Abstract
Null mutations in the genes white and brown, but not scarlet, enhance a rough eye phenotype in a Drosophila melanogaster model of tauopathy; however, adding rosy mutations suppresses these effects. Interaction with nucleotide-derived pigments or increased lysosomal dysregulation are potential mechanisms. Finally, tau toxicity correlates with increased GSK-3β activity, but not with tau phosphorylation at Ser202/Thr205.
IN transgenic models of tauopathy in Drosophila melanogaster, transgenes are often introduced into a “white” genetic background, which is a homozygous null allele of the white gene classically known for its role in eye pigmentation. Here, we demonstrate that white, as well as brown and rosy, two other pigment-related genes, dose dependently affect the tau-induced eye phenotype, tau phosphorylation, and GSK-3β activity. The effects of these pigment-related genes are not light dependent, suggesting involvement of other cellular mechanisms, such as increased lysosomal dysregulation or interaction of tau with pigment or pigment precursor molecules, e.g., drosopterins, which might induce tau seeding and aggregation. Additionally, tau toxicity correlates with increased GSK-3β/Shaggy activity, but not with tau phosphorylation at Ser202/Thr205, suggesting a role of GSK-3β activity in regulating tau toxicity independent from its ability to phosphorylate tau at S202/T205 and also implying the ability of white, brown, and rosy to regulate GSK-3β activity.
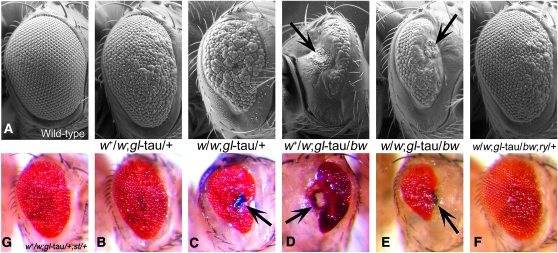
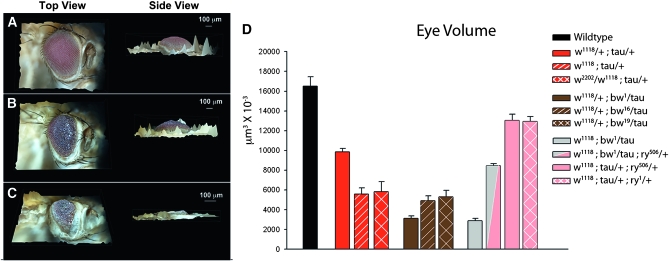
The red eye of D. melanogaster is rendered white by homozygous mutation of the white (w) gene. P elements, naturally occurring transposable elements in Drosophila, can be modified to carry transgenes (Rubin and Spradling 1983) and used for mutagenesis by inserting into genomic regions (Cooley et al. 1998a,b). The white mutant background is commonly used for insertion of transgenes that carry a cDNA sequence of the wild-type white gene, mini-white (w+mC), which serves as a positive marker of transgene incorporation (Klemenz et al. 1987). One copy of w+mC is sufficient to induce red eye development in a white homozygous mutant, although the degree of pigmentation may vary depending on insertion position. We created a model of tau-induced toxicity in Drosophila by expressing full-length, wild-type human tau in the eye of the fly, directly fusing the tau cDNA to the eye-specific glass (gl) promoter (“gl-tau” fly), which yields a rough eye phenotype (Jackson et al. 2002). This transgene is in a w1118 homozygous background and carries one copy of w+mC. While conducting a genetic screen for modifiers of tau toxicity using P-element insertion mutants, we observed that the rough eye phenotype was affected by the P elements themselves and hypothesized that this effect was due to the additional w+mC carried on the P element. To test this hypothesis, we crossed our gl-tau fly to a w+ strain (Canton-S) and compared the gl-tau phenotype in a w1118 homozygous background to that obtained in a w+/w1118 heterozygous background. The w1118 homozygotes showed a significant reduction in eye size and increased ommatidial disorganization as compared to w+/w1118 heterozygous flies (Figure 1, B and C). Furthermore, w1118 homozygous eyes often had necrotic plaques, which were never observed in w+/w1118 heterozygotes. To more accurately describe levels of degeneration, we utilized the Nikon AZ100M microscope NIS-Elements AR 3.0 software (Nikon Instruments, Melville, NY), which features an “extended depth of focus” (EDF) algorithm that allows for three-dimensional reconstruction, as demonstrated in Figure 2, A–C. This imaging allows for estimated eye-volume calculations and can be used in our tauopathy model as a metric for degeneration. Figure 2D plots the mean total eye volumes of all the genotypes used in this study, with the actual volume means and P values for each pairwise genotypic comparison listed in Table 1.
