Abstract
Three independent mutations involving the apoptosis-1 (APO-1)/Fas receptor or its putative ligand have led to lupuslike diseases associated with lymphadenopathy in different strains of mice. To determine whether humans with SLE also have a defect in this apotosis pathway, we analyzed the expression of APO-1 on freshly isolated blood mononuclear cells and on lymphocytes activated in vitro using flow cytometry and the monoclonal antibody anti-APO-1. Significantly higher level of APO-1 expression were detected on freshly isolated peripheral B cells and both CD4+ and CD8+ T lymphocyte populations obtained from lupus patients when compared with normal controls (P < 0.001). Almost 90% of the cells that stained positive for APO-1 also expressed the CD29 antigen, suggesting that APO-1 was upregulated after lymphocyte activation in vivo. No defect in APO-1 regulation was detected after activation of SLE T (with anti-CD3) or B (with Staphylococcus aureus Cowan 1) lymphocytes in the presence of IL-2 in vitro. Similarly, the anti-APO-1 antibody induced apoptosis in 74 +/- 5% of activated SLE T cells in vitro compared with 79 +/- 6% of the normal controls (P > 0.05). These results reveal that, while APO-1/Fas may play an important role in the regulation of lymphocyte survival in SLE, no consistent defect in the expression or function of the receptor could be detected in these studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Marshall J. D., Roths J. B., Sidman C. L. Differences defined by bone marrow transplantation suggest that lpr and gld are mutations of genes encoding an interacting pair of molecules. J Exp Med. 1990 Nov 1;172(5):1367–1375. doi: 10.1084/jem.172.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Ashany D., Hines J., Gharavi A., Mouradian J., Elkon K. B. Analysis of autoantibody production in SCID-systemic lupus erythematosus (SLE) chimeras. Clin Exp Immunol. 1992 Apr;88(1):84–90. doi: 10.1111/j.1365-2249.1992.tb03043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bini P., Chu J. L., Okolo C., Elkon K. Analysis of autoantibodies to recombinant La (SS-B) peptides in systemic lupus erythematosus and primary Sjogren's syndrome. J Clin Invest. 