Abstract
The basolateral amygdala is a nodal structure within a distributed and interconnected network that regulates anxiety states and anxiety-related behavior. Administration of multiple anxiogenic drugs increases cellular responses (i.e., increases c-Fos expression) in a subregion of the basolateral amygdala, but the neurochemical phenotypes of these cells are not known. The basolateral amygdala contains glutamatergic projection neurons and several populations of γ-aminobutyric acid-synthesizing (GABAergic) interneurons, including a population of parvalbumin (PV)-expressing GABAergic interneurons that co-express the excitatory 5-HT2A receptor. The role for these PV-expressing GABAergic interneurons in anxiety-states is unclear. In this experiment we examined the effects of multiple anxiogenic drugs including the 5-HT2A/2C receptor agonist m-chlorophenyl piperazine (mCPP), the adenosine receptor antagonist caffeine, the α2-adrenoreceptor antagonist yohimbine and the partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor, N-methyl-beta-carboline-3-carboxamide (FG-7142), on c-Fos expression in PV-immunoreactive (PV-ir) interneurons in subdivisions of the basolateral amygdala. All drugs with the exception of mCPP increased c-Fos expression in PV-ir neurons in the basolateral amygdaloid nucleus, anterior part (BLA). The numbers of c-Fos-immunoreactive (c-Fos-ir)/PV-ir GABAergic interneurons in the BLA were positively correlated with the numbers of c-Fos-ir serotonergic neurons in the mid-rostrocaudal dorsal raphe nucleus (DR) and with a measure of anxiety-related behavior. All four drugs increased c-Fos expression in non-PV-ir cells in most of the subdivisions of the basolateral amygdala that were sampled, compared with vehicle-injected controls. Together, these data suggest that the PV/5-HT2A receptor expressing GABAergic interneurons in the basolateral amygdala are part of a DR-basolateral amygdala neuronal circuit modulating anxiety-states and anxiety-related behavior.
Keywords: anxiety, basolateral amygdala, c-Fos, dorsal raphe nucleus, serotonin
Introduction
Anxiety is a complex emotional state associated with heightened physiological and behavioral arousal. Although the mechanisms underlying the regulation of anxiety states and anxiety-related behaviors are not well understood, the physiological and behavioral arousal appears to be regulated by a distributed and interconnected system of forebrain and hindbrain structures that include the dorsal raphe nucleus (DR) and the basolateral amygdala. Consistent with this hypothesis, administration of multiple anxiogenic drugs with diverse pharmacological mechanisms increases c-Fos expression in serotonergic neurons in the DR and in cells within the basolateral amygdala, however the neurochemical phenotypes of the c-Fos-immunopositive cells in the basolateral amygdala are not known.
The basolateral amygdala is an important component of anxiety-related neuronal circuitry. We have reported that exposure of rats to a novel open-field arena increases c-Fos expression in the basolateral amygdala and in a neuronal circuit projecting to the basolateral amygdala including the DR. Activation of the basolateral amygdala induces an increase in anxiety-state, for example, administration of the stress-related neuropeptides corticotropin-releasing factor (CRF) or the CRF-related neuropeptide urocortin 1 (Ucn1) into the basolateral amygdala increases anxiety-related behavior in the social interaction (SI) test. In contrast, inhibition of the basolateral amygdala with N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor antagonists or administration of the benzodiazepine receptor agonist, midazolam, decreases anxiety-like behavior in the SI test. Drugs acting at serotonin receptors in the basolateral amygdala may have anxiolytic or anxiogenic effects depending on the receptor subtype involved and the paradigm used to measure anxiety-related behaviors.
Several lines of evidence suggest the involvement of a serotonergic DR–basolateral amygdala neuronal circuit in the regulation of anxiety states and anxiety-related behavior. The DR sends dense projections to the basolateral amygdala. We have previously reported that most but not all projections from the DR to the basolateral amygdala are serotonergic and mostly located in the mid-rostrocaudal region of the dorsal raphe nucleus, dorsal part (DRD). This region of the DR has been consistently shown to be activated in response to stress and anxiety-related stimuli, including anxiogenic drugs, social defeat, uncontrollable stress, and intracerebroventricular administration of the stress and anxiety-related neuropeptide urocortin 2 (Ucn 2). Consistent with the hypothesis of a DR-basolateral amygdala anxiety-related neuronal circuit, direct microinjection of Ucn 2 into the DRD increases c-Fos expression in serotonergic neurons in the DRD and is associated with increases in extracelluar serotonin concentrations in the basolateral amygdala. In addition, exposure to uncontrollable tail shock increases extracellular serotonin concentrations in the basolateral amygdala in response to a juvenile social interaction test measured 24 h later in a model of learned helplessness. Although these data provide evidence for a serotonergic DR–basolateral amygdala circuit regulating anxiety states, the identity of the specific neurons within the basolateral amygdala through which serotonin regulates anxiety-related behavior or anxiety states is not known.
The basolateral amygdala contains two major classes of neurons, the large pyramidal cells (projection neurons) and the smaller non-pyramidal cells (interneurons). These interneurons are primarily GABAergic and four subgroups can be distinguished based on calcium binding protein and neuropeptide content, including a subset of GABAergic interneurons that express the calcium binding protein, parvalbumin (PV). The PV subset of interneurons has been shown to express the excitatory 5-HT2A receptor and, upon activation of 5-HT2A receptors, provides inhibitory drive to basolateral amygdala projection neurons. Several lines of evidence suggest that a dominant effect of serotonin in the basolateral amygdala is a 5-HT2A-mediated inhibition of projection neurons. Nevertheless, the role for the PV/5-HT2A expressing subset of GABAergic interneurons in the regulation of anxiety states remains unclear. In this experiment we test the hypothesis that the PV/5-HT2A receptor-expressing subset of GABAergic interneurons in the basolateral amygdala is an important component of an anxiety-related neuronal circuit. We examined the effects of four anxiogenic drugs including the 5-HT2C/2A receptor agonist m-chlorophenyl piperazine (mCPP), the adenosine receptor antagonist caffeine, the α2-adrenoreceptor antagonist yohimbine and the partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor, N-methyl-beta-carboline-3-carboxamide (FG-7142), on c-Fos expression in PV-immunoreactive (PV-ir) interneurons in subdivision of the basolateral amygdaloid complex. Subdivisions that were analyzed included the lateral amygdaloid nucleus, dorsolateral part (LaDL), ventrolateral part (LaVL) and ventromedial part (LaVM) and basolateral amygdaloid nucleus, anterior part (BLA) and posterior part (BLP) across three rostrocaudal levels. As we have previously shown that exposure to an anxiety test (open-field) increases c-Fos expression in the basolateral amygdala, we predicted that c-Fos treatment with anxiogenic drugs would also increase c-Fos expression in the basolateral amygdala.