Figure 1.—
Scanning electron micrographs (SEMs) and color light micrographs demonstrating null alleles of white (w) and brown (bw) enhance tau-induced toxicity. Arrows: necrotic patches. (A) Wild-type (Canton-S). (B) white heterozygote: w+/w1118; gl-tau/+. (C) white homozygote: w1118; gl-tau/+. (D) brown allele: w+/w1118; bw1/gl-tau. (E) white homozygote + brown: w1118; bw1/gl-tau. (F) Null allele of rosy revert white and brown enhanced toxicity: w1118; bw1/gl-tau; ry506/+. (G) Null mutations in scarlet (st) do not affect tau-induced toxicity: w+/w1118; gl-tau/+; st1/+. Flies were anesthetized with carbon dioxide for light microscopy images, taken with a digital-camera equipped Zeiss dissecting microscope. Flies were dehydrated in hexamethyldisilazane prior to mounting for SEM, as previously described in Jackson et al. (2002). SEM images were taken on a Hitachi S-2460N scanning electron microscope. Stocks and crosses were maintained on a standard yeast-molasses-cornmeal medium at 23° or 25°.
Figure 2.—
Three-dimensional reconstructions of eye volumes (A–C) were obtained using a Nikon AZ100M light microscope and Nikon DS-Fi1 digital camera with EDF algorithm with Nikon NIS-Elements AR 3.0 software on Z-stack planar images. Stacks were created by 10-μm intervals between planes; area and volume per plane were obtained by software analysis determined by region of interest boundaries. Top-down and side views are shown to demonstrate 3D reconstructions and observable differences in eye volume due to tau toxicity. (A) Wild-type eye. (B) white heterozygote eye: w+/w1118; gl-tau/+. (C) white homozygote: w1118; gl-tau/+. (D) Mean total eye-volume plot of genotypes described in this study. P values for each pairwise comparison are found in Table 1. Graph was created with SigmaPlot 9.0 (Systat, San Jose, CA).
TABLE 1.
Eye-volume statistics
| A. Volume measurements (μm3 × 10−3) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | w/+ | w1118 | w2202 | bw1 | bw16 | bw19 | w,bw | w,bw,ry | w,ry506 | w,ry1 | |
| Mean | 16514.7 | 9860.0 | 5595.6 | 5815.7 | 3133.6 | 4903.0 | 5302.5 | 2881.0 | 8446.9 | 13048.7 | 12936.8 |
| n | 5 | 8 | 8 | 5 | 7 | 8 | 9 | 7 | 8 | 9 | 8 |
| B. Pairwise comparisons P-values: ANOVA with Holm–Sidak test for significance (P < 0.01) | |||||||||||
| Wild type |
w/+ |
w1118 |
w2202 |
bw1 |
bw16 |
bw19 |
w,bw |
w,bw,ry |
w,ry506 |
w,ry1 |
|
| Wild type | – | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| w/+ | – | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.063 | <0.001 | <0.001 | |
| w1118 | – | 0.797 | 0.002 | 0.358 | 0.689 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| w2202 | – | 0.003 | 0.289 | 0.541 | 0.001 | 0.003 | <0.001 | <0.001 | |||
| bw1 | – | 0.026 | 0.005 | 0.753 | <0.001 | <0.001 | <0.001 | ||||
| bw16 | – | 0.585 | 0.011 | <0.001 | <0.001 | <0.001 | |||||
| bw19 | – | 0.002 | <0.001 | <0.001 | <0.001 | ||||||
| w,bw | – | <0.001 | <0.001 | <0.001 | |||||||
| w,bw,ry | – | <0.001 | <0.001 | ||||||||
| w,ry506 | – | 0.878 | |||||||||
| w,ry1 | – | ||||||||||
Statistical analysis performed with SigmaPlot 9.0. All listed genotypes other than “Wild type” contain 1 copy of gl-tau transgene.
