows fluorescence intensity (at 517 nm) ratios of P1, P2, and P3 from pH 3 to pH 10. F0 is the fluorescence intensity at pH 10. Excitation wavelength for emission spectra was 405 nm.
Among the three sensing films, the pKa of P3 was close to 7, suggesting possible applications of the sensing films to monitor extracellular pH changes in biological systems. As the sensing moieties were chemically bonded with their matrices which are chemically grafted on the quartz substrates, there was no leaching of the sensors from their matrices into aqueous solutions.
3.5 Comparison of the sensors reported herein with some typical reported sensors
Table 1 summarized the absorbance maxima (λabs, max), emission maxima (λem, max), Stokes shifts (λem, max − λabs, max), pKa values, and the sensitivity (Fmax/Fmin) of our developed sensors and a few typical widely studied and applied intracellular and extracellular pH sensors.
Table 1.
Photophysical properties of some typical pH indicators and the sensors reported herein.
| Sensors a) | Types of fluorophores | λmax, abs (nm) | λmax, em (nm) | Stokes Shift (nm) b) | pKa | Fmax/Fminc) | Specificity | ref |
|---|---|---|---|---|---|---|---|---|
| Fluorescein | Fluorescein | 472 (pH 5.0) 490 (pH 9.0) |
515 (pH 5.0) 515 (pH 9.0) |
43 (pH 5.0) 25 (pH 9.0) |
6.4 | ~10 | Cell impermeable | 49 |
| Oregon Green 488 Carboxylic acid | Fluorescein | 472 (pH 3.0) 490 (pH 8.1) |
513 (pH 3.0) 515 (pH 9.0) |
41 (pH 3.0) 25 (pH 9.0) |
4.7 | ~8 | Cell impermeable | 49 |
| Intracellular pH sensors | ||||||||
| Carboxylic SNARF-1 | Seminaphtharh odafluor | 515 (pH 6.0) 575 (pH 9.0) |
590 (pH 6.0) 650 (pH 9.0) |
75 (pH 6.0) 75 (pH 9.0) |
7.5 | ~8 | Physiological pH | 49, 50 |
| LysoTracker Red DND 99 | Dipyrromethen e-boron difluoride | 577 (pH 4.0) 577 (pH 8.0) |
590 (pH 4.0) 590 (pH 8.0) |
13 (pH 4.0) 13 (pH 8.0) |
NA | ~1 | Lysosomes | 35, 49, 51 |
| LysoTracker Green DND 26 | Dipyrromethen e-boron difluoride | 504 (pH 4.0) 504 (pH 8.0) |
511 (pH 4.0) 511 (pH 8.0) |
7 (pH 4.0) 7 (pH 8.0) |
NA | ~1 | Lysosomes | 35, 49, 51 |
| LysoSensor Blue DND 167 | Anthracene | 373 (pH 3.1) 373 (pH 6.1) |
425 (pH 3.1) 425 (pH 6.1) |
52 (pH 3.1) 52 (pH 6.1) |
5.1 | ~6 | Lysosomes | 35, 49, 51 |
| LysoSensor Green DND 153 | Benzimidazo-[2, 1, a]-benz-[d,e]-isoquinolin-7-one | 442 (pH 4.1) 442 (pH 8.9) |
505 (pH 4.1) 505 (pH 8.9) |
63 (pH 4.1) 63 (pH 8.9) |
7.5 | ~5 | Lysosomes | 35, 49, 51 |
| LysoSensor Green DND 189 | Benzimidazo-[2, 1, a]-benz-[d,e]-isoquinolin-7-one | 452 (pH 2.9) 442 (pH 7.1) |
505 (pH 2.9) 505 (pH 7.1) |
53 (pH 2.9) 63 (pH 7.1) |
5.2 | ~7 | Lysosomes | 35, 49, 51 |
| 4 | Naphthalimide | 387 (pH 3.0) 402 (pH 9.0) |
527 (pH 3.0) 527 (pH 9.0) |
140 (pH 3.0) 125 (pH 9.0) |
6.8 | 182 | Lysosomes | This work |
| SM1 | Naphthalimide | 387 (pH 3.0) 402 (pH 9.0) |
527 (pH 3.0) 527 (pH 9.0) |
140 (pH 3.0) 125 (pH 9.0) |
6.0 | 180 | Lysosomes | This work |
| SM2 | Naphthalimide | 387 (pH 3.0) 402 (pH 9.0) |
527 (pH 3.0) 527 (pH 9.0) |
140 (pH 3.0) 125 (pH 9.0) |
5.9 | 64 | Lysosomes | This work |
| SM3 | Naphthalimide | 387 (pH 3.0) 402 (pH 9.0) |
527 (pH 3.0) 527 (pH 9.0) |
140 (pH 3.0) 125 (pH 9.0) |
6.8 | 100 | Lysosomes | This work |
| Extracellular pH sensors | ||||||||
| CHFOE | Fluorescein | 470 (pH 5.0) 526 (pH 10.0) |
523 (pH 5.0) 545 (pH 10.0) |
53 (pH 5.0) 19 (pH 10.0) |
7.1 | ~10 | 7 | |
| DHFA | Fluorescein | 470 (pH 5.0) 516 (pH 10.0) |
516 (pH 5.0) 540 (pH 10.0) |
46 (pH 5.0) 24 (pH 10.0) |
8.4 | ~10 | 7 | |
| AMPN | Naphthalimide | 400 (pH 3.0) 432 (pH 9.0) |
517 (pH 3.0) 517 (pH 9.0) |
117 (pH 3.0) 85 (pH 9.0) |
7.5 | ~5 | 8 | |
| P1 | Naphthalimide | 388 (pH 3.0) 412 (pH 9.0) |
512 (pH 3.0) 521 (pH 9.0) |
114 (pH 3.0) 109 (pH 9.0) |
5.2 | 11 | This work | |
| P2 | Naphthalimide | 388 (pH 3.0) 412 (pH 9.0) |
512 (pH 3.0) 521 (pH 9.0) |
114 (pH 3.0) 109 (pH 9.0) |
5.2 | 5.5 | This work | |
| P3 | Naphthalimide | 388 (pH 3.0) 412 (pH 9.0) |
512 (pH 3.0) 521 (pH 9.0) |
114 (pH 3.0) 109 (pH 9.0) |
7.0 | 13.5 | This work | |
Chemical structures of the sensors reported by other scientists were given in supplementary data (S-Figure 1).
