Abstract
Cell hyperproliferation, inflammation, and angiogenesis are biological processes central to the pathogenesis of corneal disease, as well as other conditions including tumorigenesis and chronic inflammatory disorders. Due to the number of disease conditions that arise as a result of these abnormalities, identifying the molecular mechanisms underlying these processes is critical. The avascular and transparent cornea serves as a good in vivo model to study the pathogenesis of cell hyperproliferation, inflammation, and angiogenesis. Corneal disease 1 (Dstncorn1) mice are homozygous for a spontaneous null allele of the destrin (Dstn) gene, which is also known as actin depolymerizing factor (ADF). These mice exhibit abnormalities in the cornea including epithelial cell hyperproliferation, stromal inflammation, and neovascularization. We previously identified that the transcription factor, serum response factor (SRF) and a number of its target genes are upregulated in the cornea of these mice. In this study, we show that conditional ablation of Srf in the corneal epithelium of a diseased Dstncorn1 cornea results in the rescue of the epithelial cell hyperproliferation, inflammation, and neovascularization phenotypes, delineating an epithelial cell-specific role for SRF in the development of all of these abnormalities. Our study also demonstrates that Dstn is genetically upstream of Srf and defines a new functional role for SRF as the master regulator of a hyperproliferative, inflammatory phenotype accompanied by neovascularization.
CELL hyperproliferation, inflammation, and angiogenesis are biological processes known to play a central role in the pathogenesis of corneal disease, as well as several other conditions including tumorigenesis and chronic inflammatory disorders. These abnormalities often occur simultaneously and affect each other to promote disease progression. The development of corneal irregularities, such as thickening of the epithelial layer as well as stromal neovascularization and inflammation, compromises corneal transparency and results in blindness (reviewed in Cursiefen 2007; Notara et al. 2010). In the case of tumor pathology, hyperproliferative tissue is provided a means of survival and metastasis by invading blood vessels signaled for growth by tumor tissue and its associated inflammatory cells (reviewed in Allavena et al. 2008). Conditions of chronic inflammation that exist in autoimmune disorders such as rheumatoid arthritis and inflammatory bowel disease can lead to tissue destruction (D'Aura Swanson et al. 2009) and susceptibility to neoplastic growth (Lewis et al. 1999). Due to the number of disease conditions that arise as a result of abnormal cell proliferation, inflammation, and angiogenesis, identifying the molecular mechanisms underlying these conditions is critical. Although much work has been done in an effort to understand the molecular underpinnings of each phenotype, it is not well understood whether there is a primary molecular change that leads to the development of all of these abnormalities. Therefore, identification of a molecule responsible for driving the development of cell hyperproliferation, inflammation, as well as angiogenesis is important in understanding disease pathogenesis.
The cornea serves as a good model to study abnormalities such as changes in cell proliferation, inflammation, and the development of new blood vessels because these processes are tightly regulated in the normal cornea. A low, yet constant rate of basal cell proliferation ensures the constant self-renewal of the corneal epithelium (Lehrer et al. 1998). The cornea also lacks vasculature, a feature that is important to the maintenance of corneal transparency. Due to the fact that strict signaling mechanisms are in place to maintain tissue integrity, any aberration in these processes can readily be detected and studied. Animal models with corneal epithelial defects and neovascularization could provide useful experimental systems for this purpose.
Corneal disease 1 (Dstncorn1) mice are homozygous for a spontaneous null allele of the destrin (Dstn) gene, which is also known as actin depolymerizing factor (ADF). These mice exhibit abnormalities in the cornea including epithelial cell hyperproliferation, accumulation of actin stress fibers in the epithelial cells, as well as inflammation and neovascularization in the stroma (Smith et al. 1996; Ikeda et al. 2003). Significant epithelial abnormalities are obvious at 2 weeks of age (Smith et al. 1996), which coincides with the onset of inflammation (Verdoni et al. 2008b) and angiogenesis (Cursiefen et al. 2005). Complete vascularization is present at 45 days of age and does not regress, even after 1 year of life (Smith et al. 1996; Cursiefen et al. 2005).
Our recent study involving the genome-wide interrogation of differentially expressed genes in the cornea of Dstncorn1 mice revealed that >1200 genes show changes in transcription, with a large portion of the upregulated genes being associated with cytoskeletal dynamics (Verdoni et al. 2008a). Almost half of these genes were targets of the MADS domain-containing transcription factor, serum response factor (SRF), an essential regulator of the actin cytoskeleton (Miano et al. 2007). Because of the large change in transcription in Dstncorn1 mice that was potentially SRF dependent, we hypothesized that overexpression of SRF may play a role in the development of the corneal pathology in Dstncorn1 mice.
In this study, we report that the conditional ablation of Srf in the corneal epithelium of a diseased Dstncorn1 cornea results in the rescue of the epithelial cell hyperproliferation, inflammation, and neovascularization phenotypes, delineating an epithelial cell-specific role for SRF in the development of all of these abnormalities. Due to the fact that SRF ablation rescues the corneal abnormalities caused by a loss of DSTN expression, this study demonstrates that Dstn is genetically upstream of Srf. It also defines a new functional role for SRF as the master regulator of a hyperproliferative, inflammatory condition accompanied by neovascularization, which is observed in an array of detrimental diseases.
MATERIALS AND METHODS
Mice:
A. BY H2bc H2-T18f/SnJ [A. BY wild type (WT)], A. BY H2bc H2-T18f/SnJ-Dstncorn1/J (Dstncorn1), B6.129-Srftm1Rmn/J (Srff/f) (Miano et al. 2004), and FVB. Cg-Tg(tetO-cre) 1Jaw/J (TetO-cre+) (Perl et al. 2002) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in the animal facility at the University of Wisconsin-Madison. Krt12rtTA/rtTA mice were generated as described previously (Chikama et al. 2005). All mouse procedures were performed in accordance with the protocols approved by the Animal Care and Use Committee at the University of Wisconsin-Madison and conform to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research.
Doxycycline administration:
Doxycycline (Dox) (Clontech Laboratories, Mountain View, CA) was dissolved in phosphate buffered saline (PBS) at a concentration of 10 mg/ml, filter sterilized, and administered to the mice by intraperitoneal injection at a final concentration of 80 μg/g body weight. Mice were injected once every other day for a 30-day period beginning at 28 days of age. All mice that were sacrificed at postnatal day (P)58, including Dstncorn1/corn1 control mice, were treated with Dox from P28 to P58 to control for its known inhibitory effect on angiogenesis (Fife et al. 2000). Mice used in this study that were sacrificed at P28 were not treated with Dox.