To rule out background effects in the w1118 line, the gl-tau fly was crossed to another white allele line, w2202, to create white trans-homozygotes (w1118/w2202); tau enhancing effects comparable to those seen in w1118 homozygous flies were observed (Figure 2D; Table 1). This effect is dose dependent, as increasing copies of w+mC further suppressed the white mutation-induced toxicity. The white gene encodes an ATP binding cassette cotransporter (ABC transporter) that is expressed in many tissues in Drosophila (O'Hare et al. 1984; Mount 1987; http://flyatlas.org). In the eye, White is coupled to either Brown or Scarlet—both also ABC transporters—to transport one of two types of pigment molecules into pigment granules. White and Brown transport guanine-derived drosopterin precursors, while White and Scarlet transport tryptophan-derived xanthommatin precursors (Dreesen et al. 1988; Tearle et al. 1989; Mackenzie et al. 2000). We tested whether mutations in brown (bw) or scarlet (st) exerted effects similar to those of white mutations. Although no significant effect of st1 was found (Figure 1G), the bw1 allele greatly enhanced tau-induced toxicity, producing severe eye reduction and large necrotic patches (Figure 1D), demonstrating specificity of the enhanced toxicity to brown and white, but not scarlet. One copy of bw1 was sufficient to induce this phenotype in a w+/w1118 background. A similar degree of toxicity was observed with a single bw1 null allele in a w1118 homozygous background (“white + brown”) (Figure 1E; Figure 2D). Although mutant homozygous white pigment phenotypes are epistatic to mutant brown and scarlet pigment phenotypes, the lack of an epistatic effect of white with brown with tau, in addition to the lack of a phenotype with a st mutation, indicates a specific role of drosopterins or drosopterin precursors transported by Brown in enhancing tau toxicity and not general effects by all pigment precursors or pigment granules. To rule out potential background effects from the bw1 line, other brown alleles were tested and also showed enhanced toxicity, albeit less severe than those observed with bw1 (bw19 and bw16; Figure 2D and Table 1). From these observations, it can be concluded that loss-of-function mutations in white and brown enhance tau-induced toxicity.
We hypothesized that reductions in White and Brown impair transport of drosopterins or their precursors into pigment granules, causing their cytosolic accumulation. The gene rosy (ry) encodes xanthine dehydrogenase (XDH), which is found in pigment granules that contain Brown but not those that contain only Scarlet (Reaume et al. 1991). Although the role of XDH in pigmentation is complex, it is clear that rosy mutations are associated with decreased drosopterin levels, suggesting that rosy is involved in producing pterins transported by Brown and White. One copy of mutant rosy (ry506) was sufficient to revert the enhanced toxicity of white homozygotes and white + brown flies (Figure 1F), although white + brown + rosy flies were not completely rescued to the levels of white + rosy alone (Figure 2D), emphasizing the strength of degeneration that brown induces. To rule out potential background effects from the ry506 line, a different rosy allele, ry1, was tested; it suppressed the enhanced toxicity of white homozygotes flies nearly identically to the ry506 allele (Figure 2D and Table 1).