Method
Animals
The rat brain sections used for immunohistochemical staining of PV and c-Fos were derived from rat brains collected in a previously published study examining the effects of anxiogenic drugs on c-Fos expression in serotonergic neurons in the dorsal raphe complex. For detailed descriptions of the methods see,. Briefly, adult male Wistar rats (250–300 g; B&K Universal, Hull, UK) were acclimatized to the animal facility for 1 week in group housing (four/cage), then single-housed on a 14:10-h light/dark cycle (lights on at 05:00 h) and habituated to the experimental room (36–48 h) before the experiment. Food and water were provided ad libitum. Injections were performed using a randomized experimental design utilizing 16 rats each day on 2 separate days (during the rats’ inactive phase). Time-matched groups of rats were injected between 09:00 and 17:00 h. The experiment was approved by the University of Bristol Ethical Review Group, was carried out according to the UK Animals (Scientific Procedures) Act 1986, and was consistent with the NIH Guide for the Care and Use of Laboratory Animals (N.I.H. Publication No. 85-23).
Drugs
On the test days rats were injected i.p. with either saline vehicle (n = 8), the 5-HT2A/2C receptor agonist m-chlorophenyl piperazine (mCPP; Sigma-Aldrich, Dorset, UK; 5 mg/kg; n = 6), the adenosine receptor antagonist caffeine (Fluka, Dorset, UK; 50 mg/kg; n = 6), the α2-adrenoreceptor antagonist yohimbine (Sigma-Aldrich; 5 mg/kg; n = 6), or the partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor, FG-7142 (Tocris, Avonmouth, UK; 7.5 mg/kg; n = 6). These drugs and doses were selected based on previous research examining the effects of anxiogenic drugs on c-Fos expression in forebrain and hindbrain components of an anxiety and fear-related neuronal circuit and, at the doses used, all of the drugs have all been shown to be anxiogenic in animal studies: mCPP 5, mg/kg, caffeine, 50 mg/kg, yohimbine, 5 mg/kg and FG-7142, 7.5 mg/kg. All drugs were dissolved in 0.9% saline, except for FG-7142, which was dissolved in 0.9% saline/40% 2-hydroxypropyl-cyclodextrin (Tocris) to increase solubility as in previous studies. We have previously reported that each of the anxiogenic drugs increased vigilance and arousal behaviors in the rats’ home cage environments from 30 – 90 min following drug administration (90 min before perfusion) compared with vehicle-injected controls.
Experimental Procedure
Two hours following drug injection, rats were deeply anesthetized with sodium pentobarbital (0.65 mg/kg i.p.; Sagatal, Rhone-Merieux, Dublin, Ireland) and transcardially perfused with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB; pH 7.4). Following fixation, the brains were removed and post-fixed in the 4% paraformaldehyde solution for 12 h at 4 °C and were then rinsed in 0.1 M PB twice for 12 h. The brains were then placed in 30% sucrose in 0.1 M PB for 12 h. Brains were then blocked into two pieces with a cut in the coronal plane at the caudal border of the mammillary bodies (approximately −5.30 mm bregma) using a rat brain matrix (RBM-4000C, ASI Instruments, Warren, MI, USA) and rapidly frozen in isopentane cooled with liquid nitrogen. The brains were then stored at −80 °C. Forebrain sections including the basolateral amygdala (30 µm) were then prepared using a cryostat (Leica CM1900, Leica Microsystems Nussloch GmbH, Nussloch, Germany) and stored as 6 alternate sets of sections. The tissue was stored at −20 °C in cryoprotectant (30% ethylene glycol (w/w), 20% glycerol (w/w), 0.05 M phosphate buffered saline) until immunohistochemical procedures were conducted.
Immunohistochemistry
One set of brain sections, including the basolateral amygdala, was used for double immunostaining using primary antibodies directed against the protein product of the immediate early gene c-fos (rabbit anti-c-Fos polyclonal antibody, Cat# PC-38, Lot #D00007099, Calbiochem, EMD Chemicals, Gibbstown, NJ, USA) and against parvalbumin (PV; mouse anti-PV, Cat#P3088, Lot#033K4846, Sigma-Aldrich, St. Louis, MO, USA). All washes and incubation steps were performed in 12-well plates (Costar, Corning, NY, USA) with mesh well inserts (Costar) except as noted. First, tissue was washed three times for 15 min in 0.1 M phosphate buffered saline (PBS) and then washed for 20 min in 1% H2O2 in 0.1 M PBS to inhibit endogenous peroxidase activity. Tissue was then rinsed twice for 15 min each time in 0.1 M PBS and preincubated with 0.3% Triton X-100 (Cat# BP151-500, Fisher Scientific, Pittsburg, PA, USA) in 0.1 M PBS (0.3% PBST) for 15 min. The sections were then incubated overnight in rabbit anti-c-Fos primary antibody (1:8,000) in 0.1 M PBS. The following day tissue was rinsed three times for 15 min each time in 0.3% PBST and then incubated with goat anti-rabbit biotinylated secondary antibody (1:500; Cat #BA-1000, Lot#T1101; Vector Laboratories, Burlingame, CA, USA) in 0.1% Triton X-100 in PBS (0.1% PBST) for 60 min. The tissue was again washed three times for 15 min each time in 0.3% PBST and then incubated with an avidin-biotin-peroxidase complex (Vectastain ABC reagent, Cat#PK-6100; 1:1000; Vector Laboratories). The tissue was then washed for 15 min in 0.3% PBST and then twice for 15 min each time in 0.1 M PBS followed by incubation with peroxidase chromogen substrate (Vector SG; Cat#SK4700; Vector Laboratories; diluted according to manufacturer’s instructions; 3 ml volume per well) for 20 min in 12-well plates without the mesh well inserts. Following the chromogen reaction tissue was rinsed for 15 min in PBS and then twice for 15 min each time in 0.3% PBST. The tissue was then incubated in mouse anti-PV primary antibody (1:80,000) in 0.3% PBST overnight at room temperature. All subsequent steps were identical to those described above for the immunoperoxidase localization of c-Fos-immunoreactivity, except for the secondary antibody and chromogen reaction steps; these used a goat anti-mouse secondary antibody (Cat#BA-9200 1:500, Vector Laboratories), and a peroxidase chromogen substrate solution consisting of a 10 mg tablet of 3,3’-diaminobenzidine tetrahydrochloride (DAB; Cat#D5905-50TAB; Sigma-Aldrich, St. Louis, MO, USA) and 0.01% H2O2 in solution in 100 ml PBS (20 min). Finally, sections were washed for 15 min in PBS to stop the reaction and twice in 0.3% PBST. Brain sections were briefly placed into a 0.15% gelatin in distilled water solution then mounted on microscope slides (Cat#12-550-15; Fisherbrand, Pittsburg, PA, USA); dehydrated through an alcohol series; and cleared with xylene. The slides were then mounted with cover slips using mounting medium (Cat#360294H, BDH; Poole, UK). The color reaction of the c-Fos immunostaining was blue/black and localized in cell nuclei and the color reaction for the PV immunostaining was orange–brown and localized in fibers and cell bodies.