1990 Feb;85(2):325–333. doi: 10.1172/JCI114441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992 Jun;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- Carlsten H., Tarkowski A. Expression of heterozygous lpr gene in MRL mice. I. Defective T-cell reactivity and polyclonal B-cell activation. Scand J Immunol. 1989 Oct;30(4):457–462. doi: 10.1111/j.1365-3083.1989.tb02450.x. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., MacDonald H. R. The cellular basis of T-cell memory. Annu Rev Immunol. 1989;7:77–89. doi: 10.1146/annurev.iy.07.040189.000453. [DOI] [PubMed] [Google Scholar]
- Chu J. L., Drappa J., Parnassa A., Elkon K. B. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. J Exp Med. 1993 Aug 1;178(2):723–730. doi: 10.1084/jem.178.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Crow M. K., Jover J. A., Friedman S. M. Direct T helper-B cell interactions induce an early B cell activation antigen. J Exp Med. 1986 Nov 1;164(5):1760–1772. doi: 10.1084/jem.164.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
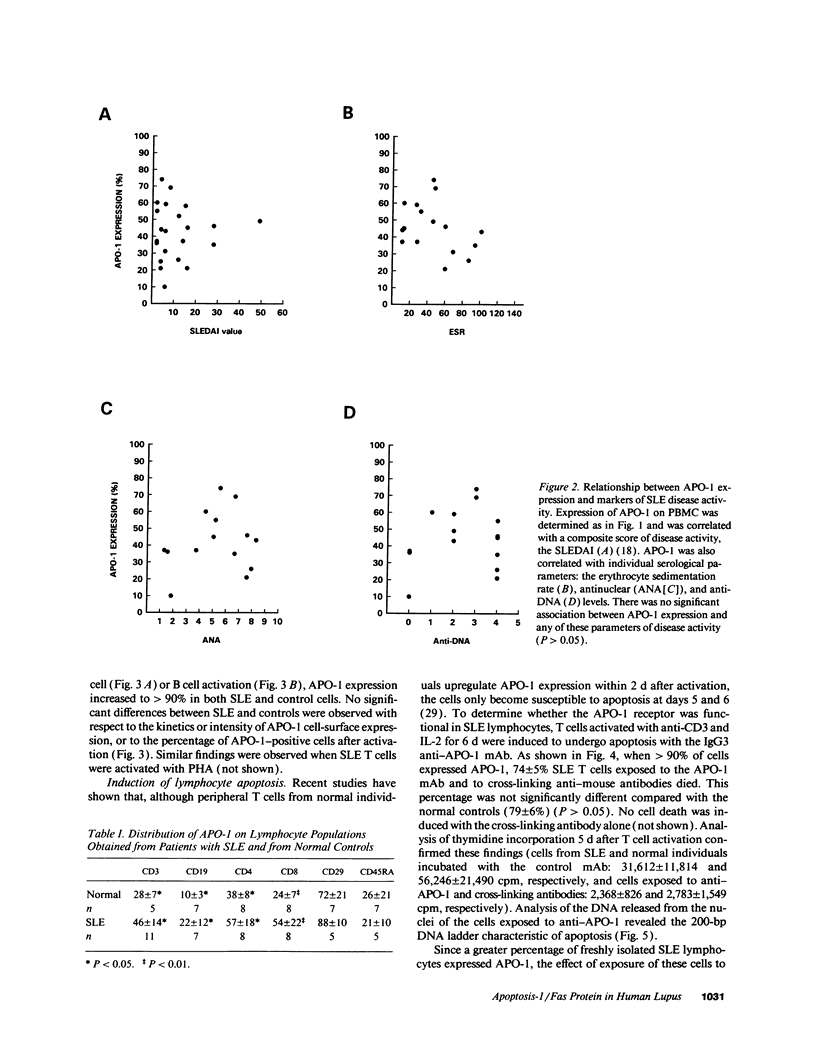
- Dhein J., Daniel P. T., Trauth B. C., Oehm A., Möller P., Krammer P. H. Induction of apoptosis by monoclonal antibody anti-APO-1 class switch variants is dependent on cross-linking of APO-1 cell surface antigens. J Immunol. 1992 Nov 15;149(10):3166–3173. [PubMed] [Google Scholar]
- Drappa J., Brot N., Elkon K. B. The Fas protein is expressed at high levels on CD4+CD8+ thymocytes and activated mature lymphocytes in normal mice but not in the lupus-prone strain, MRL lpr/lpr. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10340–10344. doi: 10.1073/pnas.90.21.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., Torpier G., Monté D., Mouton Y., Capron A., Ameisen J. C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992 Feb 1;175(2):331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Ishii A., Yonehara S. The E1b oncogene of adenovirus confers cellular resistance to cytotoxicity of tumor necrosis factor and monoclonal anti-Fas antibody. Int Immunol. 1991 Apr;3(4):343–351. doi: 10.1093/intimm/3.4.343. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Kammer G. M., Stein R. L. T lymphocyte immune dysfunctions in systemic lupus erythematosus. J Lab Clin Med. 1990 Mar;115(3):273–282. [PubMed] [Google Scholar]