Analysis of F/G-actin ratio:
The ratio of F-actin and G-actin in the cornea was analyzed using an F-actin/G-actin in vivo assay kit (Cytoskeleton, Denver, CO), based on the protocol described in Verdoni et al. (2008a). To measure the F/G-actin ratio, equal amounts of both the supernatant (G-actin) and the resuspended pellet (F-actin) for each genotype were subjected to immunoblot analysis on 10% Bis-Tris gels (Invitrogen, Carlsbad, CA) with the use of an actin antibody (1:500; Cytoskeleton). Fractionation was performed in triplicate for each genotype (total nine mice assayed/genotype). The F/G-actin ratio was determined by scanning densitometry using ImageJ software (http://rsb.info.nih.gov/ij).
Immunohistochemistry:
For immunohistochemistry on frozen sections, tissues were prepared and immunohistochemistry performed as described in Verdoni et al. (2008a). The primary antibodies and dilutions used were SRF (1:400; Santa Cruz Biotechnology, Santa Cruz, CA), KRT12 (1:200; Santa Cruz Biotechnology), Ki67 (1:100; Thermo Scientific, Fremont, CA), CXCL5 (1:200, ImmunoKontact, Oxon, UK), Myeloperoxidase (1:200, R&D Systems, Minneapolis, MN), and CD45 (1:100, BD Pharmingen, San Diego, CA). Sections were incubated with Alexa Fluor 488 or 568 conjugated secondary antibody (1:400, Invitrogen) and Alexa Fluor 568 conjugated phalloidin (1:50; Invitrogen) and were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1:200; Sigma-Aldrich, St. Louis, MO).
Whole mount immunohistochemistry was also performed as described in Verdoni et al. (2008a). Primary antibody and its dilution used was CD31 (1:50; BD Pharmingen). Corneas were transferred to block solution containing phalloidin (1:50; Invitrogen) and an Alexa Fluor 488 conjugated secondary antibody (1:400; Invitrogen). Corneas were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1:200; Sigma-Aldrich).
Imaging:
Images acquired on sections and whole mount cornea were captured on an Eclipse E600 microscope (Nikon; Tokyo, Japan) using a SPOT camera (Spot Diagnostics; Sterling Heights, MI) or a Zeiss 510 confocal laser scanning system and Axio Imager microscope using LSM 510 software (release 4.2) (Carl Zeiss MicroImaging, Thornwood, NY). For images obtained for the surface of eye, mice were killed and immediately placed under the objective of a Zeiss Stemi SV11 dissecting scope attached to a Nikon COOLPIX 995 digital camera.
Western blotting:
Both corneas from three mice were pooled and homogenized in RIPA buffer (1× PBS with 1% NP-40 and 0.1% SDS) containing a protease inhibitor cocktail. Protein concentrations were determined using the Precision Red protein assay reagent (Cytoskeleton) according to the manufacturer's instructions. Equal amounts of protein were subjected to SDS–PAGE using 4–12% Bis-Tris gels and antibodies against SRF (1:500; Santa Cruz Biotechnology), FMR1 (1:500; Millipore, Billerica, MA), and FLT1 (1:1,000; Angiobio, Del Mar, CA). Horseradish peroxidase conjugated secondary antibodies were used (1:2,000; Jackson Immunoresearch, West Grove, PA) prior to detection with a chemiluminescent reagent (Amersham ECL Plus Western blotting detection system, General Electric, Buckinghamshire, UK) and exposure to X-ray film (Thermo Scientific, Rockford, IL). Western blot analyses were performed in triplicate experiments for each genotype (total nine mice assayed/genotype). FMR1 (fragile X mental retardation syndrome 1 homolog) was chosen as a loading control due to the fact it is the most stably expressed gene between WT and Dstncorn1 cornea (Verdoni et al. 2008a).
Histological quantification of proliferating cells, inflammatory cells, and cell layers:
Corneal frozen sections were stained for the proliferation marker Ki67, the pan-leukocyte marker CD45, and the nuclear marker DAPI. To assess the average number of cells per given length in the cornea, we quantified the number of cells in the entirety of the cornea and normalized it to a unit length of 300 μm. For quantification of the cell layers in the corneal epithelium, the number of nuclei was measured basally to apically across the entirety of the corneal epithelium. To account for the focal nature of epithelial thickening in the Dstncorn/corn1 cornea (Smith et al. 1996), the maximal and minimal numbers of nuclei (cell layers) were recorded for each section, although the area within 250 μm from the limbus was not considered in this analysis because of the maintenance of SRF expression and epithelial abnormalities in Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice at the periphery (see results). Cells were counted using ImageJ software (http://rsb.info.nih.gov/ij) on digital images taken using the Spot Image Analysis system. Four animals were examined per Dox-treated genotypic group at 58 days of age (WT control, Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce). Three P28 Dstncorn1/corn1/SrfΔce/Δce animals (not Dox treated) were examined per group. Two separate sections were analyzed for each eye.
Quantification of the vascularized area of the cornea:
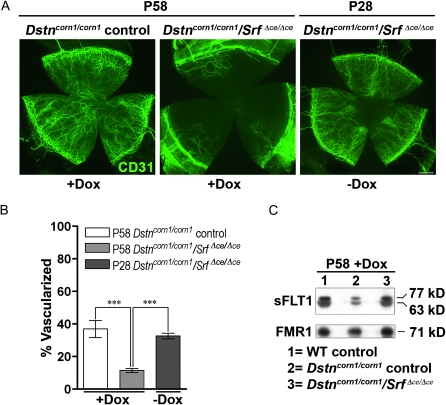
Digital images of all corneal flat mounts were collected using the Spot Image Analysis system. Vascularized area and total corneal area were measured using ImageJ software with a method similar to that of Bock et al. (2008). Filters were applied to subtract background, reduce noise, and enhance contrast. The total corneal area was outlined using the innermost vessel of the limbal arcade as the border, and the area of CD31-positive vessels within the cornea was then calculated and normalized to the total corneal area (expressed as the percentage of vascularized cornea) using thresholding analysis. Seven corneas, each derived from a different animal, were examined for P58 Dox-treated Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce mice and three corneas were examined for untreated P28 Dstncorn1/corn1/SrfΔce/Δce mice.
Statistical analyses:
One way ANOVA followed by Tukey's multiple comparison post test was performed for statistical comparison of all numerical data between P58 Dox-treated WT control, Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce mice. P-values following post-testing are reported in the manuscript. Raw P-values that could be calculated for each data set are available in supporting information, File S1. For comparison of two values only, a two-tailed, unpaired t-test was used. GraphPad Prism software (GraphPad, San Diego, CA) was used for statistical analysis and to create all graphs reporting numerical values. *P < 0.05, **P < 0.01, ***P < 0.001.