Cell counting
Cell counting for the c-Fos/PV immunostaining was conducted using a Leica DME microscope. The numbers of c-Fos-immunoreactive (c-Fos-ir)/PV-immunoreactive (PV-ir) neurons, the numbers of c-Fos-ir/PV-immunonegative cells and the total numbers of PV-ir neurons were counted in subdivisions of the basolateral amygdala at three rostrocaudal levels, including −2.12 mm, −3.30 mm and −3.80 mm bregma. Multiple rostrocaudal levels were selected for analysis as we have previously shown that the mid-rostrocaudal basolateral amygdala (at approximately −3.30 mm bregma) is preferentially activated in response to exposure to an open-field arena in low- and high-light conditions. In addition, previous research has demonstrated that injections of the benzodiazepine, midazolam, into the most rostral or most caudal regions of basolateral amygdaloid complex have either no anxiolytic effects, or inconsistent effects in the Vogel conflict test, whereas injections into the mid-rostrocaudal basolateral amygdaloid complex produce highly significant anxiolytic effects. The subdivisions selected for analysis included the LaDL and BLA at −2.12 mm bregma, the LaDL, LaVM, LaVL, BLA and BLP at −3.30 mm bregma and the LaDL, LaVM and BLP at −3.80 mm bregma (Fig. 1). Immunostaining for PV showed a greater density of PV-ir fibers in the LaDL, BLA and BLP compared with the LaVL and LaVM, delineating the borders between the subdivisions. The borders between the LaVL and LaVM and the BLA and BLP were estimated using a template from a standard rat brain atlas. Both left and right hemispheres were counted for each rat and the cell counts in the two hemispheres were summed. Cell counts were conducted by an experimenter (AMW) blind to treatment groups. One brain section was counted for each rat using a 10× objective lens (100× total magnification). Double immunostained cells were confirmed using a 40× objective lens (400× total magnification).
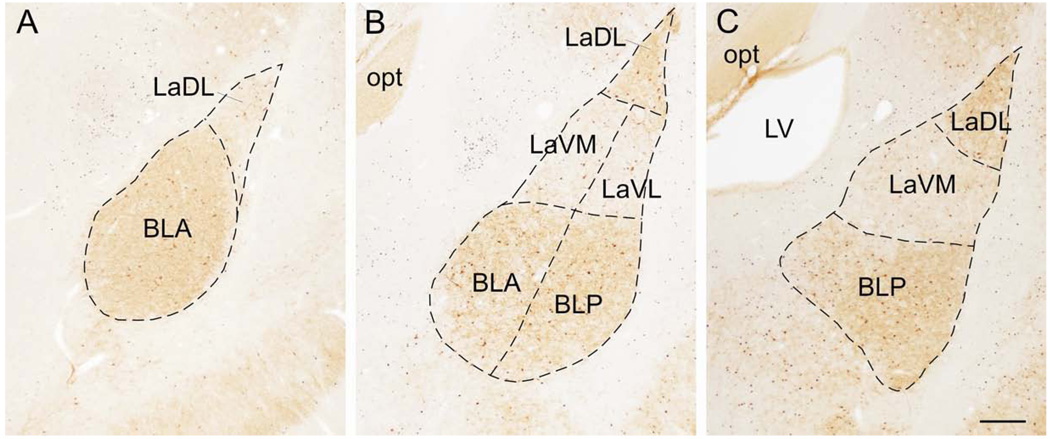
Figure 1.
Photomicrographs showing parvalbumin (PV) and c-Fos immunostaining in the subregions of the basolateral amygdaloid complex at −2.12 mm, −3.30 mm and −3.80 mm from bregma. A) Low power photomicrograph showing the basolateral amygdaloid complex at approximately −2.12 mm bregma. Dashed lines show the borders of the subregions including the lateral amygdaloid nucleus, dorsolateral part (LaDL) and basolateral amygdaloid nucleus, anterior part (BLA). B) Low power photomicrograph showing the basolateral amygdaloid complex at approximately −3.30 mm bregma. Dashed lines show the borders of the subregions including the LaDL, lateral amygdaloid nucleus, ventrolateral part (LaVL), lateral amygdaloid nucleus, ventromedial part, BLA, and basolateral amygdaloid nucleus, posterior part (BLP). C) Low power photomicrograph showing the basolateral amygdaloid complex at approximately −3.80 mm bregma. Dashed lines show the borders of the subregions including the LaDL, LaVM and BLP. Abbreviations: LV, lateral ventricle; opt, optic tract. Scale bar, 250 µm.
Image capture
Photomicrographs were taken using a Nikon 90i microscope and a Nikon DS-Fi1 digital camera linked to a computer with NIS Elements 3.00 imaging software (A.G. Heinze Inc., Lake Forest, CA, USA). Photographic plates were prepared in CorelDraw for Windows 12.0 (Viglen Ltd., Wembley, UK).