Stokes shift is the difference of the emission maximum (λmax, em) and absorption maximum (λmax, abs), e.g. λmax, em − λmax, abs.
The ratios of the maximum fluorescence intensity (Fmax) divided by the minimum fluorescence intensity (Fmin) indicate the sensors’ sensitivities.
Fluorescein and its derivative Oregon Green 488 carboxylic acid do not readily enter cells [49]. However, their acetoxymethyl (AM) esters, diacetate derivatives, and dextran conjugates are cell permeable and can be used to measure pH in acidic organelles [22, 23, 49]. SNARF derivatives are cell permeable, exhibit ratiometric spectral characteristics (e.g., the fuorophores can be excited to generate dual-emission spectral peaks that are pH-dependent) to improve the measurement accuracy and alleviate environmental influence on the pH measurement, and can be used to measure intracellular physiological pH values [20, 21, 49, 50]. Compared with the four investigated naphthalimide derived sensors (4, SM1, SM2, and SM3), the Stokes shifts and sensitivities of the fluorescein and SNARF derivatives are quite small. The widely used LysoTrackers and LysoSensors shown in Table 1 are cell permeable and show high specificity for Lysosomes [13, 35, 49, 51]. However, their Stokes shifts and sensitivities are still smaller than our developed sensors.
The fluorescein derivatives CHFOE and DHFA were used for extracellular pH sensing studies [30]. They exhibited sensitivities close to that of P1. Similar to the fluorescein based intracellular pH sensors, the fluorescein based extracellular pH probes show small Stokes shifts. The sensing film made of the naphthalimide derived AMPN [8] showed large Stokes shift similar to those of P1, P2 and P3. Structures influences on the sensitivities and pKa values among the naphthalimide derivatives were observed again.
In summary, Table 1 listed a few typical pH probes, structure influences on their absorptions, emissions, sensitivities, Stokes shifts, and pKa values related with their application ranges were clearly observed from the table. Highly sensitive pH indicators with large Stokes shifts based on the naphthalimide derivatives were developed.
3.6 Applications of 4, SM1, SM2 and SM3 for lysosome sensing (intracellular sensing)
Because the pKa values of the four compounds are between 5.9 and 6.8 and the sensors show stronger fluorescence intensities at acidic conditions of pH lower than 5.0 than at neutral and basic conditions at pH higher than 7.4, they are expected to be suitable lysosome sensors. A few commercially available lysosome sensors were reported to have pKa values between 3.9 and 7.5 [35]. Recall that lysosomes are spherical organelles with sizes varying from 0.1 to 1.2 μm and having pH values of 4.5 – 5, which is much more acidic than in the cytoplasm (pH 7 – 7.4).
We used human brain glioblastoma U87MG cells, cervical cancer HeLa cells, and Barrett’s esophagus premalignant CP-A and CP-D cells to study the subcellular distributions of the pH probes. After cells were cultured in 96 well microplate at 10,000 cells/well for 24 hours, sensors dissolved in DMSO were added into their culture medium. The concentration of the sensor molecules in the medium was controlled to be in a range of 100 nM to 5 μM. DMSO in the medium was controlled to be less than 3% by volume. The sensors with concentrations of 100 nM could stain the cells, but the signal was weak. The fluorescence became much stronger with an increase of sensor concentration (S-Figures 2 and 3 in supplementary data). To achieve images with satisfactory signal-to-noise ratios, a sensor concentration of 1 μM was usually used for cell internalization study. Subcellular distribution of the sensors was studied using confocal fluorescence microscopy. To confirm that the sensors (4, SM1, SM2, and SM3) could sense lysosomes located in the cytoplasm, the commercially available lysosome specific staining probe LysoTracker Red® and the nuclei specific staining probe Hoechst 33342 were used to co-stain the cells with 4, SM1, SM2, and SM3, and their colocalizations were studied.
