Abstract
We report the parallel synthesis of two natural cyclopeptides, isolated from the seeds of Annona squamosa, cyclosquamosin D (A1) and Met-cherimolacyclopeptide B (B) and their analogs. All of the compounds were screened for anti-inflammatory activity by evaluating their inhibitory effects on the production of pro-inflammatory cytokines using the lipopolysaccharide stimulated macrophage J774A.1 cell line. Compounds having significant anti-inflammatory activity in suppressing the secretion of IL-6 and TNF-α have been identified, some of which exhibit activity superior to that observed with the natural products.
Keywords: Parallel synthesis, Solid phase synthesis, natural cyclopeptides, antiinflammatory activity
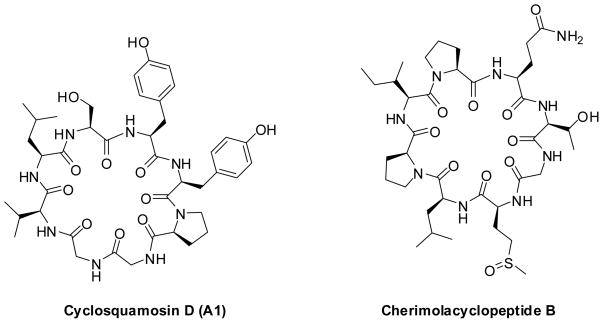
Cyclic peptides constitute an important group of natural product compounds.1 Drugs such as vancomycin, cyclosporin, daptomycin, gramicidin S and bleomycin belong to this group and their successes on the market demonstrate that these types of molecules can be antibiotics and antifungal compounds, as well as immunosuppressive and even anti-cancer drugs.2 Naturally occurring cyclic peptides continue to hold the attention of synthetic chemists and biologists alike for their intriguing chemical structures and potent biological activities.3 Cyclic peptides obtained from latex, seeds and roots of plants possess a wide array of bioactivities including cytotoxic activity,4,5 immunosuppressive activity,6 antimalarial activity,7 vasorelaxant activity,8 cyclooxygenase, ACE and tyrosinase inhibitory activity 9, 10 and anti-inflammatory activity11. Recently, cyclic peptides cyclosquamosin D (A1) and cherimolacyclopeptide B have been isolated from seeds of Annona squamosa (Figure 1). The cyclic peptide cyclosquamosin D (A1) was reported to have an inhibitory effect on the production of pro-inflammatory cytokines within lipopolysaccharide and Pam3Cys-stimulated J774A.1 macrophages.11 However, the cyclic peptide cherimolacyclopeptide B was not active in these assays. In this paper, we report our results on the parallel synthesis of the cyclic peptides cyclosquamosin D (A1) and met-cherimolacyclopeptide B (B) and their analogs. All of the synthetically-prepared peptides were tested for anti-inflammatory activity.
Figure 1.
Natural cyclic peptides: Cyclosquamosin D and Cherimolacyclopeptide B
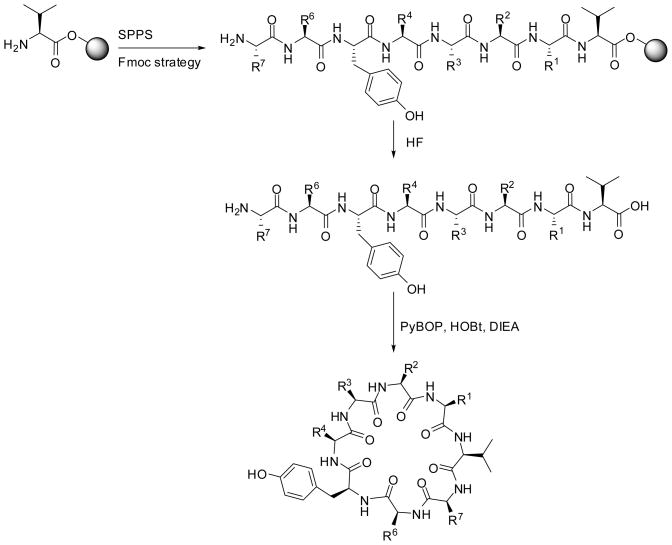
Using the T-bag technology,15 the parallel solid phase peptide synthesis 12 of Cylosquamosin D (A1) and its 14 analogs (A2–A15) was performed starting from Wang-resin bound valine,13 using Fmoc strategy.14 As outlined in Scheme 1, all syntheses were performed using standard coupling deprotection cycles in the presence of DIC, HOBt as activating agents and 20% piperidine in DMF for Fmoc deprotection. Following deprotection of the N-terminal protected amino acid, the solid support and all protecting groups were cleaved using HF.