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) with repeated measures followed, when appropriate, by post hoc Dunnett’s test for multiple comparisons with a single control using PASW Statistics (17.0.2 for Windows, SPSS Inc., Chicago, IL, USA). A Greenhouse-Geisser correction epsilon (ε) was used for repeated measures analysis to correct for potential violation of the sphericity assumption. The cell counts for the numbers of c-Fos-ir/ PV-ir neurons, the numbers of c-Fos-ir/PV-immunonegative cells, and the total numbers of PV-ir neurons were analyzed separately using treatment group (5 levels: saline, mCPP, caffeine, yohimbine and FG-7142) as a between-subjects factor and brain region (10 levels; LaDL and BLA at −2.12 mm bregma, LaDL, LaVL, LaVM, BLA and BLP at −3.30 mm bregma, LaDL, LaVM and BLP at −3.80 mm bregma) as a within-subjects factor. Correlation analysis using Pearson’s correlation was conducted for the numbers of c-Fos-ir/PV-ir neurons in the BLA at −3.30 mm bregma from the present study and the numbers of c-Fos-ir/tryptophan hydroxylase-immunoreactive neurons (i.e. c-Fos-ir serotonergic neurons) in the DR and the percentage of time engaged in spontaneous non-ambulatory locomotor activity (SNAMA), a behavior that may represent an increase in vigilance and risk assessment in the absence of a clearly identifiable threat in a home cage environment, in the 60–90 min time period following drug administration from data reported in a previous publication. Significance was accepted for the ANOVAs and post hoc Dunnett’s tests when p < 0.05. For the correlation analysis, a Bonferroni correction was used to allow for multiple comparisons and significance was accepted when p < 0.025.
Outliers (2.6% of the total data) were identified using Grubbs test and excluded. For the repeated measures ANOVA, replacement values for the excluded outliers and for missing data (6.5% of the total data) were calculated using the Petersen method. Replacement values were not included in post hoc analyses and are not represented in graphical representations of the data.
Results
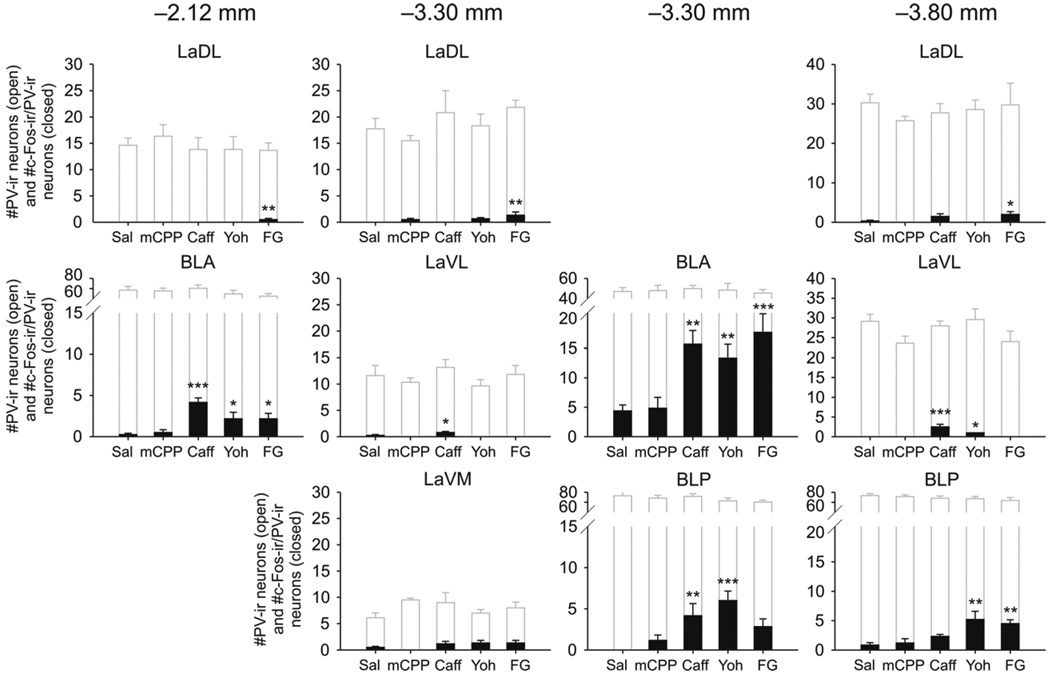
Injection of anxiogenic drugs increased c-Fos expression in PV-ir neurons in the basolateral amygdala (drug × region interaction, F(36, 243) = 6.47, p < 0.001, ε = 0.213; drug main effect, F(4,27) = 11.44, p < 0.001; region main effect, F(9, 243) = 95.03, p < 0.001, ε = 0.213; Fig. 2 (closed bars), Fig. 3). Post hoc Dunnett’s tests showed increases in the numbers of c-Fos-ir/PV-ir neurons in rats treated with caffeine, yohimbine and FG-7142 compared with saline-treated controls in the BLA at −2.12 mm and −3.30 mm bregma, however the greatest increase in the numbers of c-Fos-ir/PV-ir neurons was in the BLA at −3.30 mm bregma. Caffeine also increased the numbers of c-Fos-ir/PV-ir neurons in the LaVL at −3.30 mm bregma and caffeine and yohimbine increased c-Fos-ir/PV-ir in the LaVL at −3.80 mm bregma. In the BLP, caffeine and yohimbine increased the numbers of c-Fos-ir/PV-ir neurons at −3.30 mm bregma and yohimbine and FG-7142 increased the numbers of c-Fos-ir/PV-ir neurons at −3.80 mm bregma. There was a small but statistically significant increase in c-Fos-ir/PV-ir neurons in FG-7142-treated rats compared with saline-treated rats in the LaDL at all three rostrocaudal levels sampled. There were no differences in the numbers of c-Fos-ir/PV-ir neurons in rats treated with mCPP compared with saline-treated controls in any subdivision.
Figure 2.
Anxiogenic drugs increased c-Fos expression in parvalbumin (PV)-immunoreactive (ir) neurons in the basolateral amygdaloid nucleus complex. Graphs show the numbers of c-Fos-ir/PV-ir neurons and total numbers of PV-ir neurons in subdivisions of the basolateral amygdaloid complex at −2.12 mm bregma (left column), −3.30 mm bregma (middle columns) and −3.80 mm bregma (right column). Closed bars represent the numbers of c-Fos-immunoreactive (c-Fos-ir)/PV-ir neurons. Open bars represent the total numbers of PV-ir neurons within each subdivision. *p < 0.05, **p < 0.01, ***p < 0.001 versus saline-treated controls, post hoc Dunnett’s tests, (saline, n = 8; mCPP, n = 6; caffeine, n = 6; yohimbine, n = 6; FG-7142, n = 6). Abbreviations: BLA, basolateral amygdaloid nucleus, anterior part; BLP, basolateral amygdaloid nucleus, posterior part; Caff, caffeine; FG, N-methyl-beta-carboline-3-carboxamide (FG-7142); LaDL, lateral amygdaloid nucleus, dorsolateral part; LaVL, lateral amygdaloid nucleus, ventrolateral part; LaVM, lateral amygdaloid nucleus, ventromedial part; mCPP, m-chlorophenyl piperazine; Sal, saline; Yoh, yohimbine.