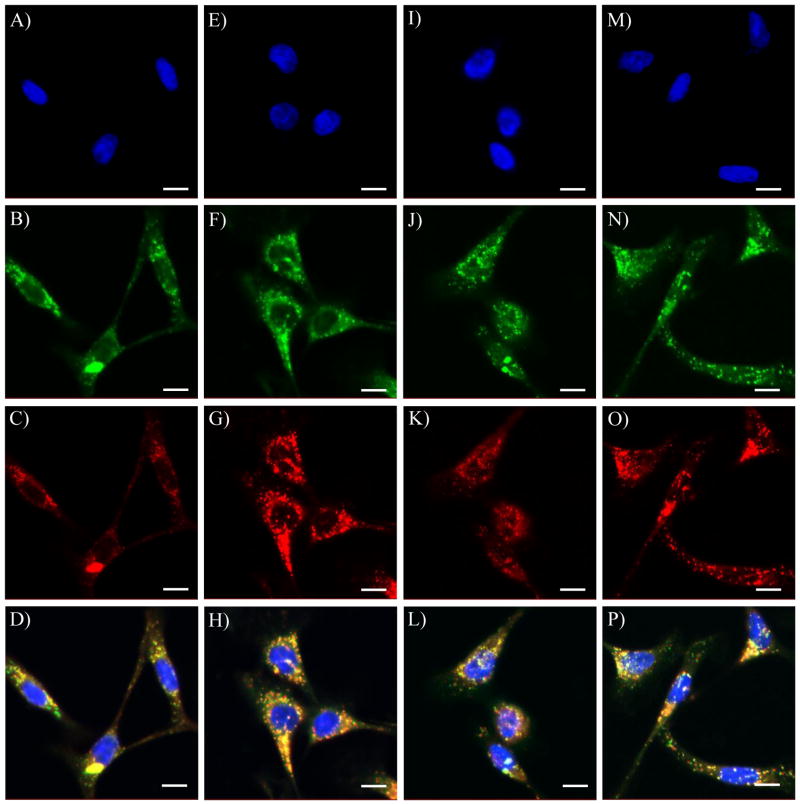
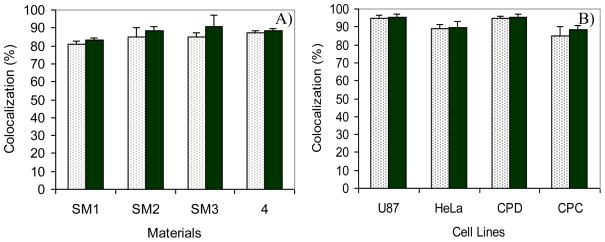
Figures 5B, F, J, and N showed the subcellular distribution of SM1, SM2, SM3 and 4 in live CP-A cells, respectively. Small spherical organelles with bright green emissions distributed mainly in the cytoplasm region were observed under confocal microscopy. These were confirmed because of only minimal co-localization of the green emissions (from sensors) with blue emissions (from Hoechst 33342) (A, E, I, and M). On the other hand, significant overlays of the green emissions (B, F, J, and N) with the red emissions (C, G, K, and O) from the LysoTracker Red® were observed (D, H, L, and P). The yellow colors in D, H, L and P indicated the same subcellular localizations of the LysoTracker Red® with SM1, SM2, SM3 and 4, respectively. The corresponding Pearson’s sample correlation factor (Rr) and Overlap Coefficient (R) are given in Figure 6A. The significant co-localizations (> 82%) of the sensors and LysoTracker Red® showed that the compounds SM1, SM2, SM3 and 4 are potential lysosome sensors.
Figure 5.
Confocal fluorescence images of SM1 (B), SM2 (F), SM3 (J), and 4 (N) in CP-A cells co-stained with nuclei staining Hoechst 33342 (A, E, I and M) and LysoTracker Red® (C, G, K and O), respectively. The scale bar represents 10 μm. D is the overlay of A, B and C. H is the overlay of E, F and G. L is the overlay of I, J and K. P is the overlay of M, N and O.
Figure 6.
A) Colocalization of SM1, SM2, SM3 and 4 with LysoTracker Red® in CP-A cells. B) Colocalization of SM2 with LysoTracker Red® in U87, HeLa, CP-D and CP-A cells. Rr is in the light color column with dots. R is in the dark color column.