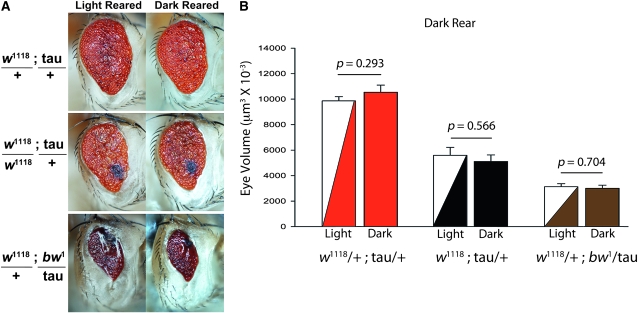
The use of the glass promoter induces expression in many cell types of the eye (Ellis et al. 1993), including photoreceptors, cone cells, and pigment cells, in which the majority of pigment granules are found. Pigment granules function to optically isolate each ommatidium and reduce excess exposure to light (Kirschfeld and Franceschini 1969; Franceschini and Kirschfeld 1976). To test whether the effects on tau toxicity due to mutations in white and brown were due to a reduced facility for light absorption, or were photoreceptor-activity dependent, flies were reared in 24 hr darkness from embryo until 2–3 days post-eclosion (dark reared) and compared to flies grown in a 12-hr light/12-hr dark cycle but kept in otherwise identical environmental conditions (light reared). As seen in Figure 3A, the effects of white and brown were identical in dark reared and light reared gl-tau flies; eye-volume calculations between groups demonstrate no difference between dark- and light-reared flies; moreover the enhanced toxic effects of white and brown are present even in the absence of light (Figure 3B). We conclude that tau toxicity itself, and the effect of white and brown mutations on tau, are light independent, suggesting that cellular functions of white and brown apart from photoreceptor isolation and protection modify tau toxicity (discussed further below). As the majority of pigment granules are in pigment cells, it is reasonable to conclude that the synergistic toxic tau effects are more abundant in pigment cells. However, given that a small number of pigment granules are also found in photoreceptors (Kirschfeld 1979; Hofstee and Stavenga 1996), as well as the light independence of the phenotypes, we cannot rule out that the tau-white–brown-enhanced degeneration is present in photoreceptors and other cell types as well, nor that nonautonomous cell-induced degeneration is also occurring. Indeed, mRNA for white, rosy, and brown is enriched in Malpighian tubules (Chintapalli et al. 2007), and the white gene product, at least, is involved in the transport of important regulatory molecules (Evans et al. 2008). Thus, it is entirely possible that “eye-color” genes affect retinal degeneration indirectly via their influence on synthesis and transport of molecules that circulate in hemolymph and are taken up by tau-producing cells.
Figure 3.—
Tau toxicity modulation by white and brown is light independent. (A) Light micrographs of gl-tau flies reared in 24 hr darkness from embryo to 2–3 days posteclosion (dark reared) as compared to gl-tau flies reared in 12-hr light/12-hr dark cycle under identical environmental conditions (22°, ambient humidity). No phenotypic difference due to light was observed in white heterozygotes (top row, w+/w1118; gl-tau/+), white homozygotes (middle row, w1118; gl-tau/+), or brown genotypes (bottom row, w+/w1118; bw1/gl-tau). Images taken with Nikon AZ100M microscope equipped with Nikon DS-Fi1 digital camera. (B) Eye-volume measurements show no statistical difference within genotypes between light vs. dark reared flies. P values were determined by t-test (SigmaStat 11.0, Systat, San Jose, CA) and graphs were created using SigmaPlot 9.0.
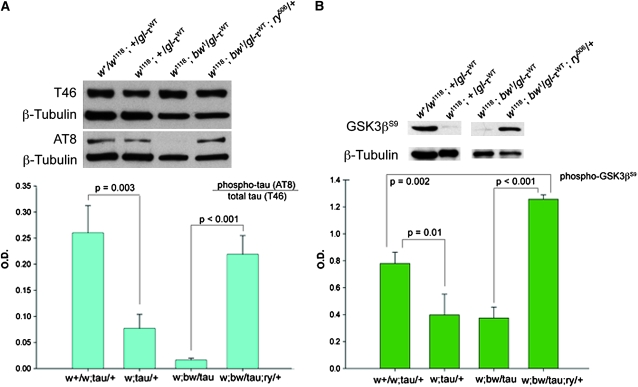
Tauopathies are neurodegenerative diseases characterized in part by hyperphosphorylated intracellular aggregates of the microtubule-associated protein tau. Tau phosphorylation at S202/T205, as detected by the AT8 antibody (Biernat et al. 1992), is a common feature in tauopathies and accumulates in fairly late neurofibrillary tangle development (Ikura et al. 1998; Wada et al. 1998; Augustinack et al. 2002; Ferrer et al. 2002; Wray et al. 2008; Han et al. 2009). Thus, increased AT8 signal is predicted to correlate with enhanced toxicity found in white and white + brown backgrounds; however, AT8 immunoreactivity was reduced in w1118 homozygous flies as compared to w+/w1118 heterozygotes (Figure 4A). Furthermore, AT8 signal was barely detectable in white + brown flies—a genotype with the strongest level of degeneration; however, one copy of ry506, which reduced cellular degeneration, again surprisingly increased AT8 signal in white + brown flies to levels comparable to those observed in w+/w1118 flies (Figure 4A). Total tau levels as detected by the T46 antibody (Kosik et al. 1988; Bramblett et al. 1993) were similar between genotypes.