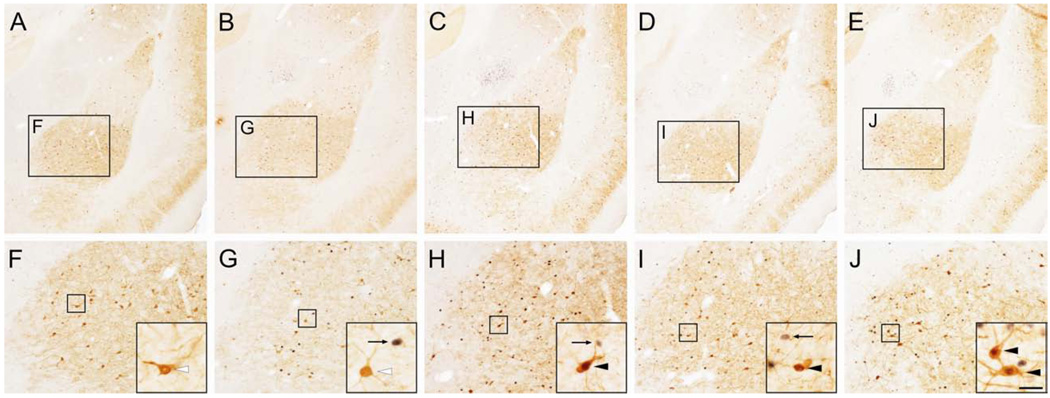
Figure 3.
Photomicrographs illustrating c-Fos expression in parvalbumin (PV)-immunoreactive (ir) neurons in the basolateral amygdaloid nucleus, anterior part (BLA) at approximately −3.30 mm bregma in rats injected with A) saline; B) m-chlorophenyl piperazine (mCPP); C) caffeine; D) yohimbine; and E) N-methyl-beta-carboline-3-carboxamide (FG-7142). A–E) Low power photomicrographs showing the basolateral amygdaloid complex. Black boxes in A–E are shown at higher magnification in F–J. F–J) Higher power photomicrographs showing the basolateral amygdaloid nucleus. Black boxes in F–J are shown at higher magnification in insets in the lower right corner of each panel. Black arrows indicate c-Fos-ir/PV-immunonegative cells (blue/black nuclear staining), white arrowheads indicate c-Fos-immunonegative/PV-ir neurons (brown/orange cytoplasmic staining) and black arrowheads indicate c-Fos-ir/PV-ir (double-immunostained) neurons. Scale bar, 250 µm (A–E), 100 µm (F–J), 25 µm (insets).
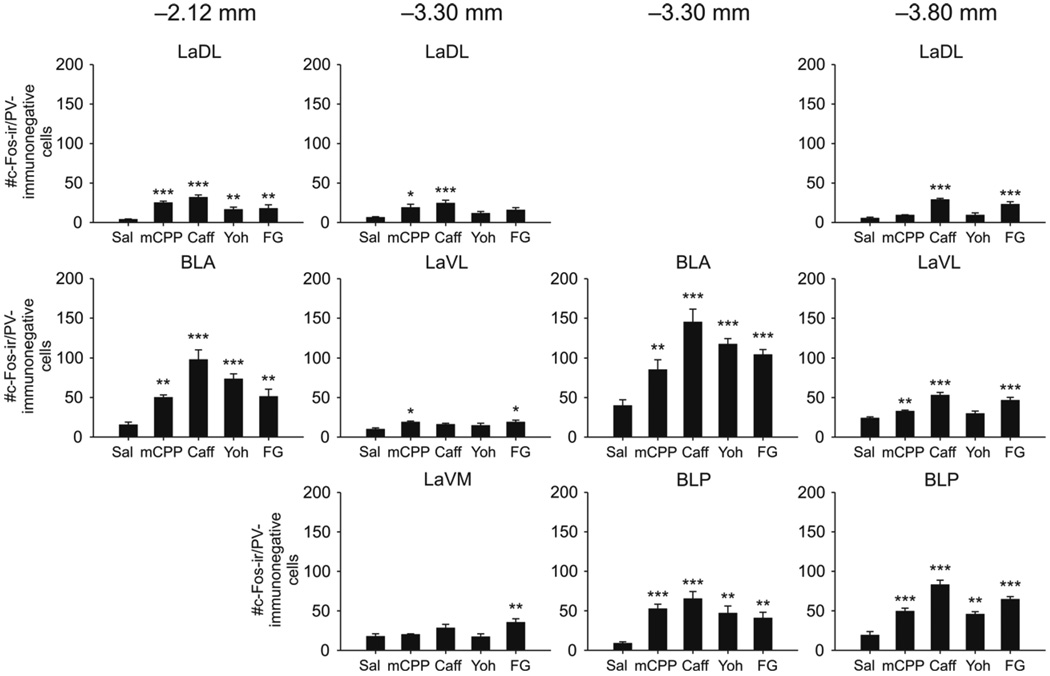
Anxiogenic drugs also increased c-Fos expression in PV-immunonegative cells in all of the subdivisions that were sampled within the basolateral amygdala (drug × region interaction, F(36, 243) = 9.49, P < 0.001, ε = 0.323; drug main effect, F(4,27) = 19.96, p < 0.001; region main effect, F(9, 243) = 177.49, p < 0.001, e = 0.323; Fig 3, 4). All four anxiogenic drugs increased c-Fos expression in PV-immunonegative cells in the LaDL and BLA at −2.12 mm bregma, the BLA and BLP at −3.30 mm bregma, and the BLP at −3.80 mm bregma. The greatest increase in the numbers of c-Fos-ir/PV-immunonegative cells was in the BLA at −3.30 mm bregma. mCPP additionally increased c-Fos expression in PV-immunonegative cells in the LaDL at −3.30 mm bregma and the LaVL at −3.30 and −3.80 mm bregma. Caffeine additionally increased c-Fos expression in PV-immunonegative cells in the LaDL at −3.30 and −3.80 mm bregma and the LaVL at −3.80 mm bregma. Finally, FG-7142 additionally increased c-Fos expression in PV-immunonegative cells in the LaVL and LaVM at −3.30 mm bregma and the LaDL and LaVL at −3.80 mm bregma.
Figure 4.