Scheme 1.
Parallel synthesis of Cylosquamosin D (A1) and its analogs.
Following extraction, and lyophilization, the identity of the linear peptides were confirmed by LC-MS. The crude peptides were treated overnight in DMF with PyBOP and HOBt in the presence of DIEA to generate the corresponding cyclic peptides in good yields.19 All of the desired compounds were purified using reversed-phase high-performance liquid chromatography (RP HPLC). The purity of these compounds is higher than 90% for all the compounds, based upon analytical traces at λ = 214 nm and 254 nm.
In addition to the natural peptide Cylosquamosin D (A1), 14 analogs were synthesized. We performed an alanine screen to investigate the effect and contribution of the amino acids Xaa1, Xaa2, Xaa4, Xaa6 and Xaa7 on the anti-inflammatory activity (Table 1). Thus, five cyclic peptide analogs (A9, A11–13, and A15) were synthesized. We also performed the synthesis of cyclic peptide analogs where we modified or permuted the order of some amino acids. In analog A4, the tyrosine at position R4 and the serine at position R6 were reversed, while in analog A8, the glycine at R1 was replaced with tyrosine and the order of amino acids at R2 and R3 was reversed. In analog A14, the proline and glycine at positions R2 and R3 were reversed. We also addressed the amino acids bearing functional groups on their side chains. Thus, in analog A2, the serine at position 6 was replaced by the threonine and in analog A3, the tyrosine at position R4 and the serine at R6 were both substituted by the threonine. In analogs A5 and A10, we addressed the importance of tyrosine at position R4 by replacing it with serine and O-methyl tyrosine. We also addressed the importance of having glycine at position R1 and R2 by replacing them with tyrosine in analogs A6 and A7, respectively.
Table 1.
Amino acids sequences of the cyclic Cyclosquamosin D (A1) and its analogs.
| Xaa1 | Xaa2 | Xaa3 | Xaa4 | Xaa5 | Xaa6 | Xaa7 | ||
|---|---|---|---|---|---|---|---|---|
| Cyclosquamosin D | ||||||||
| (A1) | Val | Gly | Gly | Pro | Tyr | Tyr | Ser | Leu |
| A2 | Val | Gly | Gly | Pro | Tyr | Tyr | Thr | Leu |
| A3 | Val | Gly | Gly | Pro | Thr | Tyr | Thr | Leu |
| A4 | Val | Gly | Gly | Pro | Ser | Tyr | Tyr | Leu |
| A5 | Val | Gly | Gly | Pro | Ser | Tyr | Ser | Leu |
| A6 | Val | Tyr | Gly | Pro | Tyr | Tyr | Ser | Leu |
| A7 | Val | Gly | Tyr | Pro | Tyr | Tyr | Ser | Leu |
| A8 | Val | Tyr | Pro | Gly | Tyr | Tyr | Ser | Leu |
| A9 | Val | Gly | Gly | Pro | Tyr | Tyr | Ser | Ala |
| A10 | Val | Gly | Gly | Pro | Tyr(OMe) | Tyr | Ser | Leu |
| A11 | Val | Ala | Gly | Pro | Tyr | Tyr | Ser | Leu |
| A12 | Val | Gly | Ala | Pro | Tyr | Tyr | Ser | Leu |
| A13 | Val | Gly | Gly | Pro | Ala | Tyr | Ser | Leu |
| A14 | Val | Gly | Pro | Gly | Tyr | Tyr | Ser | Leu |
| A14 | Val | Gly | Gly | Pro | Tyr | Tyr | Ala | Leu |
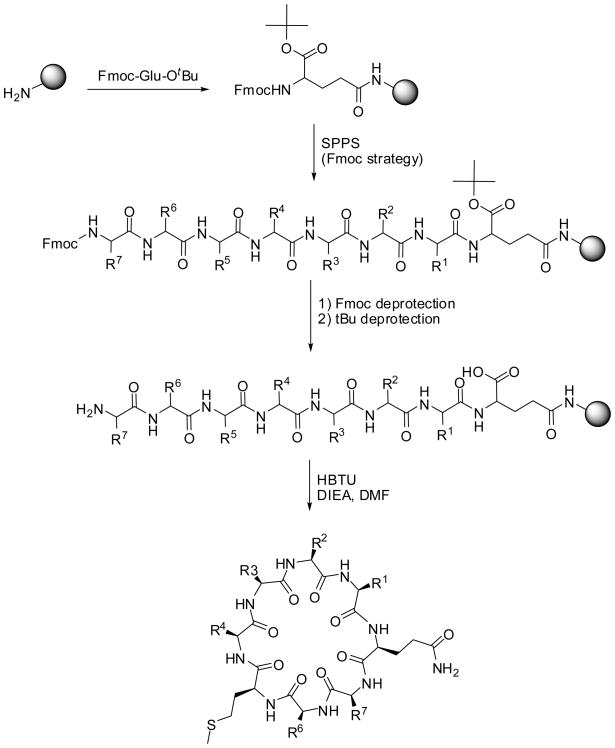
Similarly, we performed the parallel solid phase synthesis 15 of 13 analogs of Cherimolacyclopeptide B (B). In comparison to the natural cyclic peptide we used the methionine instead of the sulfoxide methionine (B1–B13). As outlined in Scheme 2, the parallel synthesis was performed using Fmoc-chemistry.
