Abstract
We present three examples of key genes that function in arterial specification that have recently been implicated in lymphatic development; ephrinB2, FoxC2, and Notch. In arterial cell fate determination, Foxc2 regulates both Notch and Notch ligand expression. In turn, Notch signal activation in arteries drives expression of ephrinB2. It will be interesting to determine if the regulatory relationships between these pathways found in arterial development are relevant to understanding lymphatic development, that is, we ask whether arterial regulators are also key regulators of lymphatic development.
Introduction
Both arterial and lymphatic specification involve bi-potential cell fate decisions, arterial/venous and lymphatic/venous. Arteries function to hold blood tight and deliver it under pressure and force. Lymphatics collect fluid and are not subject to the high pressure of blood flow.1 Although these two types of vasculature differ greatly in structure and function, they may utilize some common factors to promote differentiation and specialization. Here, we will discuss recent studies suggesting that pathways known to regulate arterial specification, such as Notch and ephrinB2, may have unexpected but important roles during specialization of the lymphatic vasculature.
In arterial/venous endothelial specification, it is thought that EphB4-expressing venous and ephrinB2-expressing arterial endothelial cells originate from a common progenitor.2 Polarized expression of the master regulator of lymphatic endothelial specification, Prox1, is thought to initiate lymphangiogenesis, reviewed in Oliver and Alitalo,1 that is, Prox1 in venous endothelial cells marks the initiation of the bi-potential cell fate decision that occurs to differentiate lymphatic endothelial cells from venous cells.3 Regulation of bi-potential cell fate determination steps, such as arterial/venous specification or lymphatic differentiation from veins, is often the job of the Notch signaling pathway. Thus, it is worthwhile to consider a potential function of Notch in these processes.
In the arterial/venous bi-potential cell fate decision, Notch promotes arterial endothelial differentiation, while suppressing venous specification.4 This activity is carried out in part through regulation of ephrinB2 expression in arterial endothelium. Interestingly, this same signaling molecule, ephrinB2, has also been shown to function in remodeling of the lymphatic plexus into lymphatic collecting ducts and capillaries.5
Once lymphatic specification is initiated by Prox1, the lymphatic endothelial cell comes to depend on VEGF receptor 3 (VEGFR-3) and the ligands VEGF-C and VEGF-D to promote further growth.6 In fact, VEGFR-3 is considered the most important growth factor receptor for lymphatic development. Notch signaling is now known to be a direct regulator of VEGFR-3 expression in blood endothelial cells,7 leading to speculation of a similar relationship between Notch and VEGFR-3 in lymphatic endothelial cells. Notch1 and Notch4 are both expressed in some murine lymphatics, including those found in neonatal dermis.7 In humans, Notch1 and Notch4 are expressed in the extratumoral lymphatics of human micropapillary mammary carcinomas.7 Thus, the receptors that function to mediate endothelial function of Notch signaling are expressed in select normal and pathological lymphatics. Equally compelling is the finding that an activated peptide of Notch1 is present in tumoral lymphatics associated with such mammary carcinomas. Together, these data suggest that it is important to consider if Notch signaling may participate and regulate different cell fate determination steps of physiological and pathological lymphangiogenesis. Further, one might ask if Notch, a known regulator of ephrinB2, functions upstream of ephrinB2 in remodeling lymphatic vasculature.
Notch signaling is not thought to be active in venous endothelium. In fact, the COUP-TFII orphan nuclear receptor is actively involved in maintaining venous cell fate by repressing Notch signaling, thus maintaining vein identity.8 It is not currently known whether COUP-TFII functions in lymphatics and, if so, if it similarly functions to repress Notch signaling. Thus, the potential role of Notch on lymphatic development may depend on defining expression patterns in lymphatics of the factors that regulate arterial (Notch), venous (COUP-TFII), and lymphatic cell fate.