Figure 4.—
rosy restores tau phosphorylation and decreases inhibitory GSK-3β phosphorylation. (A) white homozygote and white + brown show reduced S202/T205 phosphorylation (AT8 antibody, Pierce/Thermo Scientific, Rockford, IL), which is restored with rosy506 allele. Total tau levels are similar (T46 antibody, Invitrogen, Carlsbad, CA). (B) Phosphorylation of GSK-3β at Ser9, which inactivates GSK3β, is reduced in white homozygotes and white + brown, indicating increased GSK-3β activity; Ser9 phosphorylation is restored by a mutation in rosy (phospho-GSK3β-Ser9 antibody, GeneTex, Irvine, CA). Protein was extracted from fly heads and processed in TBS buffer with phosphatase and protease inhibitors (Roche Diagnostics, Manheim, Germany) and run on 10–20% SDS–PAGE gels (Bio-Rad, San Diego, CA). β-Tubulin is shown as loading control (Accurate Chemical, Westbury, NY). P values were determined by t-test (SigmaStat 11.0) and graphs were created with SigmaPlot 9.0 (Systat, San Jose, CA).
This lack of correlation of tau phosphorylation with phenotype prompted us to investigate the activation state of glycogen synthase kinase-3β beta (GSK-3β), which is known to target the Ser202/Thr205 site recognized by AT8 (Mandelkow et al. 1992). GSK-3β is a constitutively active kinase that is inactivated when phosphorylated at serine-9 (Sutherland et al. 1993). Immunoblots using an antibody specific to phospho-GSK-3βSer9 revealed strong decreases in inhibitory GSK-3β phosphorylation in white and white + brown flies, indicating increased activity. However, one copy of ry506 greatly increased inhibitory phosphorylation of GSK-3β in a white + brown background (P < 0.001; Figure 4B).
We derive four conclusions from these data:
S202/T205 phosphorylation does not correlate well with severity of tau phenotypes in our model. Recent studies using tau constructs resistant to phosphorylation also demonstrated uncoupling of tau phosphorylation at S202/T205 and toxicity (Steinhilb et al. 2007; Chatterjee et al. 2009), suggesting that other mechanisms, such as increased microtubule binding affinity by tau (Chatterjee et al. 2009), or alternatively, tau oligomerization (Kayed and Jackson 2009), may have more direct toxic effects.
GSK-3β activity does not correlate with in vivo phosphorylation of S202/T205, suggesting that other kinases may outcompete GSK-3β in vivo for tau phosphorylation at the S202/T205 sites. Some putative competing kinases are cyclin-dependent kinase 5 (Paudel et al. 1993) or extracellular regulated kinase (Drewes et al. 1992); each has been shown to also target the S202/T205 sites.