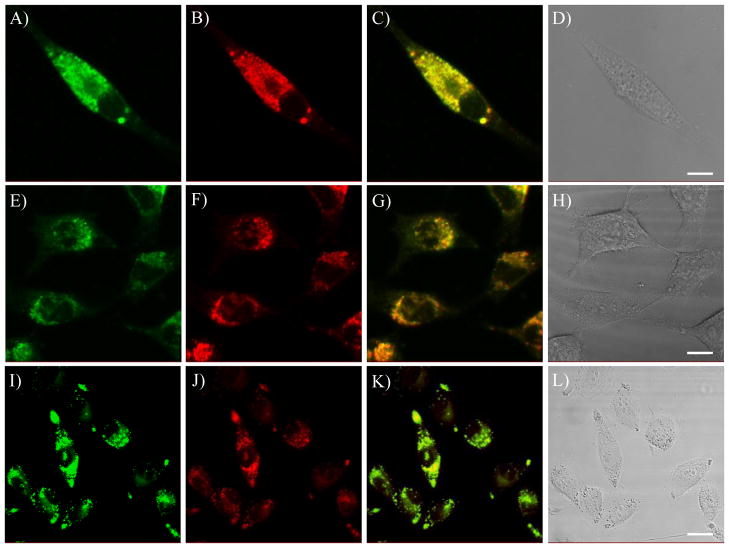
SM2 was used as a typical example for further studying the subcellular distribution in live cells of U87MG, HeLa and CP-D. Colocalizations of SM2 with LysoTracker Red® in these three cell lines are given in Figure 7. The corresponding Rr and R are given in Figure 6B. Significant overlays (82 – 95%) of the green emissions from SM2 and the red emissions from LysoTracker Red® were also observed in these three cell lines, showing its characteristics for good intracellular probes for lysosomes.
Figure 7.
Confocal fluorescence images of SM2 in U87MG (A), HeLa (E) and CP-D (I) cells co-stained with LysoTracker Red® (B, F and J), respectively. C is the overlay of A and B. G is the overlay of E and F. K is the overlay of I and J. D, H and L are the bright field images of SM2 and LysoTracker Red® in U87, HeLa and CP-D cells, respectively. The scale bar represents 10 μm.
The above imaging results clearly showed that the four pH sensors, SM1, SM2, SM3, and 4, are cell permeable and can accumulate in the acidic organelles. Most likely, the amino groups are protonated by lysosomes, relieving the fluorescence quenching of the naphthalimide fluorophores by the amino groups. The protonated amino groups enable a high cell permeability and selective accumulation of the probes in the acidic organelles. Since the probes are pH sensitive, this indicates that they are most likely new LysoSensors.
It is known that lysosomal accumulation of active molecules is a determinant for cellular, biological and therapeutic events [52, 53]. On the other hand, some amino-containing naphthalimide derivatives have been demonstrated to be efficient drugs [54, 55]. Therefore, associating the simple structures and the highly Stokes shifted fluorescence properties of these reported naphtalimide derivatives in acidic cellular compartments, they are expected to be used for understanding cellular and biological mechanisms as well as antitumor drug mechanisms of delivery and efficacy.
The toxicity of the sensors to cells under our experimental conditions was studied using Trypan blue staining. After 30 minutes of internalization of the sensors with concentrations at 5 μM, more than 97% cells were viable, showing the non-toxicity of the sensing materials to cells at our experimental conditions.
3.7 Using sensing film P3 to monitor extracellular pH change during the growth of Escherichia coli (E. coli) bacteria
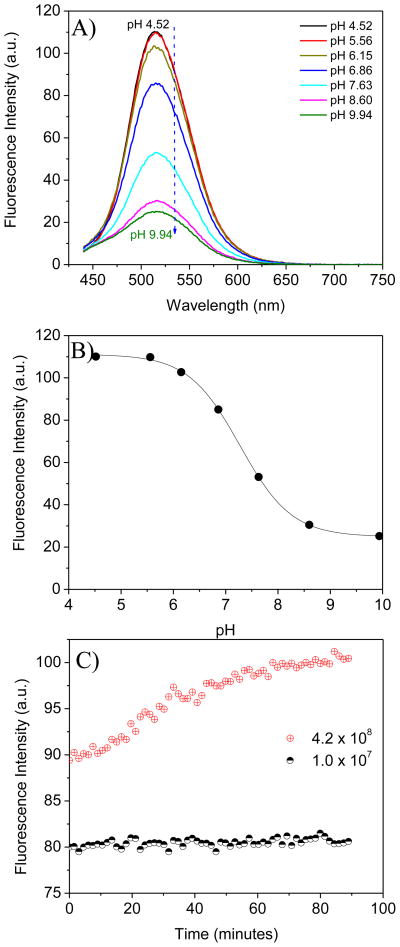
Because prokaryotic E. coli lack lysosomes, extremely weak green emission was observed under confocal fluorescence microscopy after internalization of the pH probes of 4, SM1, SM2 and SM3 with E. coli. A typical imaging was given in supplementary materials S-Figure 4. Most likely, the intracellular pH of E. coli is neutral, lacking the ability to protonate the amino groups of the pH probes to increase their cell permeability and relieve the fluorescence from the fluorophores by PET. However, the generation time of E. coli is much shorter than that of the eukaryotic cells. At optimized culture conditions, the generation time of E. coli is about 20 minutes. However, depending on the culture medium, temperature and concentration of the cultured bacteria, the generation times of E. coli can range from 20 to 90 minutes [56, 57]. E. coli can grow either in the presence or absence of oxygen because of its ability to use different sources for energy. During the growth process, metabolism of E. coli generates carbon dioxide (CO2), which makes the extracellular environment more acidic. The P3 film was used to monitor the pH change induced by the metabolism of E. coli since its pKa is close to the biological pH of 7. For efficiently monitoring pH change, a layer of mineral oil was added on the top of the LB medium to prevent the escape of the generated CO2 to the air. The use of mineral oil to prevent oxygen and carbon dioxide leaching for bacteria studies was reported in the literature [58, 59]. The sensor thin film P3 was placed diagonally into a quartz cuvette containing 2.5 mL of one of two concentrations of E. coli culture in sterilized LB medium, with the two concentrations being 1 × 107 and 4.2 × 108 cells/mL. Time dependent emission intensity measurements at 517 nm were performed at intervals of 30 s for 90 minutes at room temperature. Because the E. coli were cultured in LB medium, the sensor thin film P3 was titrated in sterilized LB medium before this bio-experiment. The measured fluorescence change by pH is given in Figure 8A. Emission intensity at 517 nm against pH values and its sigmoidal curve fitting are shown in Figure 8B, which was used as the calibration curve for pH value determination. The fluorescence intensity change measured during the E. coli growth is given in Figure 8C.