Anxiogenic drugs increased c-Fos expression in non-parvalbumin (PV)-immunoreactive (ir) cells in the basolateral amygdaloid complex. Graphs show the numbers of c-Fos-ir/PV-immunonegative cells in subdivisions of the basolateral amygdaloid complex at −2.12 mm bregma (left column), −3.30 mm bregma (middle columns) and −3.80 mm bregma (right column). *p < 0.05, **p < 0.01, ***p < 0.001 versus saline-treated controls, post hoc Dunnett’s tests, (saline, n = 8; mCPP, n = 6; caffeine, n = 6; yohimbine, n = 6; FG-7142, n = 6). For abbreviations see Figure 2 legend.
The numbers of PV-ir neurons varied across the subdivisions of the basolateral amygdala sampled (region main effect, F(9,243) = 229.77, p < 0.001, ε = 0.336; Fig 2. open bars). However there was no difference in the numbers of PV-ir neurons across treatments.
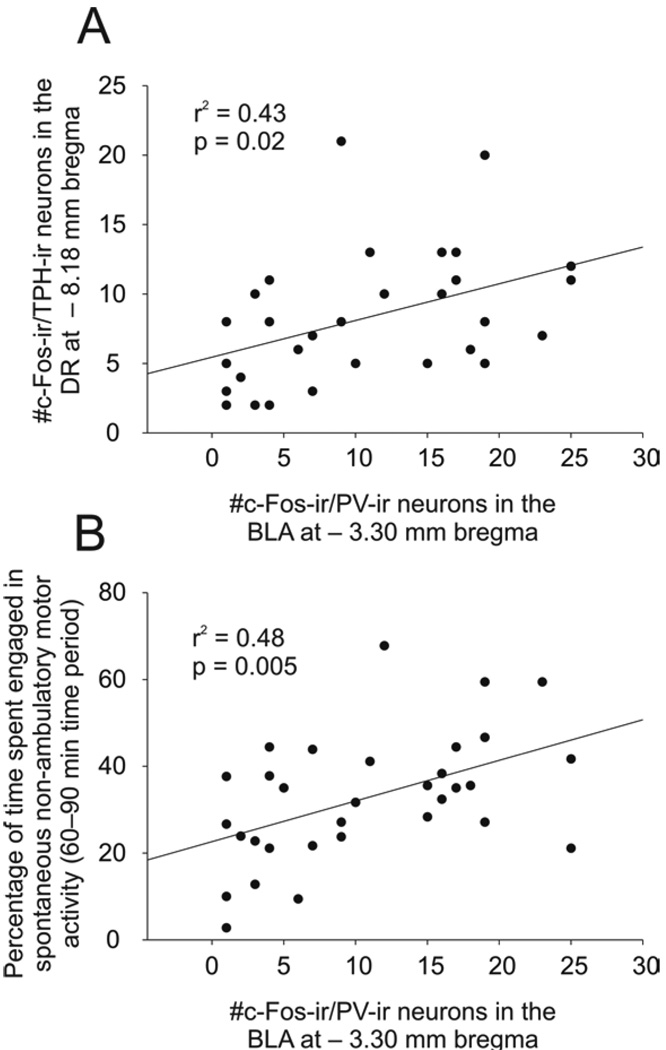
The numbers of c-Fos-ir/PV-ir neurons in the BLA were positively correlated with the numbers of c-Fos-ir serotonergic neurons (c-Fos-ir/tryptophan hydroxylase (TPH)-ir neurons; based on data from a previously published manuscript) in the DR at −8.18 mm bregma (r2 = 0.43, p = 0.020; Fig. 5A). The numbers of c-Fos-ir/PV-ir neurons in the BLA were also correlated with anxiety-related behavior (r2 = 0.48, p = 0.005; Fig. 5B). Anxiety-related behavior (based on data from a previously published manuscript; Abrams et al. 2005) was measured in the rats’ home cages as the percentage of time rats spent engaged in SNAMA between 60 and 90 min following drug administration.
Figure 5.
Activation of parvalbumin (PV)-expressing γ-aminobutyric acid (GABA)ergic interneurons in the basolateral amygdaloid nucleus, anterior part (BLA) was associated with activation of serotonergic neurons in the dorsal raphe nucleus, dorsal part (DR) and anxiety-related behavior. A) Scatter and line plot showing a positive correlation between the numbers of c-Fos-immunoreactive (ir)/PV-ir neurons in the BLA and the numbers of c-Fos-ir/tryptophan hydroxylase-ir neurons in the dorsal raphe nucleus (DR) at −8.18 mm bregma. B) Scatter and line plot showing a positive correlation between the numbers of c-Fos-ir/PV-ir neurons in the BLA and the percentage of time spent engaged in spontaneous non-ambulatory motor activity (SNAMA) in the 60–90 min time period post drug injection.
Discussion
Multiple anxiogenic drugs with diverse pharmacological properties increased c-Fos expression in PV-expressing GABAergic interneurons in the basolateral amygdala. The greatest increases were observed in mid-rostrocaudal region of the BLA. All drugs increased c-Fos expression in PV-ir interneurons within the mid-rostrocaudal BLA with the exception of the 5-HT2A/2C receptor agonist mCPP. The number of c-Fos-ir/PV-expressing GABAergic interneurons in the mid-rostrocaudal BLA was positively correlated with the number of c-Fos-ir serotonergic neurons in the mid-rostrocaudal DR, a region that is known to give rise to dense serotonergic projections to the basolateral amygdala and with a measure of anxiety-related behavior measured between 60 and 90 min following drug administration. All four drug treatments also increased c-Fos expression in non-PV-ir cells in multiple subdivisions across the rostrocaudal extent of the basolateral amygdala, compared with saline-injected controls. Previous studies have demonstrated that serotonin selectively activates a subset of GABAergic interneurons within the BLA via activation of 5-HT2A receptors. Together, these data suggest that the PV-expressing, 5-HT2A receptor expressing GABAergic interneurons in the BLA are part of a DR-BLA neuronal circuit modulating anxiety-states and anxiety-related behavior.