RESULTS
SRF expression is substantially increased throughout development of Dstncorn1 corneal abnormalities:
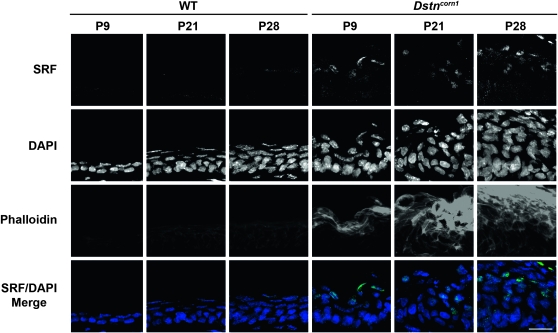
SRF expression was previously observed in Dstncorn1 corneal epithelium at P14 (Verdoni et al. 2008a), a timepoint coincident with abnormal thickening of the epithelial layer (Smith et al. 1996), accumulation of filamentous actin (F-actin) in the epithelial cells (Verdoni et al. 2008a), and the onset of inflammation (Verdoni et al. 2008b) and angiogenesis (Cursiefen et al. 2005). We examined the time course of SRF expression in the cornea of Dstncorn1 mice. Immunohistochemistry showed SRF positive nuclei in Dstncorn1 cornea as early as P9 (Figure 1). SRF positive cells are found within the suprabasal thickened areas of epithelium that also showed an increased level of F-actin. P21 and P28 timepoints also show SRF staining in Dstncorn1 epithelium, where thickening of the epithelial layer and F-actin accumulation increases with age. SRF expression is not detectable in WT cornea at any time point examined by immunohistochemistry (Figure 1). This result shows that increased SRF expression occurs simultaneously with the development of severe corneal abnormalities and SRF remains highly expressed during the transition from a mild to severe phenotype.
Figure 1.—
SRF expression and F-actin distribution in WT and Dstncorn1 cornea. Immunofluorescence shows that SRF (green) is detectable within the suprabasal nuclei of Dstncorn1 corneal epithelium as early as P9 and persists throughout the thickening of the epithelial layer and F-actin (phalloidin, red) accumulation. SRF is not detectable in WT cornea at any timepoint tested. All slides were counterstained with DAPI (blue). Bar, 20 μm.
Ablation of Srf in Dstncorn1 corneal epithelium rescues cell hyperproliferation, inflammation, and neovascularization:
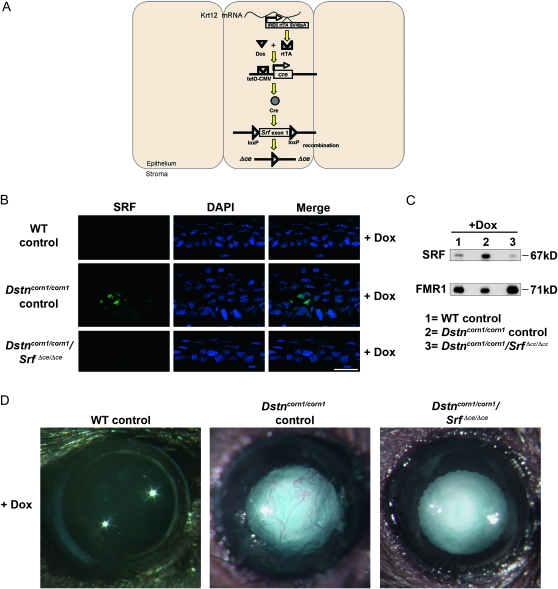
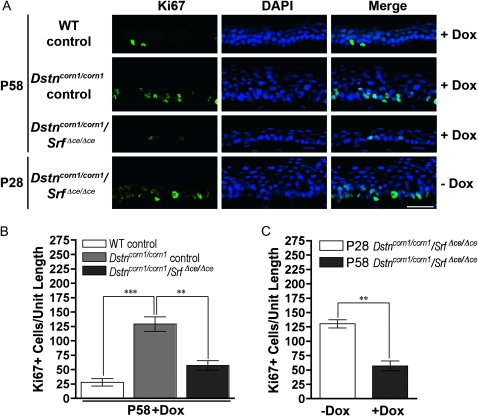
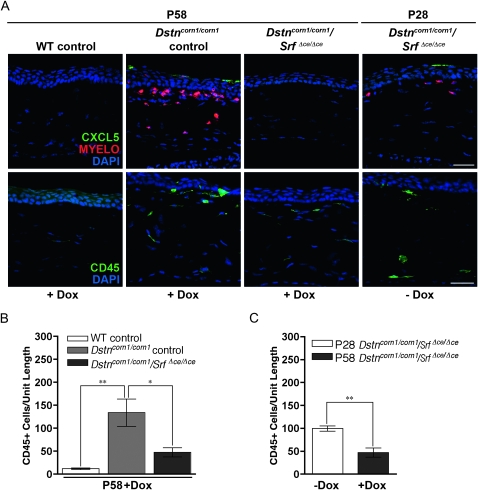
To test the possibility that overexpression of SRF plays a role in the development of the corneal pathology in Dstncorn1 mice, we generated double mutant mice for Dstn and Srf in the corneal epithelium (Dstncorn1/corn1/Srff/f/Krt12rtTA/wt/TetO-cre+, which are referred to as Dstncorn1/corn1/SrfΔce/Δce) using a Dox-inducible system as shown in Figure 2A. Dstncorn1/corn1/SrfΔce/Δce mice will have an ablation of Srf in the corneal epithelium (ce) only in the presence of Dox. Dstncorn1/wt/Srff/f/Krt12rtTA/wt and Dstncorn1/corn1/Srff/f/Krt12rtTA/wt animals were treated with doxycycline and served as WT and Dstncorn1/corn1 mutant controls, respectively. Mice were aged for 28 days to allow the development of severe corneal abnormalities in Dstncorn1 mutant mice (Cursiefen et al. 2005; Verdoni et al. 2008b). The animals were then injected with Dox every other day for a 30-day period to accomplish SRF ablation in the corneal epithelium. Immunohistochemistry showed an efficient ablation for SRF throughout the Dstncorn1/corn1/SrfΔce/Δce epithelium following Dox exposure (Figure 2B), with small areas of the peripheral epithelium maintaining a few SRF positive cells (data not shown). Western blot analysis demonstrated a small level of SRF expression in WT control cornea that was not detectable at the immunohistochemical level, a significant upregulation in Dstncorn1/corn1 control cornea, and an efficient ablation in Dstncorn1/corn1/SrfΔce/Δce cornea (Figure 2C). Examination of the corneal surface revealed that SRF ablation in the corneal epithelium significantly altered the appearance of the Dstncorn1/corn1 cornea. Surface smoothness and transparency resembled that of a WT appearance, and stromal vasculature was significantly less obvious in Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice (Figure 2D). We sought to further investigate the changes that occurred in Dstncorn1/corn1 cornea due to SRF ablation. One apparent change that occurred in SRF ablated Dstncorn1 cornea was the reduction of cell layers of the corneal epithelium in comparison to that of the Dstncorn1/corn1 control mice (Figure 2B). Quantification of the cell layers by counting the number of DAPI-stained nuclei confirmed that the maximal number of cell layers was significantly reduced in Dox-induced Dstncorn1/corn1/Srf Δce/Δce mice (5.3 ± 0.5) as compared to that of Dox-induced Dstncorn1/corn1 control mice (9.5 ± 1.7) (P < 0.05) and was comparable to that in Dox-induced WT control mice (5.0 ± 0) (P > 0.05), while the minimal number of layers was not significantly different between any group (data not shown). To determine whether changes in proliferation accounted for the reduction of cell layers in Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice, Ki67-positive cells were quantified. This analysis showed a very significant decrease in proliferating epithelial cells of Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice compared to that of Dox-induced Dstncorn1/corn1 control mice to a level that was comparable to that of WT control mice (Figure 3, A and B). The level of proliferating epithelial cells in P58 Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice was also significantly less than P28 Dstncorn1/corn1/SrfΔce/Δce mice (without Dox induction), demonstrating a reversal of the hyperproliferative condition (Figure 3, A and C). Next, we examined the effect of SRF ablation on the other phenotypes characteristic of Dstncorn1 mice. We previously demonstrated that Dstncorn1 mice show an autoinflammatory condition in the cornea characterized by the aberrant expression of the neutrophil chemoattractant, CXCL5, and the recruitment of inflammatory cells (Verdoni et al. 2008b). When SRF is ablated from the epithelial cells of Dstncorn1/corn1/SrfΔce/Δce mice, CXCL5 signal and clustered areas of recruited neutrophils are no longer observed (Figure 4A, top). We also detected a significant decrease of CD45 positive leukocytes when SRF is ablated from the corneal epithelium of Dstncorn1/corn1/Srf Δce/Δce mice, which was significantly less than that of P28 Dstncorn1/corn1/SrfΔce/Δce mice (without Dox induction) (Figure 4, A, bottom, B, and C). Examination of the vasculature showed that SRF ablation also leads to the regression of angiogenesis (Figure 5, A and B). This result shows that the ablation of SRF in the corneal epithelium can reverse hyperproliferation, inflammation, and neovascularization caused by the Dstncorn1 mutation.