Figure 8.
A) Fluorescence intensity of a P3 film at different pH values in LB medium using 405 nm excitation wavelength. B) Boltzmann fitting curve for the fluorescence intensity change at 517 nm, which was used for pH determination. C) Fluorescence intensity change of the P3 film during the process of E. coli growth. The numbers shown in C are the initial E. coli concentration (cells/mL) before the experiment started.
When the concentration of E. coli was relatively small (1 × 107 E. coli/mL), no obvious pH change was observed during the growth process as the generated CO2 was not enough to significantly change the pH of the LB medium. Based on the fluorescence intensity, the pH values were calculated to be 6.98 before and 6.92 after the 90 minutes of growth (Table 2). The pH values before and after the E. coli growth were also measured using a micro pH glass electrode (Fisher Scientific, Houston, TX). These values were 6.84 before and 6.72 after, corresponding well to the pH values measured using P3 film with an experimental error ≤ ± 0.20. OD600 measurements indicated that the E. coli concentrations increased to 2.3 × 107 E. coli/mL. When the E. coli concentration was large (4.2 × 108 E. coli/mL), the pH changed from 6.73 to 6.26 measured by P3 film and from 6.71 to 6.07 determined by pH electrode after 90 minutes of growth. OD600 measurements indicated that E. coli growth at this condition was much slower (Table 2), probably because a large number of E. coli in our experimental design significantly consumed oxygen resulting in an aerobic condition, thereby depressing the E. coli growth rate. This study demonstrates the potential application of the sensing film for monitoring pH changes of biological environments. Further experimentation using these sensing films integrated with an optical oxygen sensor is in progress to investigate the respiration and metabolism of cells and bacteria.
Table 2.
pH determination of the extracellular environment during E. coli growth in LB medium before and after 90 minutes experiment.
| t = 0 min | t = 90 min | |||||
|---|---|---|---|---|---|---|
| concentration (E. coli/mL) | pH by P3 | pH by electrode | concentration (E. coli/mL) | pH by P3 | pH by electrode | |
| Run 1 | 1 × 107 | 6.98 | 6.84 | 2.3 × 107 | 6.92 | 6.72 |
| Run 2 | 4.2 × 108 | 6.73 | 6.71 | 5.1 × 108 | 6.26 | 6.07 |
Conclusion
A series of naphthalimide derivatives with methacrylates was prepared and investigated. The substituents on the imide side and on the piperazine moieties were found to affect their solution and film pKa values and the fluorescence intensity ratios from pH 3 to pH 10. Depending on their structures, pKa values were found to be in a range of 5.2 to 7.0. Four compounds (4, SM1, SM2, and SM3) were studied as intracellular lysosome sensors using U87MG, HeLa, CP-A, CP-D cells. Results showed that the four pH probes were cell permeable and accumulated majorly in the acidic organelles. Significant colocalizations of the pH probes with their counterpart LysoTracker® in the ranges of 82 to 95% were observed under confocal fluorescence microscopy. As the three sensor monomers, SM1, SM2, and SM3, possess methacrylate active groups, they were polymerized with 2-hydroxylethyl methacrylate and acrylamide to form pH sensitive thin films. The sensing thin film P3 containing the SM3 moiety was used to monitor extracellular pH changes induced by the metabolism of E. coli in LB culture medium. Results showed that the pH values measured using the optical approach correlated well with those measured using a glass pH electrode before and after the E. coli growth experiments. This showed a reliability of the noninvasive optical approach. Thus, the reported naphthalimde derivatives were first time demonstrated to be new intracellular and extracellular pH sensors.