Anxiogenic drugs including caffeine, yohimbine and FG-7142 had convergent effects to increase c-Fos expression in PV-containing GABAergic interneurons in the BLA. The BLA is an important component of the neural circuitry that regulates anxiety-states and anxiety-related behavior. The basolateral amygdala contains two major classes of neurons, the large pyramidal cells (projection neurons) and the smaller non-pyramidal cells (interneurons). The interneurons are primarily GABAergic and four subgroups can be distinguished based on calcium binding protein and neuropeptide content, and anatomical evidence suggests that the subset of interneurons that express PV co-expresses the 5-HT2A receptor. Serotonergic terminals form synaptic contacts with both pyramidal and non-pyramidal (including PV-ir) neurons in the basolateral amygdala, however electrophysiological evidence suggests that the effects of serotonin on projection neurons within the basolateral amygdala are mostly inhibitory, through activation of 5-HT2A receptors located on PV-expressing GABAergic interneurons. This PV-expressing population of GABAergic interneurons provides a robust inhibition of basolateral amygdala projections neurons, and is therefore important in regulating basolateral amygdala output and is potentially important in the regulation of anxiety-states and anxiety-related behavior.
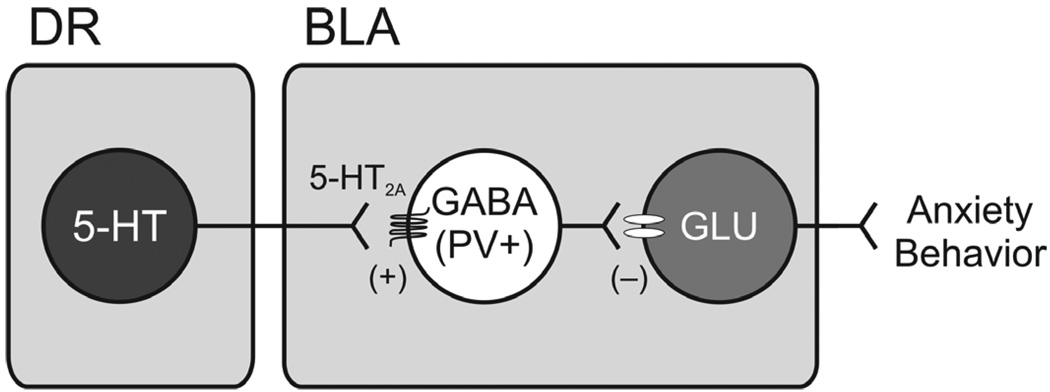
Increases in neuronal activity of PV-containing neurons in the BLA could be dependent on direct actions of the anxiogenic drugs in the BLA, indirect actions on BLA afferent regions including serotonergic neurons in the DR or both. The BLA receives dense projections from the DRD, a region that has consistently been shown to be associated with stress- and anxiety-like responses. We have previously shown that serotonergic neurons in the DRD are activated following administration of anxiogenic drugs, social defeat and intracerebroventricular administration Ucn 2. Direct microinjection of Ucn 2 into a region of the caudal DR including the DRD increases c-Fos expression in serotonergic neurons in the caudal DR and is associated with increases in extracelluar serotonin in the basolateral amygdala. In the present study, the increases in c-Fos expression in PV-containing interneurons in the BLA was correlated with increased c-Fos expression in serotonergic neurons in the DR. Together, these data are consistent with a hypothetical model in which activation of DR serotonergic neurons inhibit BLA projection neurons via activation of the 5-HT2A receptor expressing subpopulation of PV-expressing GABAergic interneurons (Fig. 6).
Figure 6.
Hypothetical model in which activation of dorsal raphe nucleus (DR) serotonergic neurons results in inhibition of projection neurons within the basolateral amygdala, anterior part (BLA) via activation of the 5-HT2A receptor-expressing subpopulation of local parvalbumin (PV)-expressing γ-aminobutyric acid (GABA)ergic interneurons, resulting in a reduction of excitatory output to circuits mediating anxiety-related behavior.
Alternatively, anxiogenic drugs may also act directly in the BLA. For example FG-7142 may act directly on PV-ir interneurons in the BLA. Although it is not specific, FG-7142 has the highest affinity for the α1 subunit of the GABAA receptor. Anatomical evidence has shown that most (96.4%) of PV-expressing interneurons in the basolateral amygdala are immunoreactive for the α1 subunit of the GABAA receptor, while the somata of basolateral amygdala pyramidal neurons are not. Therefore, although it is possible that FG-7142 and 5-HT both activate PV-expressing interneurons, further research is required to confirm such interactions following peripheral injections of FG-7142.
The numbers of c-Fos-ir/PV-expressing GABAergic interneurons in the BLA were positively correlated with the numbers of c-Fos-ir serotonergic neurons in the mid-rostrocaudal DR, a region that is known to give rise to serotonergic projections to the BLA and with SNAMA, a measure of anxiety-related behavior, measured between 60 and 90 min following drug administration. SNAMA has been characterized as a measure of increased vigilance and risk assessment in the absence of novelty or clearly identifiable threat and includes behaviors such as visual scanning of the environment, head movements associated with sniffing, shifts in body position and non-ambulatory limb movements. For a discussion of the increases in anxiety-related behaviors following administration of these anxiogenic drugs, see our previous publication. These data suggest that the DR–BLA neuronal circuit may be important in the regulation of anxiety-related behaviors. However it should be noted here that these correlations do not necessarily imply that the DR and BLA are functionally connected; it is also possible that both regions are modulated similarly by a third structure, or that the anxiogenic drugs act on different sites within a distributed system regulating anxiety states, and increase the activity of multiple nodal structures within anxiety circuits in a parallel manner.
All anxiogenic drugs studied increased c-Fos expression in non-PV-expressing cells within multiple subdivisions across the rostrocaudal extent of the basolateral amygdaloid complex. Although we have not directly confirmed the phenotype of the non-PV-expressing cells, it is likely that they include BLA projection neurons, which would be consistent with the anxiogenic effects of each of the drugs. Previous research has indicated that both PV-expressing GABAergic interneurons and BLA-projection neurons expressing calcium-calmodulin dependent protein kinase II (CaMKII) are activated following acute restraint stress. Based on the time point selected in this study, it is likely that c-Fos expression in non-PV-expressing cells represents activation of non-PV-ir GABAergic interneurons as well as glutamatergic projection neurons involved in the anxiety response itself. Activation of PV-expressing interneurons, potentially via serotonin acting at 5-HT2A receptors on these PV-expressing interneurons, may occur after activation of glutamatergic projection neurons and contribute to termination of the anxiety-response.