Figure 2.—
Generation of Dstncorn1 mice with a corneal epithelial specific deletion of Srf. (A) Schematic diagram demonstrating how Srf excision is achieved in the corneal epithelium in the presence of doxycycline (Dox). A knock in allele of rtTA (reverse tetracycline transcriptional activator) within the Krt12 gene becomes translated due to an internal ribosomal entry site (IRES). The rtTA protein binds Dox and forms a complex that initiates transcription of cre recombinase downstream of a tetracycline-inducible promoter (tetO-CMV). Excision of the loxP flanked first exon of Srf occurs, preventing its expression. (B) Immunofluorescence for SRF (green) demonstrates efficient ablation in the corneal epithelium of Dox-treated Dstncorn1/corn1/SrfΔce/Δce mice. Slides were counterstained with DAPI (blue). Bar, 20 μm. (C) Western blot analysis confirms the upregulation of SRF in Dstncorn1/corn1 control mice, as well as the efficient ablation of SRF in Dox treated Dstncorn1/corn1/SrfΔce/Δce cornea. FMR1, loading control. (D) Examination of eyes following Dox induction demonstrates the restoration of a smoothened and clear cornea in Dstncorn1/corn1/SrfΔce/Δce mice as compared to Dstncorn1/corn1 control mice that display a roughened and vascularized cornea. Note that cataract formation (opacification of the lens) that develops due to the Dstn mutation is not affected by Srf inactivation in the corneal epithelial cells.
Figure 3.—
Corneal epithelial cell proliferation in WT control, Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce corneal epithelium. (A) Immunofluorescent staining for the cell proliferation marker Ki67 (green) demonstrates an obvious decrease in the number of proliferating cells in Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice as compared to Dox-induced Dstncorn1/corn1 control mice. This is also decreased compared to Dstncorn1/corn1/SrfΔce/Δce mice prior to Dox treatment, demonstrating a reversal of the hyperproliferative condition. All slides were counterstained with DAPI (blue). Bar, 20 μm. (B) Quantification of the Ki67 positive cells throughout the length of the corneal epithelium confirmed a significantly smaller number of proliferating cells in Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice as compared to Dox-induced Dstncorn1/corn1 control. (C) Quantification of the Ki67 positive cells throughout the length of the corneal epithelium confirmed a significant regression of the hyperproliferative phenotype following Dox induction in Dstncorn1/corn1/SrfΔce/Δce mice. Error bars, SEM. * denotes statistical significance. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.—
CXCL5 expression and inflammatory cell recruitment in WT control, Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce cornea. (A, top) CXCL5 is detectable in the corneal epithelium and neutrophils of Dox-induced Dstncorn1/corn1 control mice and Dstncorn1/corn1/SrfΔce/Δce cornea prior to Dox induction. CXCL5 is no longer detectable in the corneal epithelium of Dstncorn1/corn1/SrfΔce/Δce mice following Dox induction, demonstrating a reversal of the expression of this neutrophil chemoattractant. (A, bottom) Immunofluorescence for the pan-leukocyte marker CD45 shows a high level of inflammation in Dox-treated Dstncorn1/corn1 control and Dstncorn1/corn1/SrfΔce/Δce cornea prior to Dox treatment. Inflammation decreases in Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice. All slides were counterstained with DAPI (blue). Bar, 20 μm. (B) Quantification of the CD45 positive cells demonstrated significantly fewer inflammatory cells in Dstncorn1/corn1/SrfΔce/Δce cornea as compared to Dstncorn1/corn1 control mice treated with Dox. (C) Quantification of the CD45 positive cells confirmed a significant regression of the inflammatory phenotype following Dox induction in Dstncorn1/corn1/SrfΔce/Δce mice. Error bars, SEM. * denotes statistical significance. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5.—
Neovascularization in the corneas of Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce mice. (A) Immunofluorescence for CD31 (green) shows significant infiltration of vessels into the cornea of Dox-induced Dstncorn1/corn1 control cornea and Dstncorn1/corn1/SrfΔce/Δce cornea prior to Dox induction. Vascularization decreases in Dstncorn1/corn1/SrfΔce/Δce mice upon Dox induction. Bar, 200 μm. (B) Quantification of the vascularized area confirms the regression of angiogenesis in Dstncorn1/corn1/SrfΔce/Δce mice following Dox induction. Error bars, SEM. * denotes statistical significance. *P < 0.05, **P < 0.01, ***P < 0.001. (C) Western blot analysis demonstrates downregulation of two isoforms (63 kDa and 77 kDa) of the soluble form of FLT1 (sFLT1) in Dstncorn1/corn1 control corneas. sFLT1 protein level in Dox-induced Dstncorn1/corn1/SrfΔce/Δce cornea is comparable to that in WT control mice. FMR1, loading control.