Scheme 2.
Parallel synthesis of met-Cherimolacyclopeptide B (B) and its analogs
Since the natural cyclic peptide Cherimolacyclopeptide B (B) contains glutamine, the methionine containing analogs of Cherimolacyclopeptide B were synthesized using amino acid side chain attachment strategy.16 Thus, starting from p-methylbenzhydrylamine hydrochloride (MBHA·HCl) resin, and following neutralization of the resin, Fmoc-Glu-OtBu was attached to the solid support in the presence of DIC and HOBt. Following Fmoc deprotection, and subsequent coupling of the amino acids using standard stepwise Fmoc chemistry, the N-terminal amine was deprotected and the tert-butyl group on the Cα of the glutamic acid was removed with a solution of TFA in DCM. The resin-bound linear peptide was treated with HBTU to generate the corresponding cyclic compounds. The desired cyclic peptides were obtained following cleavage of the solid support with HF/anisole. All the compounds were purified using RP HPLC. The purity of these compounds is higher than 90% for all the compounds, based upon the analytical traces at λ = 214 nm and 254 nm.
We performed the synthesis of the methionine analog B1 of the cyclic natural peptide Cherimolacyclopeptide B. We used an alanine substitution screen to study the effect and contribution of each amino acid to the activity (B2–B8). In addition, we synthesized five Cherimolacyclopeptide B analogs, where we substituted the threonine at position R7 by different amino acids bearing the hydroxyl group on the side chain (Ser, Tyr, D-Thr, D-Ser and D-Tyr) (B9–B13) (Table 2).
Table 2.
Amino acids sequences of Met-Cherimolacyclopeptide B (B1) and its analogs
| Xaa1 | Xaa2 | Xaa3 | Xaa4 | Xaa5 | Xaa6 | Xaa7 | ||
|---|---|---|---|---|---|---|---|---|
| B1 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Met | Gly | Thr |
| B2 | Fmoc-Glu-O-tBu | Ala | Ile | Pro | Leu | Met | Gly | Thr |
| B3 | Fmoc-Glu-O-tBu | Pro | Ala | Pro | Leu | Met | Gly | Thr |
| B4 | Fmoc-Glu-O-tBu | Pro | Ile | Ala | Leu | Met | Gly | Thr |
| B5 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Ala | Met | Gly | Thr |
| B6 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Ala | Gly | Thr |
| B7 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Met | Ala | Thr |
| B8 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Met | Gly | Ala |
| B9 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Met | Gly | Ser |
| B10 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Met | Gly | Tyr |
| B11 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Met | Gly | D-Tyr |
| B12 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Met | Gly | D-Thr |
| B13 | Fmoc-Glu-O-tBu | Pro | Ile | Pro | Leu | Met | Gly | D-Ser |
Cell Culture
Murine macrophage J774A.1 cells were purchased from the ATCC and cultured in the RPMI1640 medium supplemented with 10% heat inactivated fetal bovine serum (Hyclone), L-Glutamine, penicillin-streptomycin, sodium pyruvate, non essential amino acids, HEPES, 2-mercaptoethanol. For stimulation assays, J774A.1 cells were incubated at 37 °C with the cyclic peptides for 1 hour, followed by lipopolysaccharide (LPS) (0.1 μg/mL) treatment for additional 4 or 6 hours. Cyclic peptides were dissolved in dimethyl sulfoxide, DMSO at concentration 20–40 μg/mL.