The Notch Signaling Pathway
Notch functions in an evolutionarily conserved signaling pathway that modulates cell-fate decisions (reviewed in Ref. 9). In mammals, there are four Notch genes (Notch1—4) and five ligands, Jagged1, Jagged2 and Delta-like 1, 3, 4 (Dll). Upon ligand binding, the cytoplasmic domain of Notch is released from the cell surface by a presenilin/γ-secretase proteolytic cleavage. The cytoplasmic Notch peptide translocates to the nucleus and interacts with the CBF-1/Suppresor of Hairless/Lag-2 (CSL/rbpsuh) transcriptional repressor, converting it to an activator. Hairy Enhancer of Split (HES) and HES-Related (Hey) transcriptional repressors are direct targets of Notch/CSL signaling. Consistent with the Notch/CSL signaling pathway regulating the expression of blood endothelial cell genes involved in cell fate determination, mice deficient for Notch1, Dll4, Hey1, and Hey2 or CSL/rbpsuh are embryonic lethal prior to E10.5 and display defects in angiogenesis and arterial endothelial cell specification.10 Transgenic mice that activate Notch4 within the embryonic endothelium also die in mid-gestation with defects in vascular remodeling.11 Thus, the dosage of Notch activity within the endothelium is critical.
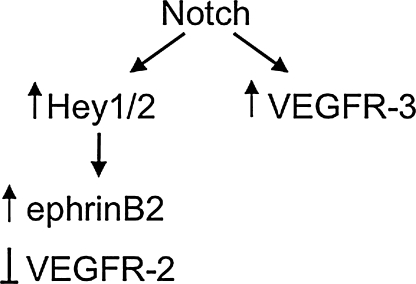
In blood endothelial cells, the Notch/CSL signaling pathway regulates cell fate decisions by regulating gene expression.12 The downstream mediators of Notch function in endothelial cells are becoming known. Hey1 and Hey2 figure prominently in models of Notch function and are directly regulated by Notch/CSL.13 However, other intriguing targets of the Notch signaling pathway consist of genes that encode members of the VEGF receptor family. Notch induces VEGFR-3 directly, that is, it functions by binding and trans-activating the VEGFR-3 promoter.7 Notch also suppresses the expression of VEGFR-2 and upregulates ephrinB2 via its induction of Hey2.12 This complex relationship is schematized in Figure 1. How important is VEGF receptor expression for Notch function in endothelial cell fate determination steps? This question is currently being addressed experimentally and we consider evidence for such regulation in the lymphatic vasculature.
FIG. 1.
Notch Signaling in Angiogenensis and Arterial Endothelial Specification
VEGF drives a variety of key steps in vascular development including growth of endothelium, specification of arteries and veins, and promotion of new angiogenic sprouts. During sprouting angiogenesis, VEGF promotes a new sprout from a pre-existing vessel. A role for Notch in sprouting angiogenesis is evident from analysis of retinal vasculature, where Notch limits angiogenic sprouting, reviewed in Refs. 14 and 15. The role of Notch signaling in this process is to limit further sprouting via a feedback loop involving downregulation of VEGFR-2 (Fig. 1).
VEGF also utilizes Notch signaling to drive the process of endothelial cell specification to an arterial fate; that is, Notch functions downstream of VEGF in arterial/venous (A/V) specification.16 In both zebrafish and mammals, VEGF induces the expression of Notch and Notch ligands, suggesting VEGF regulates arterial specification via activation of Notch signaling. In this setting, VEGF promotes Notch signaling which then in turn drives expression of ephrinB2 via Hey2 to promote the arterial cell fate (Fig. 1).
Eprhinb2 in Lymphangiogenesis Signaling
EphrinB2 is a molecule long appreciated as a protein expressed at the onset of arterial specification and in differentiated arterial endothelium.2 The arterial expression of eprhinB2 is mediated by Notch signaling.17,18 The discovery of new roles for ephrinB2 in lymphatic remodeling5 highlights the diversity of functions that can be carried out by distinct angiogenic regulators.
In mouse postnatal day 0 (P0) neonates, the dermal lymphatic vasculature is a uniform vascular plexus deep with the dermis. This lymphatic vascular plexus at this early stage of development expresses both ephrinB2 and EphB4.5 At P3, lymphatic endothelial cells sprouting from the plexus begin moving towards the upper dermis. Between P5 and P7, lymphatic capillaries that express EphB4 and are devoid of mural cells differentiate in the upper dermal layer. Deeper in the dermis, ephrinB2 expression is maintained in the lymphatic collecting ducts that are surrounded by αSMA-positive vascular smooth muscle cells. Thus, eprhinB2 comes to be associated with lymphatic collecting ducts and not lymphatic capillaries.
EphrinB2 can act both as a ligand for EphB4 and also as a receptor that depends on EphB4 binding to promote reverse signaling. EphrinB2 signaling (referred to as “reverse” signaling) depends on an intracellular PDZ domain.19 Deletion of the ephrinB2 PDZ domain in mice resulted in lymphatic defects, demonstrating a previously unappreciated role for ephrinB2 signaling in lymphangiogenesis.5 Mice lacking the PDZ domain die during the first 3 weeks of life. Analysis of the mutant mice showed a failure to remodel the primary lymphatic plexus into a network of vessels with the proper hierarchy of collecting ducts and capillaries. In addition, luminal valve formation in the lymphatic collecting ducts was defective and general hyperplasia of lymphatics was observed. Finally, new sprout formation appeared to be aborted, displaying few proper elongated sprouts and stumps where sprouts might have attempted to initiate.
Although no similar role in lymphatic remodeling has been ascribed to the Notch pathway, as that described above for ephrinB2 signaling, one may consider if Notch is involved in regulation of ephrinB2 in the lymphatic endothelium. If this was the case, one might expect that the expression pattern whereby ephrinB2 becomes restricted to collecting ducts and absent from capillaries may involve Notch. Further analysis of this concept will depend on understanding the expression of Notch and Notch ligands in lymphatic ducts. To date, no Notch mutant mouse has been described with defects in lymphatic vasculature, however, many of the Notch mutant mice die during stages of cardiovascular development prior to the onset of lymphatic vascular development.
Notch Signaling Regulates the Balance of VEGFR-2 and VEGFR-3
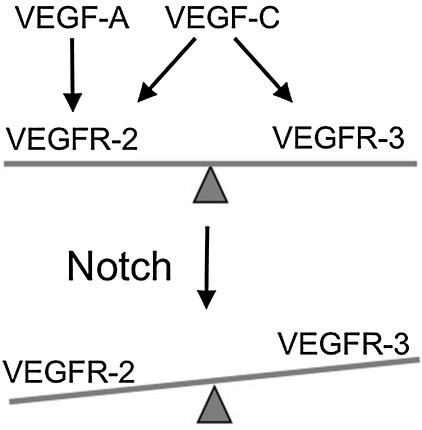
During angiogenesis, endothelial cells are exposed to a variety of external stimuli, such as those provided by bFGF, VEGF, ephrins, and angiopoietins.20,21 Notch alters endothelial cell responsiveness by changing the expression of VEGF receptors at the cell surface of endothelial cells. Notch signaling suppressed VEGFR-2 expression, while dramatically increasing VEGFR-3 in cultured primary endothelial cells,7 schematized in Figure 2. Consistent with this reciprocal change in expression, HUVEC survival in response to VEGF-A was reduced when Notch signaling was activated and increased in response to VEGF-C.7 Thus, Notch signaling may regulate blood endothelial cell fate decisions by decreasing VEGFR-2 and increasing VEGFR-3-based signaling. In vivo, Notch signaling has been found to regulate the expression of VEGF receptors during postnatal retinal angiogenesis.22 In the retina of Dll4 heterozygous mice, disruption of Notch signaling correlated with an increase in vascular density, an increase in VEGFR-2 expression and a reduction in VEGFR-1 endothelial expression.22
FIG. 2.
The alteration in the balance of VEGFR-2 and VEGFR-3 levels by Notch may affect the ability of VEGFR-2 and VEGFR-3 to form a heterodimeric complex.23 The VEGFR-2/VEGFR-3 receptor complex has been suggested to maintain vascular integrity and promote angiogenesis.24 In zebrafish, VEGFR-3 interacts with VEGFR-2 during morphogenesis of the intersomitic arteries.25 In mice, embryonic endothelial Notch promoted VEGFR-3 expression within the developing intersomitic vessels. Thus, Notch may regulate the balance of VEGFR-2 and VEGFR-3 expression during angiogenesis. VEGFR-2 and VEGFR-3 heterodimers have also been proposed to regulate different steps of lymphangiogenesis and thus, Notch signaling may also regulate their expression levels.
Notch, a Regulator of Lymphangiogenesis?
Notch1, Notch2, and Notch4 and the Notch ligand, Dll1, are all expressed by cultured rat thoracic lymphatic endothelial cells (rLECs).26 Interestingly, expression of Notch2 and Dll1 was found to be restricted to the rLEC and absent in the venous endothelial cells. Rat LEC grown in hypoxic conditions, a closer simulation of LEC physiological condition, expressed higher levels of Notch4 relative to normoxic conditions. Hey2 expression was also increased, suggesting that Notch signal activation was increased under hypoxic conditions. This correlated with a downregulation of VEGFR-2 and an increase in VEGFR-3. Under normoxic conditions, treatment with a gamma-secretase inhibitor or expression of a dominant-negative Dll-1 resulted in an increase in VEGFR-2 expression and increased LEC migration in scratch/wound assay. These data suggest that Notch functions to suppress VEGFR-2 expression in lymphatic endothelial cells, similar to blood endothelial cells. However, it is yet unclear whether Notch regulates VEGFR-3 expression in LECs.
A role for Notch in lymphangiogenesis in vivo has yet to be described. Within the murine dermis, both Notch1 and Notch4 are co-expressed with VEGFR-3 and the lymphatic endothelial cell marker, LYVE-1.7 In humans, Notch1 and Notch4 are expressed in the extratumoral lymphatic vessels of invasive mammary micropapillary ductal carcinomas. Moreover, Notch1 signaling was found to be activated with in a subset of the tumoral lymphatic endothelial cells, indicating that Notch1 is not only expressed, but is activated in tumor lymphatic vessels. In human breast cancer, VEGF-C via VEGFR-3 signal activation has been proposed to promote lymphatic endothelial cell proliferation, tumor lymphatic invasion, and tumor metastasis.27 Thus, Notch1 signaling may function in tumor lymphangiogenesis and by extension breast cancer metastasis.
A Potential FoxC-Notch Signaling Axis in the Endothelium?
FoxC2 is a transcription factor of the forkhead family that functions in differentiation of lymphatic vasculature. Specifically, FoxC2 is expressed in luminal valves of lymphatic vasculature and loss of function of FoxC2 results in failure to form valves and recruit smooth muscle cells to lymphatic collecting ducts.28
Recently, it has been found that in mouse embryonic endothelial cells (MEECs), FoxC1 and FoxC2 upregulate the expression of Notch1, Notch4, and Dll4, as well as the arterial specific markers, ephrinB2 and the Notch target, Hey2.29 The regulation of Dll4 occurs via direct binding of FoxC to fork-head binding sites in the Dll4 promoter. This suggests that the FoxC transcription factors function upstream of Notch to promote arterial specification. In fact, FoxC1 and FoxC2 double homozygous knockout mice exhibit arteriovenous malformations and a loss of arterial markers within the embryonic endothelium. As with ephrinB2, Foxc2 functions with Notch in arterial specification. Also like ephrinB2, FoxC2 is involved in lymphatic remodeling and maturation.
Another intriguing observation relates to the fact that Foxc1 heterozygous/Foxc2 homozygous double knockouts have reduced sprouting of Prox1 positive cells from the cardinal vein that was suggested to arise from reduced mesenchymal VEGF-C expression. Thus, early lymphatic specification depends to some extent on proper Foxc1 activity. However, Foxc2 was found expressed in endothelium of the cardinal vein. Could the lymphatic defects be in part due to aberrant Notch signaling as well?
Closing Comments
We present three examples of key genes that function in arterial specification that have recently been implicated in lymphatic development; ephrinB2, FoxC2, and Notch. In arterial cell fate determination, Foxc2 regulates both Notch and Notch ligand expression that in turn drives expression of ephrinB2. It will be interesting to determine if the regulatory relationships between these pathways found in arterial development are relevant to understanding lymphatic development, that is, we ask whether arterial regulators are also key regulators of lymphatic development. On the other hand, should one be asking whether key regulators of lymphatic development also function in arterial development?
Disclosure Statement
Professors Shawber and Kitajewski have no conflicts of interest or financial ties to disclose.