GSK-3β activation state correlates well with tau toxicity, with lower activity state correlated with reduced toxicity. This suggests that GSK-3β activity modulates tau-induced toxicity through mechanisms independent of direct S202/T205 phosphorylation. GSK-3β has several downstream targets and is a regulator in many pathways, including Wnt, PI3K, and hedgehog signaling (Liang and Slingerland 2003; Cadigan and Liu 2006; Wang et al. 2007). One such target is the cotranscription factor, Armadillo, which we have previously shown to modulate tau toxicity (Jackson et al. 2002). GSK-3β may also modulate tau-induced toxicity by regulating the activity of the kinase partitioning defective 1 (par-1) (Timm et al. 2008). PAR-1, also known as MARK (Microtubule-Associated Protein/Microtubule Affinity Regulating Kinase), is another known tau kinase (Drewes et al. 1995) shown to modulate tau-induced toxicity; however, reports differ as to whether PAR-1 activity enhances (Nishimura et al. 2004; Chatterjee et al. 2009) or suppresses (Shulman and Feany 2003; Chen et al. 2007; Thies and Mandelkow 2007) tau-induced toxicity.
Mutations in white, brown, and rosy can affect GSK-3β activity, although more work is needed to understand the mechanisms behind this regulation.
Human homologs of rosy/XDH and white (ABCG1) have been cloned and mapped, and both are expressed in several tissues including the brain (Ichida et al. 1993; Xu et al. 1994; Chen et al. 1996; Croop et al. 1997; Saksela et al. 1998). Mutations in ABCG1 are associated with mood and panic disorders (Nakamura et al. 1999), and mutations in white and brown have a range of neurobehavioral effects in flies, including reduced sensitivity to anesthesia, learning defects, and abnormal courtship behavior (Zhang and Odenwald 1995; Campbell and Nash 2001; Diegelmann et al. 2006). Our data fit a model in which an enzymatic product of XDH is transported by White and Brown into granules and in which interaction of this product with tau is detrimental to the cell. Drosopterins are derived from the nucleotide guanosine-5′-triphosphate (GTP), and it has been demonstrated that mammalian tau can interact directly with nucleic acids (Schröder et al. 1984; Wang et al. 2006; Sjöberg et al. 2006); thus it is conceivable that nucleotides and derivatives such as drosopterins directly interact with tau and induce aggregation. This association could also sterically inhibit the interaction of kinases with tau, causing a reduction of AT8 signal.
An alternative model is one of White reduction and tau-overexpression synergism in lysosomal dysregulation. In Drosophila, white mutants have abnormally large pigment granules. Granules with improper pigment balance due to white, brown, or scarlet mutations become autolysosomes (Shoup 1966; Stark and Sapp 1988). Lysosomal dysregulation is a characteristic feature of Niemann–Pick disease type C and Sanfillipo syndrome type B, both tauopathies (Blanchette-Mackie et al. 1988; Sokol et al. 1988; Suzuki et al. 1995; Ohmi et al. 2009). Additionally, Dermaut et al. (2005) showed that abnormal loss-of-function mutations in benchwarmer, which are associated with enlarged lysosomes, also dose dependently enhance tau toxicity. Lysosomal degradation of tau may be an important method of tau clearance; thus, dysfunctional lysosomes may exacerbate tau toxicity. It has been suggested that XDH is required for the formation of the pigment granules that contain Brown and White (Reaume et al. 1991); thus, rosy null mutations may rescue white and brown enhanced tau-induced toxicity by preventing the formation of granules that would otherwise become abnormal and autophagic. It may be that the strong reduction in Ser202/Thr205 tau phosphorylation seen with white and brown mutations is due to tau being sequestered into large but dysfunctional lysosomal/autophagosomal bodies where it is protected from kinase activity.
The results presented here identify novel genetic modifiers of tau-induced toxicity that have with human homologs; such modifiers may function to increase seeding for tau aggregation, augment lysosomal dysregulation, or both. In addition, these data also suggest a novel connection between white, brown, and rosy and GSK-3β/shaggy activity. Finally, given the common use of these eye-color mutations as genetic backgrounds for identification of P-element transformants, these results also have important implications for interpreting genetic disease models in Drosophila.
Acknowledgments
We thank S. Chatterjee, G. Lawless, A. Ratnaparkhi, T.-K. Sang, and D. E. Krantz for helpful discussions, J. Hirsh (University of Virginia) for providing the w2202 line, the Bloomington Drosophila Stock Center (Indiana University) for providing bw, ry, and st alleles, J. Olson and U. Banerjee for use of the Scanning Electron Microscope Facility, and the UCLA Fly Food Facility. The authors thank the anonymous reviewers for their constructive suggestions. National Instutes of Health Grants NS04648, AG16570, and T32 MH073526 and the American Health Assistance Foundation supported this research.