Enzyme-Linked Immunosorbent Assay (ELISA)
Anti-mouse IL-6 and TNF-α antibodies as well as recombinant mouse IL-6 and TNF-α for ELISA standards were purchased from eBioscience (San Diego). Avidin-HRP peroxidase was purchased from Vector Laboratories and ABTS peroxidase substrate system from KPL. The cytokine Elisa was performed essentially as described earlier.17,18 Anti-IL-6 or anti-TNF-α capture antibodies were diluted to 1 μg/mL in PBS. 50 μL of diluted antibody was added to the wells of Nunc Maxisorp plate. Plates were then incubated overnight at 4 °C. The next day they were washed 2X with PBS/Tween solution (0.05%) and then blocked with 200 μL of blocking buffer (10% FBS in PBS solution) and incubated at room temperature for 2 hours. Following washing 4–5 times with PBS/Tween, IL-6 or TNF-α standards and samples were added (50 μL/well). Plates were sealed overnight at 4 °C and washed next day 5 times with PBS/Tween solution. The biotinylated anti- IL-6 or anti-TNF-α detection antibodies were diluted to 1 mg/mL in PBS and added at 100 mL/well. Plates were incubated for 1 hr at room temperature and then washed 6 times with PBS/Tween. Avidin-HRP peroxidase conjugate was diluted to 2.5 mg/mL in PBS and added 100 mL/well and platee were incubated at room temperature for 30 minutes. After washing 6 times with PBS/Tween, ABTS Substrate solution and Peroxidase Substrate solutions were mixed at 1:1 ratio and added at 100 mL/well and plates covered with aluminum foil. After 10–20 minutes the optical density was read at 405 nm using a microplate reader.
Infections with pathogenic agents, including viruses and bacteria can result in overproduction of pro-inflammatory cytokines, including IL-6 and TNF-α. We have determined whether treatment with synthetic cyclic peptides has any influence on the secretion of these cytokines by a murine macrophage cell line, J 774A.1 cells. These cells were pre-treated with various synthetic cyclic peptides for 1 hr at 37 °C. Following treatment with peptides, cells were stimulated for 6 hr with LPS, a gram-negative bacterial cell wall component. LPS has been shown to induce signaling for the production of cytokines via toll-like receptor 4.
Screening results
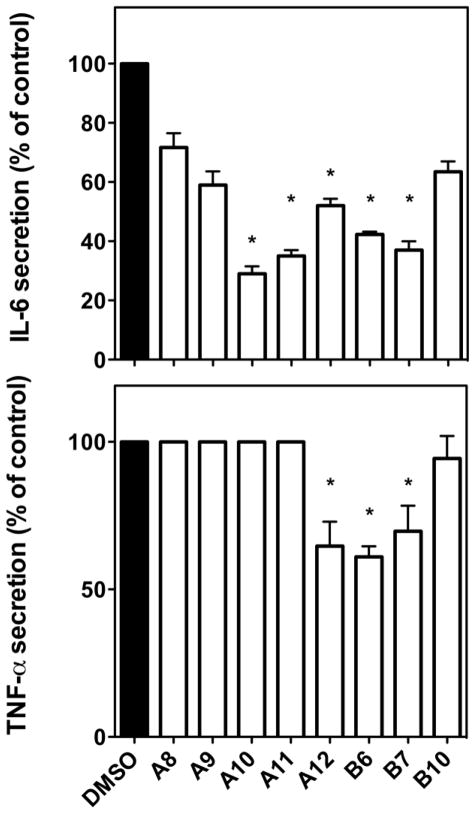
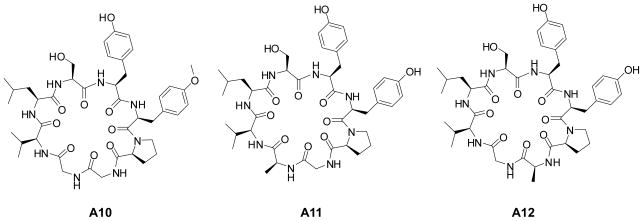
All the synthetic cyclic peptides were examined for their ability to block IL-6 or TNF-α using the J774A.1 cell line. A wide array of biological activity was observed among the analogs with some exhibiting no activity with others exhibiting activity superior to the natural products. Some analogs of both A and B peptides that were found to show suppression of inflammatory responses in bulk assays were further examined along with some that showed no activity which were included as negative controls. As shown in Figure 2, while A10, A11 and A12 significantly reduced IL-6 secretion, only A12 was able to block TNF-α secretion as well. It is interesting that, although A10 was most effective (up to ~25%) in suppressing IL-6, it had no effect on the secretion of TNF-α.
Figure 2.
Inhibitory effect of cyclic peptides on the production of TNF-α and IL-6 by LPS-stimulated J774A.1 cells. IL-6 secretion after 4 hours of LPS treatment (upper panel). Values are mean ± SD, *: p value for A10, A11, A12, B6, B7 is <0.0001.
TNF-α secretion after 4 hours of LPS treatment (lower panel). Values are mean ± SD. *, p values for A12, B6 and B7 are 0.0137, 0.0005 and 0.0268, respectively.
These data are representative of three independent experiments.
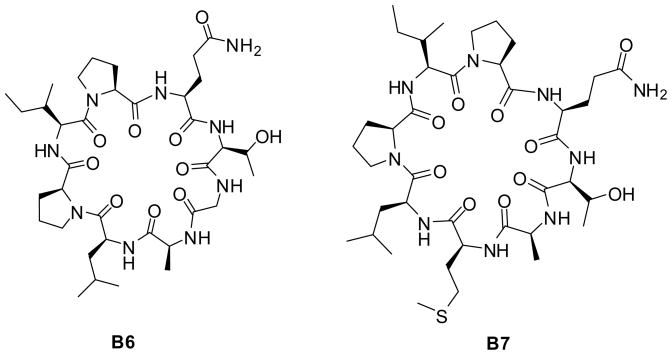
Similar to what was reported for the natural cyclic peptide cherimolacyclopeptide B, the Met-cherimolacyclopeptide B (B1) did not show activity. In contrast, both analogs of Met-cherimolacyclopeptide B (B1), cyclic peptides B6 and B7 were able to effectively suppress TNF-α and IL-6 secretion. Whereas, peptide analog B10 showed no activity in reducing LPS-induced cytokine secretion by macrophages and served as a negative control.
Structurally, these peptide analogs are very similar and yet they generate specific anti-inflammatory activity. The methylation of the hydroxyl group of the tyrosine (R4) in analog A10 significantly reduced IL-6 secretion. Similarly, the substitution of the glycine (R1) and the glycine (R2) by alanine for analogs A11 and A12, respectively, resulted in reduction of IL-6 secretion (Figure 3).
Figure 3.
Structures of the individual analogs of Cyclosquamosin D showing an inhibitory effect on the production of pro-inflammatory cytokines within lipopolysaccharide stimulated J774A.1 macrophages.
Interesting results were obtained for cyclic peptides met-cherimolacyclopeptide B analogs. The substitution of methionine (R5) and Gly (R6) by alanine for analogs B6 and B7 (Figure 4), respectively resulted in suppression of TNF-α and IL-6 secretion.
Figure 4.
Structures of the individual analogs of Cherimolacyclopeptide B showing an inhibitory effect on the production of pro-inflammatory cytokines within lipopolysaccharide stimulated J774A.1 macrophages.
In summary, we performed the parallel synthesis of two natural cyclopeptides and their analogs. We tested all the compounds for anti-inflammatory activity for their inhibitory effect on the production of pro-inflammatory cytokines using lipopolysaccharide stimulated J774A.1 macrophage line. Modified analog compounds of cyclosquamosin D (A1) showed significant anti-inflammatory activity in suppressing the secretion of IL-6 and TNF-α, in some cases superior to the activity observed with the natural product Similarly, we were able to identify active compounds analogs of the non-active natural cyclic peptide Cherimolacyclopeptide B.