To further investigate the mechanism for the rescue from angiogenesis, we examined the expression of the soluble form of VEGF receptor 1 (FMS-like tyrosine kinase 1, sFLT1). Downregulation of this protein, which acts as a sequestration sink for VEGFA in the corneal epithelium, was found to be responsible for neovascularization in Dstncorn1 mice (Ambati et al. 2006). Western blot analysis showed downregulation of two soluble forms of FLT1 in the Dstncorn1/corn1 control cornea as compared to WT control (Figure 5C). This protein is detectable again in Dstncorn1/corn1/SrfΔce/Δce cornea after Dox induction at levels comparable to the WT control (Figure 5C), showing that the return of normal sFLT1 expression may be at least partially responsible for the rescue in the angiogenesis.
Increased SRF expression is responsible for F-actin accumulation in Dstncorn1 corneal epithelial cells:
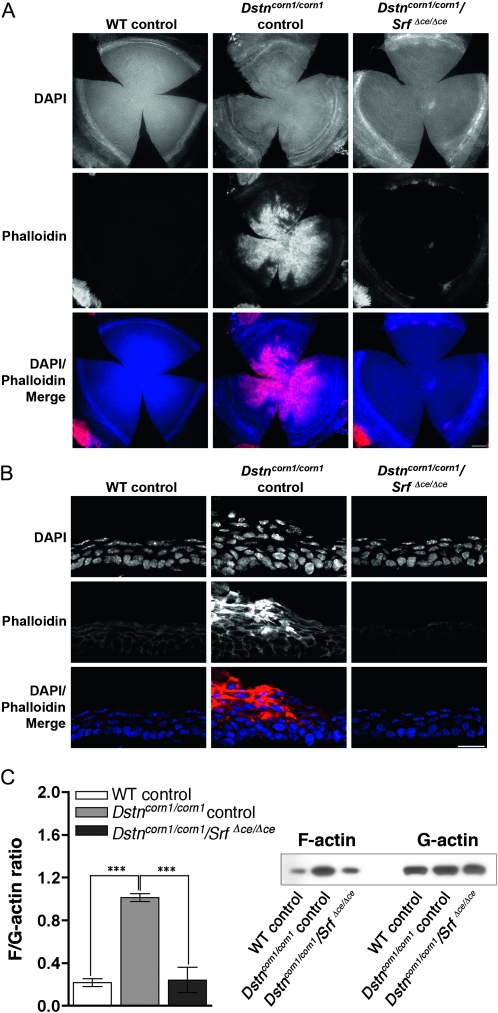
We next examined the level of F-actin in the corneas of Dox-induced WT control, Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce cornea. Analysis of flat mounted corneas stained with phalloidin showed that the F-actin signal was greatly decreased throughout Dstncorn1/corn1/SrfΔce/Δce corneal epithelium, except in regions at the periphery (Figure 6A). Stress fibers disappear in Dstncorn1/corn1/SrfΔce/Δce cornea, and cell shape has been restored to resemble that of a WT control cell in the apical and basal layers (Figure 6B). To quantify the overall change in the levels of F-actin that occur in the cornea due to SRF signaling, we performed fractionation for F-actin and G-actin pools in cornea lysate. The F/G-actin ratio is significantly increased in Dstncorn1/corn1 control mice as compared to WT and is decreased upon SRF ablation to the level similar to that in WT control mice (Figure 6C). The levels of G-actin between all samples are similar, while F-actin level fluctuates (Figure 6C). This result shows that SRF overexpression in Dstncorn1 mice influences the level of F-actin in the cornea, as was shown through immunofluorescence, rather than acting on the G-actin population. The cortical actin cytoskeleton in basal cells appears to be less populated with actin filaments in the Dstncorn1/corn1/SrfΔce/Δce rescued corneas as compared to WT (Figure 6B), which may indicate a role for SRF in regulating a subpopulation of the actin filaments found at cell borders in normal cornea. Interestingly, the F-actin localization pattern and cell–cell contacts appear normal in Dstncorn1/corn1/SrfΔce/Δce cornea as compared to WT control, demonstrating that SRF is not essential for these processes in corneal epithelium in contrast to its essential roles in other epithelia such as the epidermis (Koegel et al. 2009; Verdoni et al. 2010).
Figure 6.—
F-actin in the corneas of Dox-induced WT control, Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce cornea. (A) Immunofluorescence for F-actin (phalloidin, red) shows that F-actin accumulation is prevented upon SRF ablation throughout the corneal epithelium in Dstncorn1/corn1/SrfΔce/Δce cornea except in small areas of the most peripheral region. Bar, 200 μm. (B) Cell shape and architecture of Dox-induced Dstncorn1/corn1/SrfΔce/Δce corneal epithelium has been restored to a more normal appearance and F-actin accumulation no longer occurs. Bar, 20 μm. (C, left) Quantification of the overall changes of F-actin and G-actin in the cornea shows that SRF activation leads to significant increases in the F/G-actin ratio. Error bars, SEM. * denotes statistical significance. *P < 0.05, **P < 0.01, ***P < 0.001. (C, right) Immunoblotting showed that SRF activation affects the filamentous, but not globular actin level.
Ablation of Srf restores normal epithelial differentiation in Dstncorn1 cornea:
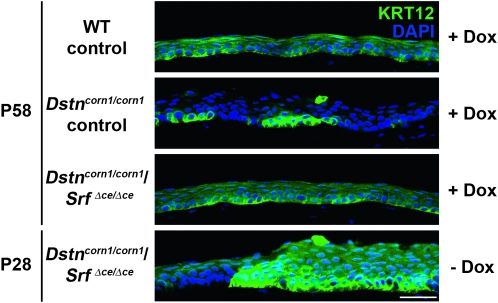
In the normal cornea, differentiated corneal epithelial cells express keratin 12 (Krt12) (Liu et al. 1993; Wu et al. 1994), and populate almost the entire corneal epithelium with the exception of basal cells in the limbal (peripheral) epithelium (Liu et al. 1993). This widespread KRT12 expression was found to be affected in Dstncorn1 mice in a previous study (Zhang et al. 2008) and is markedly lost by P58 in Dstncorn1/corn1 control mice (Figure 7), denoting a problem with epithelial cell identity in the cornea. Upon SRF ablation in Dstncorn1/corn1/SrfΔce/Δce cornea, Krt12 expression is restored to the corneal epithelium in a pattern resembling that of WT control (Figure 7). Examination of KRT12 expression in P28 Dstncorn1/corn1/SrfΔce/Δce cornea prior to Dox induction demonstrated the existence of the defect prior to SRF ablation and thus the normalization of cell differentiation in this tissue (Figure 7).