Supplementary Material
Acknowledgments
This work was supported by NIH National Human Genome Research Institute, Centers of Excellence in Genomic Science, Grant Number 5 P50 HG002360. Dr. Brian J. Reid and Tom Paulson at Fred Hutchison Cancer Research Center (Seattle, WA) were acknowledged for kindly providing us the CP-A and CP-D cell lines.
Footnotes
Available supplementary materials include: concentration dependent bioimages, quantum yields of the sensors (4, SM1, SM2, and SM3) in aqueous solutions at pH 3 and 9.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jeevarajan AS, Vani S, Taylor TD, Anderson MM. Continuous pH monitoring in a perfused bioreactor system using an optical pH sensor. Biotechnol Bioeng. 2002;78:467–472. doi: 10.1002/bit.10212. [DOI] [PubMed] [Google Scholar]
- 2.Grant SA, Glass RS. A sol-gel based fiber optic sensor for local blood pH measurements. Sens Actuators B. 1997;45:35–42. [Google Scholar]
- 3.Martz TR, Carr JJ, French CR, DeGrandpre MD. A submersible autonomous sensor for spectrophotometric pH measurements of natural waters. Anal Chem. 2003;75:1844–1850. doi: 10.1021/ac020568l. [DOI] [PubMed] [Google Scholar]
- 4.Nagl S, Wolfbei OS. Optical multiple chemical sensing: status and current challenges. Analyst. 2007;132:507–511. doi: 10.1039/b702753b. [DOI] [PubMed] [Google Scholar]
- 5.Kneipp A, Kneipp H, Wittig B, Kneipp K. One- and two-photon excited optical pH probing for cells using surface-enhanced Raman and hyper-Raman nanosensors. Nano Letters. 2007;7:2819–2823. doi: 10.1021/nl071418z. [DOI] [PubMed] [Google Scholar]
- 6.Lee YEK, Smith R, Kopelman R. Nanoparticle PEBBLE sensors in live cells and in vivo. Annu Rev Anal Chem. 2009;2:57–76. doi: 10.1146/annurev.anchem.1.031207.112823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroder CR, Polerecky L, Klimant I. Time-resolved pH/pO2 mapping with luminescent hybrid sensors. Anal Chem. 2007;79:60–70. doi: 10.1021/ac0606047. [DOI] [PubMed] [Google Scholar]
- 8.Niu CG, Gui XQ, Zeng GM, Yuan XZ. A ratiometric fluorescence sensor with broad dynamic range based on two pH-sensitive fluorophores. Analyst. 2005;130:1551–1556. doi: 10.1039/b504882f. [DOI] [PubMed] [Google Scholar]
- 9.Kneen M, Farinas J, Li Y, Verkman AS. Green Fluorescent Protein as a noninvasive intracellular pH indicator. Biophysical J. 1998;74:1591–1599. doi: 10.1016/S0006-3495(98)77870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang JS, Kostov Y. Ratiometric pH Measurements Using LysoSensor DND-192. J Biochem Mol Biology. 2002;35:384–388. doi: 10.5483/bmbrep.2002.35.4.384. [DOI] [PubMed] [Google Scholar]
- 11.Coupland PG, Briddon SJ, Aylott JW. Using fluorescent pH-sensitive nanosensors to report their intracellular location after Tat-mediated delivery. Integr Biol. 2009;1:318–323. doi: 10.1039/b822569a. [DOI] [PubMed] [Google Scholar]
- 12.Perez JF, Ruiz MC, Michelangeli F. Simultaneous measurement and imaging of intracellular Ca2+ and H+ transport in isolated rabbit gastric glands. J Physiol. 2001;537:735–745. doi: 10.1111/j.1469-7793.2001.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hector M, Urayama P. Using LysoSensor Yellow/Blue DND-160 to sense acidic pH under high hydrostatic pressures. Anal Biochem. 2009;384:359–361. doi: 10.1016/j.ab.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Fry DR, Bobbitt DR. Investigation of dynamically modified optical-fiber sensors for pH sensing at the extremes of the pH scale. Microchem J. 2001;69:123–131. [Google Scholar]
- 15.Lobnik A, Oehme I, Murkovic I, Wolfbeis OS. pH optical sensors based on sol–gels: chemical doping versus covalent immobilization. Anal Chim Acta. 1998;367:159–165. [Google Scholar]
- 16.Brasselet S, Moerner WE. Fluorescence behavior of single-molecule pH-sensors. Single Mol. 2000;1:17–23. [Google Scholar]
- 17.Ferguson JA, Healey BG, Bronk KS, Barnard SM, Walt DR. Simultaneous monitoring of pH, CO2 and O2 using an optical imaging fiber. Anal Chim Acta. 1997;340:123–131. [Google Scholar]
- 18.Lin HJ, Szmacinski H, Lakowicz JR. Lifetime-based pH sensors: indicators for acidic environments. Anal Biochem. 1999;269:162–167. doi: 10.1006/abio.1999.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNamara KP, Nguyen T, Dumitrascu G, Ji J, Rosenzweig N, Rosenzweig Z. Synthesis, characterization, and application of fluorescence sensing lipobeads for intracellular pH measurements. Anal Chem. 2001;73:3240–3246. doi: 10.1021/ac0102314. [DOI] [PubMed] [Google Scholar]