Available freely online through the author-supported open access option.
References
- Augustinack, J. C., A. Schneider, E. M. Mandelkow and B. T. Hyman, 2002. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 103 26–35. [DOI] [PubMed] [Google Scholar]
- Biernat, J., E. M. Mandelkow, C. Schroter, B. Lichtenberg-Kraag, B. Steiner et al., 1992. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 11 1593–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette-Mackie, E. J., N. K. Dwyer, L. M. Amende, H. S. Kruth, J. D. Butler et al., 1988. Type-C Niemann–Pick disease: low density lipoprotein uptake is associated with premature cholesterol accumulation in the Golgi complex and excessive cholesterol storage in lysosomes. Proc. Natl. Acad. Sci. USA 85 8022–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramblett, G. T., M. Goedert, R. Jakes, S. E. Merrick, J. Q. Trojanowski et al., 1993. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron 10 1089–1099. [DOI] [PubMed] [Google Scholar]
- Cadigan, K. M., and Y. I. Liu, 2006. Wnt signaling: complexity at the surface. J. Cell Sci. 119 395–402. [DOI] [PubMed] [Google Scholar]
- Campbell, J. L., and H. A. Nash, 2001. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J. Neurobiol. 49 339–349. [DOI] [PubMed] [Google Scholar]
- Chatterjee, S., T. K. Sang, G. M. Lawless and G. R. Jackson, 2009. Dissociation of tau toxicity and phosphorylation: role of GSK-3beta, MARK and Cdk5 in a Drosophila model. Hum. Mol. Genet. 18 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., C. Rossier, M. D. Lalioti, A. Lynn, A. Chakravarti et al., 1996. Cloning of the cDNA for a human homologue of the Drosophila white gene and mapping to chromosome 21q22.3. Am. J. Hum. Genet. 59 66–75. [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. M., Q. J. Wang, H. S. Hu, P. C. Yu, J. Zhu et al., 2006. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc. Natl. Acad. Sci. USA 103 8534–8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli, V. R., J. Wang and J. A. T. Dow, 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39 715–720. [DOI] [PubMed] [Google Scholar]
- Cooley, L., C. Berg and A. Spradling, 1988. a Controlling P element insertional mutagenesis. Trends Genet. 4 254–258. [DOI] [PubMed] [Google Scholar]
- Cooley, L., R. Kelley and A. Spradling, 1988. b Insertional mutagenesis of the Drosophila genome with single P elements. Science 239 1121–1128. [DOI] [PubMed] [Google Scholar]
- Croop, J. M., G. E. Tiller, J. A. Fletcher, M. L. Lux, E. Raab et al., 1997. Isolation and characterization of a mammalian homolog of the Drosophila white gene. Gene 185 77–85. [DOI] [PubMed] [Google Scholar]
- Dermaut, B., K. K. Norga, A. Kania, P. Verstreken, H. Pan et al., 2005. Aberrant lysosomal carbohydrate storage accompanies endocytic defects and neurodegeneration in Drosophila benchwarmer. J. Cell. Biol. 170 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann, S., M. Zars and T. Zars, 2006. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn. Mem. 13 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesen, T. D., D. H. Johnson and S. Henikoff, 1988. The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol. Cell. Biol. 8 5206–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes, G., B. Lichtenberg-Kraag, F. Doring, E. M. Mandelkow, J. Biernat et al., 1992. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 11 2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes, G., B. Trinczek, S. Illenberger, J. Biernat, G. Schmitt-Ulms et al., 1995. Microtubule-associated protein/microtubuleaffinity-regulating kinase (p110mark): a novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J. Biol. Chem. 270 7679–7688. [DOI] [PubMed] [Google Scholar]