Figure 7.—
KRT12 expression in the corneas of WT control, Dstncorn1/corn1 control, and Dstncorn1/corn1/SrfΔce/Δce mice. Immunofluorescence for KRT12 (green) demonstrates a loss of expression in portions of corneal epithelium of Dox-induced Dstncorn1/corn1 control and Dstncorn1/corn1/SrfΔce/Δce mice prior to Dox induction. KRT12 is expressed throughout the corneal epithelium in Dox-induced Dstncorn1/corn1/SrfΔce/Δce mice, demonstrating the restoration of normal cell differentiation. Corneas were counterstained with DAPI (blue). Bar, 20 μm.
As observed for SRF expression, small areas of the most peripheral region of the Dox-induced Dstncorn1/corn1/SrfΔce/Δce cornea maintained vasculature and F-actin accumulation. This could be due to the peripheral localization of epithelial cells that remain negative for Krt12 expression even after Dox induction. Theoretically, KRT12 negative cells do not go through SRF ablation with the system used in this study, which is driven by the Krt12 promoter. Indeed, Krt12 negative cells have been detected in the very peripheral region of the Dstncorn1/corn1/SrfΔce/Δce epithelium, which are positive for the SRF signal (data not shown). These cells could represent those in the stem cell niche of the corneal epithelium, which do not normally express KRT12 (Liu et al. 1993).
DISCUSSION
In this study, SRF activation was shown to be responsible for the maintenance of cell hyperproliferation, inflammation, angiogenesis, and F-actin accumulation in Dstncorn1 mice. A wide array of pathological conditions show a hyperproliferative phenotype that accompanies inflammation and angiogenesis to promote disease progression. Therefore, the data presented showing that abnormal SRF activation is the primary mediator of all of these abnormalities in the Dstncorn1 cornea is of extreme clinical significance. SRF is known to be involved in the pathogenesis of various forms of cancer, which demonstrates its potential as a disease-causing gene (Park et al. 2007; Bai et al. 2009; Choi et al. 2009; Eisenmann et al. 2009; Medjkane et al. 2009; Yamaguchi et al. 2009). Most studies demonstrate a link between SRF and the migratory and invasive potential of cancer cells, while a role for SRF in the hyperproliferative phenotype has been shown in some cases. This is the only study showing that abnormal SRF expression can result in the induction of cell hyperproliferation, inflammation, as well as angiogenesis and is the first to define a role for SRF in the development of corneal disease.
How SRF upregulation may lead to Dstncorn1corneal abnormalities:
SRF activation is likely to cause hyperproliferation, one of the downstream phenotypic changes in the Dstncorn1 cornea, in a cell autonomous manner. SRF and its target genes have been implicated in promoting cell proliferation (Gauthier-Rouviere et al. 1991; Lyulcheva et al. 2008; Yamaguchi et al. 2009) as well as F-actin accumulation (Chai et al. 2004a). Known targets of SRF that are involved with cell proliferation are upregulated in Dstncorn1 cornea (Verdoni et al. 2008a) and therefore could be influencing hyperproliferation. It is also a possibility that SRF affects cell proliferation through changes in signaling that occur due to F-actin accumulation. The filamentous actin cytoskeleton shows stress fiber formation in cancer cells (Barkan et al. 2008; Guan et al. 2008; Ren et al. 2008), which can serve as a signal to promote proliferation (Barkan et al. 2008; Gray et al. 2008). Further investigation is necessary to determine contributing roles for upregulated SRF target genes or the F-actin accumulation in the hyperproliferative phenotype in Dstncorn1 cornea.
Since ablation of SRF, which was achieved specifically in the epithelium, leads to the regression of vascular growth and reduction of inflammation, the effect of SRF on these phenotypes is not cell autonomous. One possibility is that SRF acts as a distant growth signal rather than its previously known effect to act within endothelial cells to promote vascular outgrowth (Chai et al. 2004b; Franco et al. 2008; Franco and Li 2009). The mechanism underlying the vascular growth likely involves the regulation of soluble FLT1 (sFLT1) expression in the corneal epithelium. sFLT1 has been shown to be a general regulator of corneal avascularity (Ambati et al. 2006), in addition to its role as a suppressor of angiogenesis in tumors (Zhang et al. 2005; Liu et al. 2007; Hu et al. 2008; Ramachandra et al. 2009). Our finding that SRF overexpression leads to downregulation of such a critical molecule for the maintenance of avascularity may be of extreme clinical significance and could explain why cancers associated with increases in SRF are highly tumorigenic and have significant rates of metastases (Park et al. 2007; Genin et al. 2008; Choi et al. 2009; Medjkane et al. 2009). Another possibility remains that the ablation of Srf in the corneal epithelium results in signaling changes that lead to the repression of vascular outgrowth. Further experimentation is necessary to discern the two possibilities.
Genetic interaction between Dstn and Srf:
This study proved that SRF activation in the corneal epithelium is genetically downstream of a loss of DSTN function. This brings us to the lingering question of how ablation of DSTN leads to activation of SRF. One major mechanism that is known to activate SRF is through depletion of cytoplasmic G-actin (Miralles et al. 2003). G-actin levels have been assessed in the cornea of WT and Dstncorn1 mice at P14 and P58 days of age, two timepoints shown to have SRF activation. Neither experiments showed a decrease in G-actin in Dstncorn1 cornea (Verdoni et al. 2008a) (Figure 6C). However, we cannot rule out the possibility that SRF is activated due to G-actin depletion specifically in the nucleus, which would be difficult to detect with previous experiments performed. Since all members of the ADF/Cofilin family contain a nuclear localization signal and DSTN binds actin, there is a possibility that it acts to shuttle monomeric actin to the nucleus where it normally functions to inhibit the SRF coactivator MAL through binding and sequestration (Vartiainen et al. 2007). An actin shuttling function for the ADF/Cofilin family of proteins has been postulated (Bamburg and Wiggan 2002) and should be a matter of future study.
Another area where DSTN and SRF may interact to lead to the corneal abnormalities is the F-actin accumulation in the epithelial cells. DSTN shows the strongest depolymerization activity out of all ADF/Cofilin family member proteins (Yeoh et al. 2002), making it a likely candidate that a loss of DSTN function would contribute to the F-actin accumulation. However, when SRF is ablated the filamentous actin accumulation is significantly decreased, demonstrating the necessity of SRF for this accumulation. There is a possibility that the loss of DSTN and presence of SRF both serve to lead to the accumulation. For example, the loss of DSTN may lead to the accumulation of actin filaments but stress fiber formation and actin filament stabilization may be facilitated by SRF-target gene expression of actin stabilization molecules such as α-actinin (Pellegrin and Mellor 2007) and vinculin (Zemljic-Harpf et al. 2009).