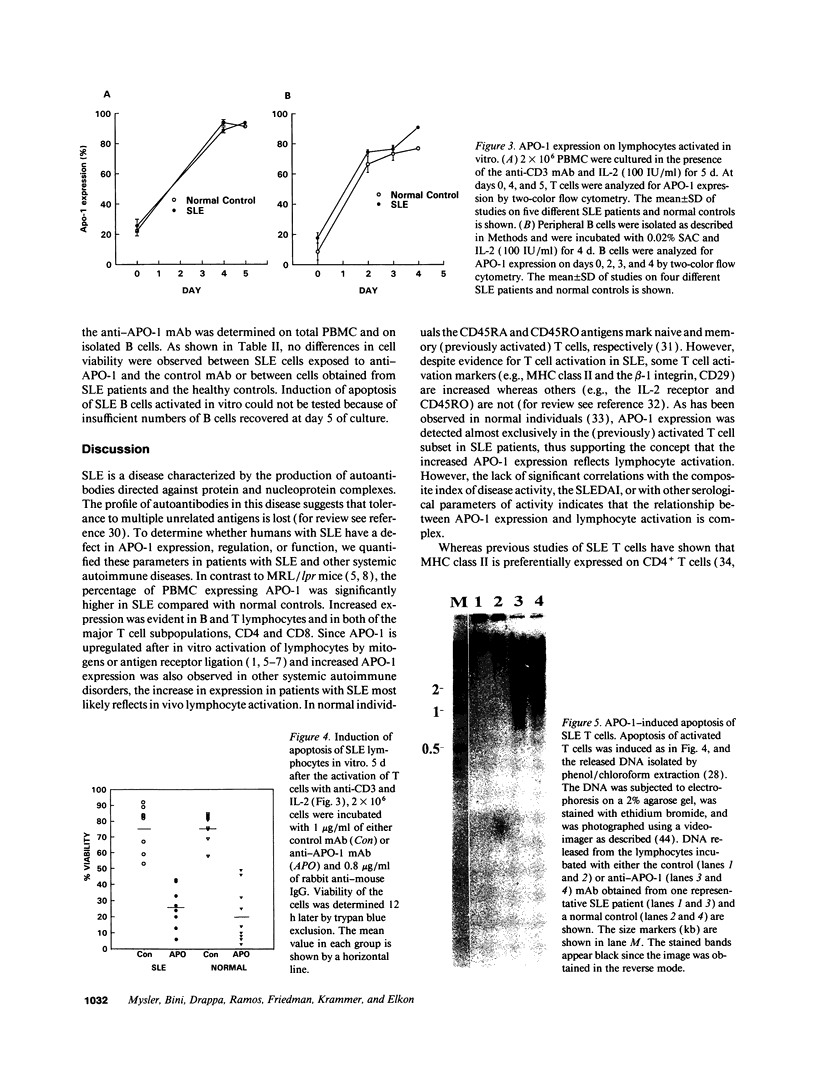
- Klas C., Debatin K. M., Jonker R. R., Krammer P. H. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993 Jun;5(6):625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- Koide J. Functional property of Ia-positive T cells in peripheral blood from patients with systemic lupus erythematosus. Scand J Immunol. 1985 Nov;22(5):577–584. doi: 10.1111/j.1365-3083.1985.tb01917.x. [DOI] [PubMed] [Google Scholar]
- Koide J., Takano M., Takeuchi T., Hosono O., Amano K., Homma M., Abe T. Direct demonstration of immunoregulatory T-cell defects in patients with systemic lupus erythematosus. Scand J Immunol. 1986 Apr;23(4):449–459. doi: 10.1111/j.1365-3083.1986.tb03076.x. [DOI] [PubMed] [Google Scholar]
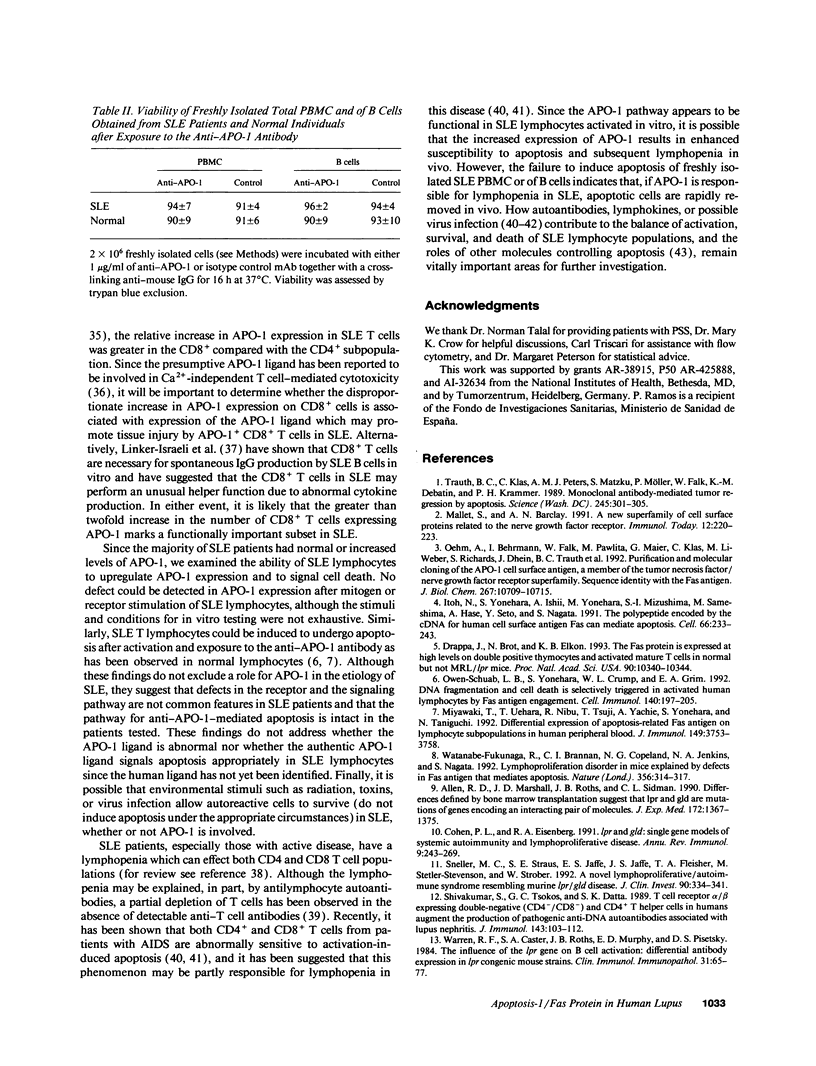
- Linker-Israeli M., Quismorio F. P., Jr, Horwitz D. A. CD8+ lymphocytes from patients with systemic lupus erythematosus sustain, rather than suppress, spontaneous polyclonal IgG production and synergize with CD4+ cells to support autoantibody synthesis. Arthritis Rheum. 1990 Aug;33(8):1216–1225. doi: 10.1002/art.1780330823. [DOI] [PubMed] [Google Scholar]
- Mallett S., Barclay A. N. A new superfamily of cell surface proteins related to the nerve growth factor receptor. Immunol Today. 1991 Jul;12(7):220–223. doi: 10.1016/0167-5699(91)90033-P. [DOI] [PubMed] [Google Scholar]
- Mapara M. Y., Bargou R., Zugck C., Döhner H., Ustaoglu F., Jonker R. R., Krammer P. H., Dörken B. APO-1 mediated apoptosis or proliferation in human chronic B lymphocytic leukemia: correlation with bcl-2 oncogene expression. Eur J Immunol. 1993 Mar;23(3):702–708. doi: 10.1002/eji.1830230320. [DOI] [PubMed] [Google Scholar]
- Meyaard L., Otto S. A., Jonker R. R., Mijnster M. J., Keet R. P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Uehara T., Nibu R., Tsuji T., Yachie A., Yonehara S., Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992 Dec 1;149(11):3753–3758. [PubMed] [Google Scholar]
- Miyawaki T., Uehara T., Nibu R., Tsuji T., Yachie A., Yonehara S., Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992 Dec 1;149(11):3753–3758. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Nadler L. M., Anderson K. C., Marti G., Bates M., Park E., Daley J. F., Schlossman S. F. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol. 1983 Jul;131(1):244–250. [PubMed] [Google Scholar]