- Ellis, M. C., E. M. O'Neill and G. M. Rubin, 1993. Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development 119 855–865. [DOI] [PubMed] [Google Scholar]
- Evans, J. M., J. P. Day, P. Cabrero, J. A. T. Dow and S.-A. Davies, 2008. A new role for a classical gene: white transports cyclic GMP. J. Exp. Biol. 211 890–899. [DOI] [PubMed] [Google Scholar]
- Ferrer, I., M. Barrachina and B. Puig, 2002. Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration. Acta Neuropathol. 104 583–591. [DOI] [PubMed] [Google Scholar]
- Franceschini, N., and K. Kirschfeld, 1976. The automatic control of the light flux in the compound eye of Diptera: spectral, statical, and dynamical properties of the mechanism. Biol. Cybernetics 21 181–203. [Google Scholar]
- Han, D., H. Y. Qureshi, Y. Lu and H. K. Paudel, 2009. Familial FTDP-17 missense mutations inhibit microtubule assembly promoting activity of tau by increasing phosphorylation at Ser202 in vitro. J. Biol. Chem. [DOI] [PMC free article] [PubMed]
- Hofstee, C. A., and D. G. Stavenga, 1996. Calcium homeostasis in photoreceptor cells of Drosophila mutants inaC and trp studied with the pupil mechanism. Vis Neurosci. 13 257–263. [DOI] [PubMed] [Google Scholar]
- Ichida, K., Y. Amaya, K. Noda, S. Minoshima, T. Hosoya et al., 1993. Cloning of the cDNA encoding human xanthine dehydrogenase (oxidase): structural analysis of the protein and chromosomal location of the gene. Gene 133 279–284. [DOI] [PubMed] [Google Scholar]
- Ikura, Y., T. Kudo, T. Tanaka, H. Tanii, I. Grundke-Iqbal et al., 1998. Levels of tau phosphorylation at different sites in Alzheimer disease brain. Neuroreport 9 2375–2379. [DOI] [PubMed] [Google Scholar]
- Jackson, G. R., M. Wiedau-Pazos, T. K. Sang, N. Wagle, C. A. Brown et al., 2002. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34 509–519. [DOI] [PubMed] [Google Scholar]
- Kayed, R., and G. R. Jackson, 2009. Prefilament tau species as potential targets for immunotherapy for Alzheimer disease and related disorders. Curr. Opin. Immunol. 21 359–363. [DOI] [PubMed] [Google Scholar]
- Kirschfeld, K., 1979. The function of photostable pigments in fly photoreceptors. Biophys. Struct. Mechanism 5 117–128. [DOI] [PubMed] [Google Scholar]
- Kirschfeld, K., and N. Franceschini, 1969. A mechanism for the control of the light flow in the rhabdomeres of the complex eye of Musca. Kybernetik 6 13–22. [DOI] [PubMed] [Google Scholar]
- Klemenz, R., U. Weber and W. J. Gehring, 1987. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 15 3947–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik, K. S., L. D. Orecchio, L. Binder, J. Q. Trojanowski, V. M. Lee et al., 1988. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron 1 817–825. [DOI] [PubMed] [Google Scholar]
- Liang, J., and J. M. Slingerland, 2003. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2 339–345. [PubMed] [Google Scholar]
- Mackenzie, S. M., A. J. Howells, G. B. Cox and G. D. Ewart, 2000. Sub-cellular localisation of the white/scarlet ABC transporter to pigment granule membranes within the compound eye of Drosophila melanogaster. Genetica 108 239–252. [DOI] [PubMed] [Google Scholar]
- Mandelkow, E. M., G. Drewes, J. Biernat, N. Gustke, J. Van Lint et al., 1992. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 314 315–321. [DOI] [PubMed] [Google Scholar]
- Mount, S. M., 1987. Sequence similarity. Nature 325 487. [DOI] [PubMed] [Google Scholar]
- Nakamura, M., S. Ueno, A. Sano and H. Tanabe, 1999. Polymorphisms of the human homologue of the Drosophila white gene are associated with mood and panic disorders. Mol. Psychiatry 4 155–162. [DOI] [PubMed] [Google Scholar]
- Nishimura, I., Y. Yang and B. Lu, 2004. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 116 671–682. [DOI] [PubMed] [Google Scholar]
- O'Hare, K., C. