Abstract
Therapeutic guidelines of intravenous thrombolysis with tissue plasminogen activator (tPA) for hyperacute ischemic stroke are very strict. Because of potential higher risk of bleeding complications, the presence of unruptured cerebral aneurysm is a contraindication for systemic thrombolysis with tPA. According to the standard CT criteria, a 66-year-old woman who suddenly developed aphasia and hemiparesis received intravenous tPA within 3 h after ischemic stroke. Magnetic resonance angiography during tPA infusion was performed and the presence of a small unruptured cerebral aneurysm was suspected at the anterior communicating artery. Delayed cerebral angiography confirmed an aneurysm with a size of 7 mm. The patient did not experience any adverse complications associated with the aneurysm. Clinical experiences of this kind of accidental off-label thrombolysis may contribute to modify the current rigid tPA guidelines for stroke.
Key Words: Aneurysm, Stroke, Thrombolysis, Tissue plasminogen activator
Introduction
Intravenous thrombolysis with tissue plasminogen activator (tPA) has been licensed for selected patients within 3 h after ischemic stroke in many countries [1, 2], and a recent clinical study demonstrated the efficacy and safety up to 4.5 h after stroke onset [3]. However, the therapeutic guidelines of intravenous thrombolysis with tPA are very strict [1,2,3,4,5].
Because of potential higher risk of bleeding complications, the presence of an unruptured cerebral aneurysm is a contraindication for systemic thrombolysis with tPA [2, 4,5,6,7]. In this case study, we report a patient receiving intravenous alteplase within 3 h after ischemic stroke according to the standard CT criteria, in whom post-tPA investigations detected an unruptured cerebral aneurysm.
Case Report
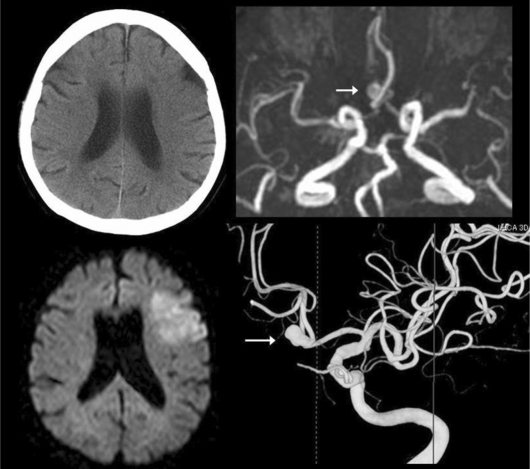
A 66-year-old woman without family history of subarachnoid hemorrhage (SAH) developed aphasia and right hemiparesis, and arrived at our hospital 135 min after symptom onset. The baseline National Institute of Health Stroke Scale was 10 points and baseline CT showed an early ischemic sign in the left frontal lobe (fig. 1). The patient met the guidelines for systemic thrombolysis with tPA [2] and received intravenous alteplase 177 min after onset. Magnetic resonance (MR) imaging during tPA infusion detected a focal territorial early ischemia in the left frontal lobe on the diffusion image, and MR angiography showed no arterial occlusion and a suspected unruptured cerebral aneurysm at the anterior communicating artery. The patient gradually improved without adverse complications associated with the aneurysm. The patient had mild aphasia without hemiparesis at 90 days, indicating the modified Rankin Scale of 1. Delayed cerebral angiography for endovascular coiling after 3-month outcome follow-up confirmed an unruptured cerebral aneurysm with a diameter of about 7 mm at the anterior communicating artery (fig. 1).
Fig. 1.
Baseline CT, MRI during thrombolysis, and delayed angiography. Baseline CT (left top) before intravenous thrombolysis shows a subtle early ischemic sign in the left frontal lobe. Diffusion image (left bottom) during tPA infusion demonstrates a hyperacute ischemia, and MR angiography (right top) shows no arterial occlusions and a suspected small aneurysm at the anterior communicating artery. Delayed cerebral angiography (right bottom) detects an unruptured aneurysm with a size of 7 mm at the anterior communicating artery.
Discussion
In this patient, the presence of an unruptured cerebral aneurysm was finally confirmed on cerebral angiography after 3-month clinical follow-up, although the presence of the aneurysm was suspected by MR angiography during tPA infusion. Therefore, the off-label use of tPA was not intentional but accidental. If this patient had undergone MR angiography before tPA infusion, she would not have received intravenous tPA [2]. However, according to the standard guidelines with the rigid 3-hour therapeutic time window [1,2,3,4,5], this patient received intravenous tPA. The patient had no complications associated with the aneurysm.
In clinical practice, standard CT-based thrombolysis with tPA for ischemic stroke may occasionally include non-stroke conditions [8]. Utilization of MRI or vascular imaging before thrombolysis can provide a higher accuracy of stroke diagnosis [9] and may detect the contra-indicated conditions such as cerebral aneurysm or other vascular anomalies. However, these additional imaging studies may delay the delivery of tPA.
In reports on off-label thrombolysis for stroke [4, 5], intentional off-label thrombolysis, such as intravenous alteplase for elderly patients over 80 years in Europe or for patients with seizure at onset, has been performed. In previous reports [4,5,6,7], only two patients in whom an incidental unruptured cerebral aneurysm was detected after intravenous tPA safely received systemic thrombolysis for stroke. Among about 70,000 patients receiving systemic thrombolysis for myocardial infarction, none experienced an aneurysmal SAH [10]. To our knowledge, only one patient developed an aneurysmal SAH after systemic thrombolysis for myocardial infarction [11]. The decision against systemic thrombolysis in the presence of an unruptured cerebral aneurysm has no scientific basis [4, 5]. In the general population, the incidence of an unruptured cerebral aneurysm has been reported to be 3.6-6% [12]. Therefore, many patients with an unruptured cerebral aneurysm might safely receive systemic thrombolysis with tPA, although unfavorable complications after off-label thrombolysis may have been underreported [5]. The safety of systemic thrombolysis with tPA in stroke patients with an unruptured cerebral aneurysm should be further examined, especially with regard to the size and location of the aneurysm.
In conclusion, data collection of both intentional and accidental off-label thrombolysis for stroke may contribute to modify the presently applied rigid guidelines.
References
- 1.Tissue plasminogen activator for acute ischemic stroke The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, Shinohara Y, for the Japan Alteplase Clinical Trial (J-ACT) Group Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 h of onset. Stroke. 2006;37:1810–1815. doi: 10.1161/01.STR.0000227191.01792.e3. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 4.De Keyser J, Gdovinová Z, Uyttenboogaart M, Vroomen PC, Luijckx GJ. Intravenous alteplase for stroke: beyond the guidelines and in particular clinical situations. Stroke. 2007;38:2612–2618. doi: 10.1161/STROKEAHA.106.480566. [DOI] [PubMed] [Google Scholar]
- 5.Aleu A, Mellado P, Lichy C, Köhrmann M, Schellinger PD. Hemorrhagic complications after off-label thrombolysis for ischemic stroke. Stroke. 2007;38:417–422. doi: 10.1161/01.STR.0000254504.71955.05. [DOI] [PubMed] [Google Scholar]
- 6.D'Olhaberriagure L, Joshi N, Chaturvedi S, et al. Tissue plasminogen activator for acute ischaemic stroke in patients with unruptured cerebral aneurysms. J Stroke Cerebrovasc Dis. 2000;9:181–184. doi: 10.1053/jscd.2000.7213. [DOI] [PubMed] [Google Scholar]
- 7.Kane I, Sandercock P, Thomas B. Can patients with unruptured intracranial aneurysms be treated with thrombolysis? Cerebrovasc Dis. 2005;20:51–52. doi: 10.1159/000086282. [DOI] [PubMed] [Google Scholar]
- 8.Scott PA, Silbergleit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42:611–618. doi: 10.1016/s0196-0644(03)00443-8. [DOI] [PubMed] [Google Scholar]
- 9.Yoneda Y, Yamamoto S, Hara Y, et al. Post-licensed 1-year experience of systemic thrombolysis with tissue plasminogen activator for ischemic stroke in a Japanese neuro-unit. Clin Neurol Neurosurg. 2007;109:567–570. doi: 10.1016/j.clineuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Gurwitz JH, Gore JM, Goldberg RJ, et al. Risk for intracranial hemorrhage after tissue plasminogen activator treatment for acute myocardial infarction. Ann Intern Med. 1998;129:597–604. doi: 10.7326/0003-4819-129-8-199810150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Lagares A, Gómez PA, Lobato RD, Alén JF, Campollo J, Benito-León J. Cerebral aneurysm rupture after r-TPA thrombolysis for acute myocardial infarction. Surg Neurol. 1999;52:623–626. doi: 10.1016/s0090-3019(99)00147-0. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123:205–221. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]