- Oehm A., Behrmann I., Falk W., Pawlita M., Maier G., Klas C., Li-Weber M., Richards S., Dhein J., Trauth B. C. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992 May 25;267(15):10709–10715. [PubMed] [Google Scholar]
- Owen-Schaub L. B., Yonehara S., Crump W. L., 3rd, Grimm E. A. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992 Mar;140(1):197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- Raziuddin S., Danial H. B., Kelley M. OKT4+ T cell abnormality in patients with active systemic lupus erythematosus: HLA-DR antigen expressions. Clin Immunol Immunopathol. 1988 Jul;48(1):42–49. doi: 10.1016/0090-1229(88)90155-9. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Hussey R. E., Schlossman S. F. A monoclonal antibody blocking human T cell function. Eur J Immunol. 1980 Oct;10(10):758–762. doi: 10.1002/eji.1830101006. [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar S., Tsokos G. C., Datta S. K. T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989 Jul 1;143(1):103–112. [PubMed] [Google Scholar]
- Sneller M. C., Straus S. E., Jaffe E. S., Jaffe J. S., Fleisher T. A., Stetler-Stevenson M., Strober W. A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest. 1992 Aug;90(2):334–341. doi: 10.1172/JCI115867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Rudd C. E., Schlossman S. F., Morimoto C. Induction of suppression following autologous mixed lymphocyte reaction; role of a novel 2H4 antigen. Eur J Immunol. 1987 Jan;17(1):97–103. doi: 10.1002/eji.1830170117. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Warren R. W., Caster S. A., Roths J. B., Murphy E. D., Pisetsky D. S. The influence of the lpr gene on B cell activation: differential antibody expression in lpr congenic mouse strains. Clin Immunol Immunopathol. 1984 Apr;31(1):65–77. doi: 10.1016/0090-1229(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G. Hormone-induced cell death. Purification ad properties of thymocytes undergoing apoptosis after glucocorticoid treatment. Am J Pathol. 1982 Oct;109(1):78–87. [PMC free article] [PubMed] [Google Scholar]