The 5-HT2C2A receptor agonist, mCPP, had no effect on c-Fos expression in PV-expressing interneurons but, consistent with the anxiogenic properties of the drug, increased c-Fos expression in non-PV-containing, possibly glutamatergic, cells in the BLA. As mCPP has affinity for 5-HT2A receptors (Callahan and Cunningham, 1994;Hoyer, 1988), it is surprising that mCPP did not activate the PV-expressing subset of GABAergic interneurons. However, mCPP has higher affinity for 5-HT2C receptors than 5-HT2A receptors, and, at the dose used, may preferentially activate 5-HT2C receptors in the BLA. The finding that mCPP activated non-PV-expressing cells in the BLA is inconsistent with a previous report that mCPP, unlike other anxiogenic drugs studied, did not increase c-Fos expression in the basolateral amygdaloid nucleus. This discrepancy could be due, in part, to the failure of mCPP to activate the PV-expressing subset of local GABAergic interneurons. We have previously reported that injection of mCPP increases c-Fos expression in serotonergic neurons in the DR and increases anxiety-like behavior in the rats’ home cages, similar to other anxiogenic drugs studied. Consequently, the reason that mCPP failed to activate PV-expressing cells in the BLA is unclear. One possibility is that mCPP acts on 5-HT2C receptors within the BLA to inhibit, through synaptic mechanisms, PV-expressing GABAergic interneurons within the BLA, but this has not been directly studied. Although 5-HT2C mRNA and protein have been described in the basolateral amygdaloid nucleus, the neurochemical phenotype of 5-HT2C-expressing cells has yet to be described. Another possibility is that, although mCPP increases c-Fos expression in DR serotonergic neurons, similar to other anxiogenic drugs (Abrams et al., 2005), it does not activate the subset of serotonergic neurons that is hypothesized to project to the BLA and activate 5-HT2A receptor/PV-expressing GABAergic interneurons. Consistent with this hypothesis, mCPP, caffeine, and FG-7142 increase c-Fos expression in serotonergic neurons in the caudal part of the DR (DRC), but only caffeine and FG-7142 increase c-Fos expression in serotonergic neurons within the mid-rostrocaudal DRD, the region with the greatest number of BL-projecting serotonergic neurons (Abrams et al., 2005). Also consistent with this hypothesis, intravenous administration of mCPP increases c-Fos expression in GABAergic neurons in the DR and decreases neuronal firing rates of some DR serotonergic neurons.
Activation of 5-HT2C receptors in the basolateral amygdaloid complex is associated with increased anxiety-related behavior. Recent evidence suggests that anxiety-like behavior observed 24 h following uncontrollable stress in a model of learned helplessness is mediated by increased extracellular concentrations of serotonin acting at 5-HT2C receptors in the basolateral amygdaloid nucleus. Exposure to uncontrollable tail shock induces a downregulation of 5-HT2A receptors in the basolateral amygdaloid nucleus, while 5-HT2C receptors remain unchanged. It is possible that uncontrollable stress alters the 5-HT2A-PV-interneuronal inhibition of BL-projection neurons and the stress coping effects of serotonin in the basolateral amygdaloid nucleus.
Conclusions
These data suggest that the GABAergic interneurons in the basolateral amygdala expressing 5-HT2A receptors form part of a distributed neuronal circuit regulating anxiety-states and anxiety-related behavior. The role of this subset of GABAergic interneurons in the basolateral amygdala in regulating anxiety states and anxiety-related behavior is unclear, however, chronic anxiety states have been associated with a decrease in GABAergic tone within the BLA. Therefore, the serotonergic DR–basolateral amygdala circuit acting on a 5-HT2A receptor/PV-expressing subset of GABAergic interneurons may be important in maintaining resilience in response to chronic stress. Consistent with this hypothesis,5HT2A gene polymorphisms have been associated with anxiety disorders in humans including obsessive compulsive disorder and panic disorder as well as affective disorders and the response to antidepressant treatment. Further characterization of this serotonergic DR–basolateral amygdala neuronal circuit may contribute to the understanding of the pathophysiology of stress-related neurological conditions or psychiatric disorders.
Research Highlights
In this study, we found that multiple anxiogenic drugs including the adenosine receptor antagonist caffeine, the α2-adrenoreceptor antagonist yohimbine and the partial inverse agonist at the benzodiazepine allosteric site on the GABAA receptor, N-methyl-beta-carboline-3-carboxamide (FG-7142), activate a subpopulation of the 5-HT2A receptor-expressing, parvalbumin-immunoreactive (PV-ir) interneurons in the basolateral amygdala, and effect that was correlated with activation of serotonergic neurons in the dorsal raphe nucleus and with anxiety-related behavior. The role of serotonergic systems in modulating anxiety states and anxiety-related behavior is complex, and this work defines for the first time a systems level understanding of how serotonergic systems acting within the amygdala may increase resilience to stress and anxiety-related stimuli. This work is important because too often we focus on mechanisms facilitating the development of anxiety states and anxiety-related behavior, rather than mechanisms underlying resilience.
Acknowledgements
Supported by a Wellcome Trust Research Fellowship to CAL (RCDF 068558/Z/02/Z) and NIH award numbers R01MH086539 (CAL) and R01MH065702 (AS and CAL). AMW was supported by an Undergraduate Research Opportunities Program (UROP)/Howard Hughes Medical Institute (HHMI) individual grant funded by the Biological Sciences Initiative (BSI) through a grant from the Howard Hughes Medical Institute (HHMI).
Abbreviations
- 5-HT2A
serotonin 2A receptor subtype
- 5-HT2C
serotonin 2C receptor subtype
- ANOVA
analysis of variance
- BLA
basolateral amygdaloid nucleus, anterior part
- BLP
basolateral amygdaloid nucleus, posterior part
- CRF
corticotropin-releasing factor
- DAB
3,3’-diaminobenzidine tetrahydrochloride
- DR
dorsal raphe nucleus
- DRC
dorsal raphe nucleus, caudal part
- DRD
dorsal raphe nucleus, dorsal part
- FG-7142
N-methyl-beta-carboline-3-carboxamide
- GABA
γ-aminobutyric acid
- LaDL
lateral amydaloid nucleus, dorsolateral part
- LaVL
lateral amydaloid nucleus, ventrolateral part
- LaVM
lateral amydaloid nucleus, ventromedial part
- mCPP
m-chlorophenyl piperazine
- PB
0.1 M sodium phosphate buffer
- PBS
0.1 M phosphate buffered saline
- PBST
0.1 M phosphate buffered saline with 0.3% Triton X-100
- PV
parvalbumin
- SI
social interaction test
- SNAMA
spontaneous non-ambulatory motor activity
- Ucn 1
urocortin 1
- Ucn 2
urocortin 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.