- 20.Venn AA, Tambutte E, Lotto S, Zoccola D, Allemand D, Tambutte S. Imaging intracellular pH in a reef coral and symbiotic anemone. Proc Natl Acad Sci USA. 2009;106:16574–16579. doi: 10.1073/pnas.0902894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang M, Walker JW. Endothelin-1 and PKC induce positive inotropy without affecting pHi in ventricular myocytes. Exp Biol Med. 2006;231:865–870. [PubMed] [Google Scholar]
- 22.Shimoda LA, Luke T, Sylvester JT, Shih HW, Jain A, Swenson ER. Inhibition of hypoxia-induced calcium responses in pulmonary arterial smooth muscle by acetazolamide is independent of carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1002–L1012. doi: 10.1152/ajplung.00161.2006. [DOI] [PubMed] [Google Scholar]
- 23.Liu YH, Dam TD, Pantano P. A pH-sensitive nanotip array imaging sensor. Anal Chim Acta. 2000;419:215–225. [Google Scholar]
- 24.Bradley M, Alexander L, Duncan K, Chenaoui M, Jones AC, Sánchez-Martín RM. pH sensing in living cells using fluorescent microspheres. Bioorganic Medicinal Chem Letter. 2008;18:313–317. doi: 10.1016/j.bmcl.2007.10.075. [DOI] [PubMed] [Google Scholar]
- 25.Peng JF, He X, Wang K, Tan W, Wang Y, Liu Y. Noninvasive monitoring of intracellular pH change induced by drug stimulation using silica nanoparticle sensors. Anal Bioanal Chem. 2007;388:645–654. doi: 10.1007/s00216-007-1244-9. [DOI] [PubMed] [Google Scholar]
- 26.Kosch U, Klimant I, Wolfbeis OS. Long-lifetime based pH micro-optodes without oxygen interference. Frese J Anal Chem. 1999;364:48–53. [Google Scholar]
- 27.Malins C, Glever HG, Keyes TE, Vos JG, Dressick WJ, MacCraith BD. Sol–gel immobilised ruthenium(II) polypyridyl complexes as chemical transducers for optical pH sensing. Sens Actuators B. 2000;67:89–95. [Google Scholar]
- 28.Lobnik A, Majcen N, Niederreiter K, Uray G. Optical pH sensor based on the absorption of antenna generated europium luminescence by bromothymolblue in a sol–gel membrane. Sens Actuators B. 2001;74:200–206. [Google Scholar]
- 29.Mock DM, Langford G, Dubois D, Criscimagna N, Horowitz P. A fluorometric assay for the biotin-avidin interaction based on displacement of the fluorescent probe 2-anilinonaphthalene-6-sulfonic acid. Anal Biochemistry. 1985;151:178–181. doi: 10.1016/0003-2697(85)90068-5. [DOI] [PubMed] [Google Scholar]
- 30.Vasylevska GS, Borisov SM, Krause C, Wolbeis OS. Indicator-loaded permeation-selective microbeads for use in fiber optic simultaneous sensing of pH and dissolved oxygen. Chem Mater. 2006;18:4609–4616. [Google Scholar]
- 31.Tang B, Liu X, Xu K, Huang H, Yang G, An L. A dual near-infrared pH fluorescent probe and its application in imaging of HepG2 cells. Chem Commun. 2007:3726–3728. doi: 10.1039/b707173f. [DOI] [PubMed] [Google Scholar]
- 32.Kim HM, An MJ, Hong JH, Jeong BH, Kwon O, Hyon JY, et al. Angew Chem. 2008;120:2263–2266. [Google Scholar]
- 33.Kuehnel W. Color atlas of cytology, histology, & microscopic anatomy. 4. Thieme; 2003. p. 34. [Google Scholar]
- 34.Mader S. Biology. 9. McGraw Hill; New York: 2007. [Google Scholar]
- 35.Haugland RP, editor. Spence MTZ. Chapter 12. Probes for organelles. 10. Carlsbad, CA: 2005. The Handbook – A guide to fluorescence probes and labeling technologies; pp. 580–583. Molecular Probes™. [Google Scholar]
- 36.Niu CG, Zeng GM, Chen LX, Shen GL, Yu RQ. Proton “off-on” behaviour of methylpiperazinyl derivative of naphthalimide: a pH sensor based on fluorescence enhancement. Analyst. 2004;129:20–24. doi: 10.1039/b309594k. [DOI] [PubMed] [Google Scholar]
- 37.Cui D, Qian X, Liu F, Zhang R. Novel fluorescence pH sensors based on intramolecular hydrogen bonding ability of naphthalimide. Org Lett. 2004;6:2757–2760. doi: 10.1021/ol049005h. [DOI] [PubMed] [Google Scholar]
- 38.Tian H, Gan J, Chen K, He J, Song QL, Hou XY. Positive and negative fluorescent imaging induced by naphthalimide polymers. J Mater Chem. 2002;12:1262–1267. [Google Scholar]