In summary, conditional ablation of SRF in the corneal epithelium of a diseased cornea lead to the regression of severe corneal inflammation, cell hyperproliferation, and abnormal angiogenesis caused by the Dstncorn1 mutation. This study delineated an epithelial cell-specific role for SRF in the induction of cell hyperproliferation, inflammation, and angiogenesis, which are phenotypes important for the pathogenesis of corneal disease and a number of other conditions. Identification of a novel pathway that serves as a primary signal for the induction of this type of condition could provide a useful therapeutic target for the treatment of these abnormalities.
Acknowledgments
The authors thank Ivan Rayment and Dima Klenchin for the use of the ultracentrifuge and technical advice, Satoshi Kinoshita for generating frozen sections, and the University of Wisconsin-Madison Genetics Confocal Facility for the use of the confocal microscope. This work was supported by a grant from the National Institutes of Health (NIH; R01-EY016108 and -EY010556). Support for A.M.V. was partially provided by the NIH predoctoral training program in Genetics (T32-GM07133).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.117309/DC1.
References
- Allavena, P., C. Garlanda, M. G. Borrello, A. Sica and A. Mantovani, 2008. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 18 3–10. [DOI] [PubMed] [Google Scholar]
- Ambati, B. K., M. Nozaki, N. Singh, A. Takeda, P. D. Jani et al., 2006. Corneal avascularity is due to soluble VEGF receptor-1. Nature 443 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, S., M. W. Nasser, B. Wang, S. H. Hsu, J. Datta et al., 2009. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to Sorafenib. J. Biol. Chem. 284(46):32015–32027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg, J. R., and O. P. Wiggan, 2002. ADF/cofilin and actin dynamics in disease. Trends Cell. Biol. 12 598–605. [DOI] [PubMed] [Google Scholar]
- Barkan, D., H. Kleinman, J. L. Simmons, H. Asmussen, A. K. Kamaraju et al., 2008. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68 6241–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, F., J. Onderka, D. Hos, F. Horn, P. Martus et al., 2008. Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp. Eye Res. 87 462–470. [DOI] [PubMed] [Google Scholar]
- Chai, J., D. Baatar and A. Tarnawski, 2004. a Serum response factor promotes re-epithelialization and muscular structure restoration during gastric ulcer healing. Gastroenterology 126 1809–1818. [DOI] [PubMed] [Google Scholar]
- Chai, J., M. K. Jones and A. S. Tarnawski, 2004. b Serum response factor is a critical requirement for VEGF signaling in endothelial cells and VEGF-induced angiogenesis. FASEB J. 18 1264–1266. [DOI] [PubMed] [Google Scholar]
- Chikama, T., Y. Hayashi, C. Y. Liu, N. Terai, K. Terai et al., 2005. Characterization of tetracycline-inducible bitransgenic Krt12rtTA/+/tet-O-LacZ mice. Invest. Ophthalmol. Vis. Sci. 46 1966–1972. [DOI] [PubMed] [Google Scholar]
- Choi, H. N., K. R. Kim, J. H. Lee, H. S. Park, K. Y. Jang et al., 2009. Serum response factor enhances liver metastasis of colorectal carcinoma via alteration of the E-cadherin/beta-catenin complex. Oncol. Rep. 21 57–63. [PubMed] [Google Scholar]
- Cursiefen, C., 2007. Immune privilege and angiogenic privilege of the cornea. Chem. Immunol. Allergy 92 50–57. [DOI] [PubMed] [Google Scholar]
- Cursiefen, C., S. Ikeda, P. M. Nishina, R. S. Smith, A. Ikeda et al., 2005. Spontaneous corneal hem- and lymphangiogenesis in mice with destrin-mutation depend on VEGFR3 signaling. Am. J. Pathol. 166 1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aura Swanson, C., R. T. Paniagua, T. M. Lindstrom and W. H. Robinson, 2009. Tyrosine kinases as targets for the treatment of rheumatoid arthritis. Nat. Rev. Rheumatol. 5 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann, K. M., K. J. Dykema, S. F. Matheson, N. F. Kent, A. D. DeWard et al., 2009. 5q- myelodysplastic syndromes: chromosome 5q genes direct a tumor-suppression network sensing actin dynamics. Oncogene 28 3429–3441. [DOI] [PubMed] [Google Scholar]
- Fife, R. S., G. W. Sledge, Jr., S. Sissons and B. Zerler, 2000. Effects of tetracyclines on angiogenesis in vitro. Cancer Lett. 153 75–78. [DOI] [PubMed] [Google Scholar]
- Franco, C. A., and Z. Li, 2009. SRF in angiogenesis: branching the vascular system. Cell Adh. Migr. 3 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, C. A., M. Mericskay, A. Parlakian, G. Gary-Bobo, J. Gao-Li et al., 2008. Serum response factor is required for sprouting angiogenesis and vascular integrity. Dev. Cell 15 448–461. [DOI] [PubMed] [Google Scholar]
- Gauthier-Rouviere, C., J. C. Cavadore, J. M. Blanchard, N. J. Lamb and A. Fernandez, 1991. p67SRF is a constitutive nuclear protein implicated in the modulation of genes required throughout the G1 period. Cell Regul. 2 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genin, O., G. Rechavi, A. Nagler, O. Ben-Itzhak, K. J. Nazemi et al., 2008. Myofibroblasts in pulmonary and brain metastases of alveolar soft-part sarcoma: A novel target for treatment? Neoplasia 10 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, D. S., W. F. Liu, C. J. Shen, K. Bhadriraju, C. M. Nelson et al., 2008. Engineering amount of cell-cell contact demonstrates biphasic proliferative regulation through RhoA and the actin cytoskeleton. Exp. Cell Res. 314 2846–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, M., V. Tripathi, X. Zhou and N. C. Popescu, 2008. Adenovirus-mediated restoration of expression of the tumor suppressor gene DLC1 inhibits the proliferation and tumorigenicity of aggressive, androgen-independent human prostate cancer cell lines: prospects for gene therapy. Cancer Gene Ther. 15 371–381. [DOI] [PubMed] [Google Scholar]
- Hu, M., J. L. Yang, H. Teng, Y. Q. Jia, R. Wang et al., 2008. Anti-angiogenesis therapy based on the bone marrow-derived stromal cells genetically engineered to express sFlt-1 in mouse tumor model. BMC Cancer 8 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, S., L. A. Cunningham, D. Boggess, N. Hawes, C. D. Hobson et al., 2003. Aberrant actin cytoskeleton leads to accelerated proliferation of corneal epithelial cells in mice deficient for destrin (actin depolymerizing factor). Hum. Mol. Genet. 12 1029–1037. [DOI] [PubMed] [Google Scholar]