References
- 1.Oliver G. Alitalo K. The lymphatic vasculature: Recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457–483. doi: 10.1146/annurev.cellbio.21.012704.132338. [DOI] [PubMed] [Google Scholar]
- 2.Wang HU. Chen ZF. Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 3.Wigle JT. Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 4.Lawson ND. Scheer N. Pham VN. Kim CH. Chitnis AB. Campos—Ortega JA. Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 5.Makinen T. Adams RH. Bailey J. Lu Q. Ziemiecki A. Alitalo K. Klein R. Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veikkola T. Jussila L. Makinen T. Karpanen T. Jeltsch M. Petrova TV. Kubo H. Thurston G. McDonald DM. Achen MG. Stacker SA. Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shawber CJ. Funahashi Y. Francisco E. Vorontchikhina M. Kitamura Y. Stowell SA. Borisenko V. Feirt N. Podgrabinska S. Shiraishi K. Chawengsaksophak K. Rossant J. Accili D. Skobe M. Kitajewski J. Notch alters VEGF responsiveness in human and murine endothelial cells by direct regulation of VEGFR-3 expression. J Clin Invest. 2007;117:3369–3382. doi: 10.1172/JCI24311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You LR. Lin FJ. Lee CT. DeMayo FJ. Tsai MJ. Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- 9.Shawber CJ. Kitajewski J. Notch function in the vasculature: insights from zebrafish, mouse and man. Bioessays. 2004;26:225–234. doi: 10.1002/bies.20004. [DOI] [PubMed] [Google Scholar]
- 10.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 11.Uyttendaele H. Ho J. Rossant J. Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc Natl Acad Sci USA. 2001;98:5643–5648. doi: 10.1073/pnas.091584598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor KL. Henderson AM. Hughes CC. Notch activation during endothelial network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc Res. 2002;64:372–383. doi: 10.1006/mvre.2002.2443. [DOI] [PubMed] [Google Scholar]
- 13.Fischer A. Schumacher N. Maier M. Sendtner M. Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holderfield MT. Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 15.Siekmann AF. Covassin L. Lawson ND. Modulation of VEGF signaling output by the Notch pathway. Bioessays. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 16.Lawson ND. Vogel AM. Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 17.Lawson ND. Weinstein BM. Arteries and veins: making a difference with zebrafish. Nat Rev Genet. 2002;3:674–682. doi: 10.1038/nrg888. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y. Watanabe T. Saito D. Takahashi T. Yoshida S. Kohyama J. Ohata E. Okano H. Takahashi Y. Notch mediates the segmental specification of angioblasts in somites and their directed migration toward the dorsal aorta in avian embryos. Dev Cell. 2008;14:890–901. doi: 10.1016/j.devcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Adams RH. Diella F. Hennig S. Helmbacher F. Deutsch U. Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 20.Saaristo A. Karpanen T. Alitalo K. Mechanisms of angiogenesis and their use in the inhibition of tumor growth and metastasis. Oncogene. 2000;19:6122–6129. doi: 10.1038/sj.onc.1203969. [DOI] [PubMed] [Google Scholar]
- 21.Yancopoulos GD. Davis S. Gale NW. Rudge JS. Weigand SJ. Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 22.Suchting S. Freitas C. Ie Noble F. Benedito R. Breant C. Duarte A. Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci USA. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixelius J. Makinen T. Wirzenius M. Karkkainen MJ. Wernstedt C. Alitalo K. Claesson-Welsh L. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura K. Hirashima M. Ogawa M. Kubo H. Hisatsune H. Kondo N. Nishikawa S. Chiba T. Modulation of VEGFR-2-mediated endothelial-cell activity by VEGF-C/VEGFR-3. Blood. 2003;101:1367–1374. doi: 10.1182/blood-2002-05-1329. [DOI] [PubMed] [Google Scholar]
- 25.Covassin LD. Villefranc JA. Kacergis MC. Weinstein BM. Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci USA. 2006;103:6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ota H. Katsube K. Ogawa J. Yanagishita M. Hypoxia/Notch signaling in primary culture of rat lymphatic endothelial cells. FEBS Lett. 2007;581:5220–5226. doi: 10.1016/j.febslet.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Li YS. Kaneko M. Amatya VJ. Takeshima Y. Arihiro K. Inai K. Expression of vascular endothelial growth factor-C and its receptor in invasive micropapillary carcinoma of the breast. Pathol Int. 2006;56:256–261. doi: 10.1111/j.1440-1827.2006.01961.x. [DOI] [PubMed] [Google Scholar]
- 28.Petrova TV. Karpanen T. Norrmen C. Mellor R. Tamakoshi T. Finegold D. Ferrell R. Kerjaschki D. Mortimer P. Yla-Herttuala S. Miura N. Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 29.Seo S. Fujita H. Nakano A. Kang M. Duarte A. Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]