- 39.Shen L, Zhu W, Meng X, Guo Z, Tian H. A hydrophilic fluorescent polymer containing naphthalimide moiety as chemosensor for microbioreactors. Science in China B Chemistry. 2009;52:821–826. [Google Scholar]
- 40.Tian YQ, Shumway BR, Youngbull C, Jen AKY, Johnson RH, Meldrum DR. Dually fluorescent sensing of pH and dissolved oxygen using a membrane made from polymerizable sensing monomers. Sens Actuators B Chemical. 2010;147:714–722. doi: 10.1016/j.snb.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian YQ, Shumway BR, Meldrum DR. A new crosslinkable oxygen sensor covalently bonded into poly(2-hydroxyethyl methacrylate)-co-polyacrylamide thin film for dissolved oxygen sensing. Chem Mater. 2010;22:2069–2078. doi: 10.1021/cm903361y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dualcolor confocal images. J Microscopy. 1993;169:375–382. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 43.Mallette MF. In: Evaluation of growth by physical and chemical means in Methods in Microbiology. Norris JR, Ribbons DW, editors. Vol. 1. Academic Press; London: 1969. pp. 521–566. [Google Scholar]
- 44.Freshney R. Culture of animal cells: a manual of basic technique. Alan R. Liss, Inc; New York: 1987. p. 117. [Google Scholar]
- 45.Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochemica et Cytochemica. 2007;40:101–111. doi: 10.1267/ahc.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valeur B. Molecular fluorescence: principles and applications. New York: Wiley; 2002. [Google Scholar]
- 47.Ramachandram B, Samanta A. Transition metal ion induced fluorescence enhancement of 4-(N,N-Dimethylethylenediamino)-7-nitrobenz-2-oxa-1,3-diazole. J Phys Chem A. 1998;102:10579–10587. [Google Scholar]
- 48.Ramachandram B, Saroja G, Sankaran B, Samanta A. Unusually high fluorescence enhancement of some 1,8-Naphthalimide derivatives induced by transition metal salts. J Phys Chem B. 2000;104:11824–11832. [Google Scholar]
- 49.Haugland RP, editor. Spence MTZ. Chapter 20. pH indicators. 10. Carlsbad, CA: 2005. The Handbook – A guide to fluorescence probes and labeling technologies; pp. 935–955. Molecular Probes™. [Google Scholar]
- 50.Seksek O, Henry-Toulme N, Sureau F, Bolard J. SNARF-1 as an intracellular pH indicator in microspectrofluorometry: a critical assessment. Anal Biochem. 1991;139:49–54. doi: 10.1016/0003-2697(91)90042-r. [DOI] [PubMed] [Google Scholar]
- 51.Lin JH, Herman P, Kang JS, Lakowicz JR. Fluorescence lifetime characterization of novel low-pH probes. Anal Biochem. 2001;294:118–125. doi: 10.1006/abio.2001.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castino R, Démoz M, Isidora C. Destination Lysosome: a target organelle for tumour cell killing? J Mol Recognit. 2003;16:337–348. doi: 10.1002/jmr.643. [DOI] [PubMed] [Google Scholar]
- 53.Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: Analysis and therapeutic implications. J Pharm Sci. 2007;96:729–746. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 54.Nitiss JL, Zhou JF, Rose A, Hsiung Y, Gale KC, Osheroff N. The bis(naphthalimide) DMP-840 causes cytotoxicity by its action against eukaryotic topoisomerase II. Biochemistry. 1998;37:3078–3085. doi: 10.1021/bi9723257. [DOI] [PubMed] [Google Scholar]
- 55.Zhu H, Miao ZH, Huang M, Feng JM, Zhang ZX, Lu JJ, et al. Naphthalimides induce G2 arrest through the ATM-activated Chk2-executed pathway in HCT116 cells. Neoplasia. 2009;11:1126–1234. doi: 10.1593/neo.09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plank LD, Harvey JD. Generation time statistics of Escherichia coli B measured by synchronous culture techniques. J General Microbiology. 1979;115:69–77. doi: 10.1099/00221287-115-1-69. [DOI] [PubMed] [Google Scholar]
- 57.Wheelis M. Principle of Modern Microbiology. Jones and Bartlett Publisher; Sudbury, MA: 2008. p. 195. [Google Scholar]
- 58.Sattley WM, Madigan MT. Cold-active acetogenic bacteria from surficial sediments of perennially ice-covered Lake Fryxell, Antarctica. FEMS Microbiol Lett. 2007;272:48–54. doi: 10.1111/j.1574-6968.2007.00737.x. [DOI] [PubMed] [Google Scholar]
- 59.O’Riordan TC, Buckley D, Ogurtsov V, O’Connor R, Papkovsky DB. A cell viability assay based on monitoring respiration by optical oxygen sensing. Anal Biochem. 2000;278:221–227. doi: 10.1006/abio.1999.4431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.