- Koegel, H., L. von Tobel, M. Schafer, S. Alberti, E. Kremmer et al., 2009. Loss of serum response factor in keratinocytes results in hyperproliferative skin disease in mice. J. Clin. Invest. 119 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer, M. S., T. T. Sun and R. M. Lavker, 1998. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 111(Pt 19): 2867–2875. [DOI] [PubMed] [Google Scholar]
- Lewis, J. D., J. J. Deren and G. R. Lichtenstein, 1999. Cancer risk in patients with inflammatory bowel disease. Gastroenterol. Clin. North Am. 28 459–477. [DOI] [PubMed] [Google Scholar]
- Liu, C. Y., G. Zhu, A. Westerhausen-Larson, R. Converse, C. W. Kao et al., 1993. Cornea-specific expression of K12 keratin during mouse development. Curr. Eye Res. 12 963–974. [DOI] [PubMed] [Google Scholar]
- Liu, J., J. Li, C. Su, B. Huang and S. Luo, 2007. Soluble Fms-like tyrosine kinase-1 expression inhibits the growth of multiple myeloma in nude mice. Acta Biochim. Biophys. Sin. (Shanghai) 39 499–506. [DOI] [PubMed] [Google Scholar]
- Lyulcheva, E., E. Taylor, M. Michael, A. Vehlow, S. Tan et al., 2008. Drosophila pico and its mammalian ortholog lamellipodin activate serum response factor and promote cell proliferation. Dev. Cell 15 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjkane, S., C. Perez-Sanchez, C. Gaggioli, E. Sahai and R. Treisman, 2009. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat. Cell Biol. 11 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano, J. M., N. Ramanan, M. A. Georger, K. L. de Mesy Bentley, R. L. Emerson et al., 2004. Restricted inactivation of serum response factor to the cardiovascular system. Proc. Natl. Acad. Sci. USA 101 17132–17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano, J. M., X. Long and K. Fujiwara, 2007. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am. J. Physiol. Cell Physiol. 292 C70–C81. [DOI] [PubMed] [Google Scholar]
- Miralles, F., G. Posern, A. I. Zaromytidou and R. Treisman, 2003. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113 329–342. [DOI] [PubMed] [Google Scholar]
- Notara, M., A. Alatza, J. Gilfillan, A. R. Harris, H. J. Levis et al., 2010. In sickness and in health: corneal epithelial stem cell biology, pathology and therapy. Exp. Eye Res. 90 188–195. [DOI] [PubMed] [Google Scholar]
- Park, M. Y., K. R. Kim, H. S. Park, B. H. Park, H. N. Choi et al., 2007. Expression of the serum response factor in hepatocellular carcinoma: implications for epithelial-mesenchymal transition. Int. J. Oncol. 31 1309–1315. [PubMed] [Google Scholar]
- Pellegrin S., and H. Mellor, 2007. Actin stress fibres. J. Cell Sci. 120 3491–3499. [DOI] [PubMed] [Google Scholar]
- Perl, A. K., S. E. Wert, A. Nagy, C. G. Lobe and J. A. Whitsett, 2002. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc. Natl. Acad. Sci. USA 99 10482–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra, S., S. S. D'Souza, A. E. Gururaj, M. S. Shaila and B. P. Salimath, 2009. Paracrine action of sFLT-1 secreted by stably-transfected Ehrlich ascites tumor cells and therapy using sFLT-1 inhibits ascites tumor growth in vivo. J. Gene Med. 11 422–434. [DOI] [PubMed] [Google Scholar]
- Ren, K., H. Jin, C. Bian, H. He, X. Liu et al., 2008. MR-1 modulates proliferation and migration of human hepatoma HepG2 cells through myosin light chains-2 (MLC2)/focal adhesion kinase (FAK)/Akt signaling pathway. J. Biol. Chem. 283 35598–35605. [DOI] [PubMed] [Google Scholar]
- Smith, R. S., N. L. Hawes, S. D. Kuhlmann, J. R. Heckenlively, B. Chang et al., 1996. Corn1: a mouse model for corneal surface disease and neovascularization. Invest. Ophthalmol. Vis. Sci. 37 397–404. [PubMed] [Google Scholar]
- Vartiainen, M. K., S. Guettler, B. Larijani and R. Treisman, 2007. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316 1749–1752. [DOI] [PubMed] [Google Scholar]
- Verdoni, A. M., N. Aoyama, A. Ikeda and S. Ikeda, 2008. a Effect of destrin mutations on the gene expression profile in vivo. Physiol. Genomics 34 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni, A. M., R. S. Smith, A. Ikeda and S. Ikeda, 2008. b Defects in actin dynamics lead to an autoinflammatory condition through the upregulation of CXCL5. PLoS One 3 e2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni, A. M., S. Ikeda and A. Ikeda, 2010. Serum response factor is essential for the proper development of skin epithelium. Mamm. Genome 21 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, R. L., G. Zhu, S. Galvin, C. Xu, T. Haseba et al., 1994. Lineage-specific and differentiation-dependent expression of K12 keratin in rabbit corneal/limbal epithelial cells: cDNA cloning and northern blot analysis. Differentiation 55 137–144. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, S., K. Asanoma, T. Takao, K. Kato and N. Wake, 2009. Homeobox gene HOPX is epigenetically silenced in human uterine endometrial cancer and suppresses estrogen-stimulated proliferation of cancer cells by inhibiting serum response factor. Int. J. Cancer 124 2577–2588. [DOI] [PubMed] [Google Scholar]
- Yeoh, S., B. Pope, H.G. Mannherz, and A. Weeds, 2002. Determining the differences in actin binding by human ADF and cofilin. J. Mol. Biol. 315 911–925. [DOI] [PubMed] [Google Scholar]
- Zemljic-Harpf A., A. M. Manso, R.S. Ross, 2009. Vinculin and talin: focus on the myocardium. J. Investig. Med. 57 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., J. Zhao, L. Chen, M. M. Urbanowicz and T. Nagasaki, 2008. Abnormal epithelial homeostasis in the cornea of mice with a destrin deletion. Mol. Vis. 14 1929–1939. [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., W. Zou, J. Wang, J. Gu, Y. Dang et al., 2005. Suppression of tumor growth by oncolytic adenovirus-mediated delivery of an antiangiogenic gene, soluble Flt-1. Mol. Ther. 11 553–562. [DOI] [PubMed] [Google Scholar]