Acknowledgments
The presented work was supported by Grants from the NIH, JDRF and MSNRC (Vipin. K). NIH (1R03DA025850-01A1, Nefzi), NIH (5P41GM081261-03, Houghten) and NIH (3P41GM079590-03S1, Houghten) and the State of Florida Funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Tamai S, Kaneda M, Nakamura S. J Antibiot. 1982;35:1130. doi: 10.7164/antibiotics.35.1130. [DOI] [PubMed] [Google Scholar]; (b) Walsh CT. Science. 1993;261:308. doi: 10.1126/science.8392747. [DOI] [PubMed] [Google Scholar]; (c) Kannan R, Harris CM, Harris TM, Waltho GP, Skelton NJ, Williams DH. J Am Chem Soc. 1988;110:2946. [Google Scholar]
- 2.(a) Wipf P. Chem Rev. 1995;95:2115. [Google Scholar]; (b) Hamada Y, Shioiri T. Chem Rev. 2005;105:4441. doi: 10.1021/cr0406312. [DOI] [PubMed] [Google Scholar]; (c) Pomilio AB, Battista ME, Vitale AA. Curr Org Chem. 2006;10:2075. [Google Scholar]
- 3.Blout ER. Biopolymers. 1981;20:1901. [Google Scholar]
- 4.Mongkolvisut W, Sutthivaiyakit S, Leutbecher H, Mika S, Klaiber I, Moller W, Rosner H, Beifuss U, Conrad J. J Nat Prod. 2006;69:14355. doi: 10.1021/np0602012. [DOI] [PubMed] [Google Scholar]
- 5.Dahiya R, Kaur K. Arch Pharm Res. 2007;30:1380. doi: 10.1007/BF02977360. [DOI] [PubMed] [Google Scholar]
- 6.Morita H, Shishido A, Matsumoto T, Itokawa H, Takeya K. Tetrahedron. 1999;55:967. [Google Scholar]
- 7.Baraguey C, Blond A, Correia I, Pousset JL, Bodo B, Auvin-Guette JL, Mahafacyclin A. Tetrahedron Lett. 2000;41:325. [Google Scholar]
- 8.Morita H, Lizuka T, Choo CY, Chan KL, Itokawa H, Takeya K. J Nat Prod. 2005;68:1686. doi: 10.1021/np050262k. [DOI] [PubMed] [Google Scholar]
- 9.Morita H, Shishido A, Kayashita T, Takeya K, Itokawa H. J Nat Prod. 1997;60:404. [Google Scholar]
- 10.Yahara S, Shigeyama C, Nohara T, Okuda H, Wakamatsu K, Yasuhara T. Tetrahedron Lett. 1989;30:6041. [Google Scholar]
- 11.Yang YL, Hua KF, Chuang PH, Wu SH, Wu KY, Chang FR, Wu YC. J Agric Food Chem. 2008;56:386. doi: 10.1021/jf072594w. [DOI] [PubMed] [Google Scholar]
- 12.a) Merrifield RB. J Am Chem Soc. 1963;85:2149. [Google Scholar]; b) Merrifield RB. Science. 1986;232:341. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell AR. Biopolymers. 2008;90:3175. doi: 10.1002/bip.20925. [DOI] [PubMed] [Google Scholar]
- 14.Fields GB, Noble RL. Int J Peptide Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 15.Houghton RA. Proc Nat Acad Sci USA. 1985;82:5131. [Google Scholar]
- 16.a) Cabrele C, Langer M, Beck-Sickinger AG. J Org Chem. 1999;64:4353. [Google Scholar]; b) Ficht S, Payne RJ, Guy RT, Wong C-H. Chemistry - A European Journal. 2008;14:3620. doi: 10.1002/chem.200701978. [DOI] [PubMed] [Google Scholar]; c) Dixon MJ, Nathubhai A, Andersen OA, van Aalten DMF, Eggleston IM. Org Biomol Chem. 2009;7:259. doi: 10.1039/b815077j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X, Maricic I, Purohit N, Bakamjian B, Reed-Loisel LM, Beeston TP, Jensen T, Kumar V. J Immunol. 2006;177:7645. doi: 10.4049/jimmunol.177.11.7645. [DOI] [PubMed] [Google Scholar]
- 18.Smith TR, Tang X, Maricic I, Garcia Z, Fanchiang S, Kumar V. J Immunol. 2009;182:6959. doi: 10.4049/jimmunol.0900316. [DOI] [PubMed] [Google Scholar]
- 19.10 mg of linear crude peptide was dissolved in 600 μL of DMF anhydrous. PyBOP (3 eq) and HOBt (3 eq) were added to the reaction vessel in the presence of DIEA (3eq). The clear solution was stirred overnight at room temperature. The completion of the cyclization was monitored by LC-MS. The DMF was evaporated and the crude product was purified using reversed-phase high-performance liquid chromatography (RP HPLC). All the compouns were obtained in good yield ranging from 50 to 60%.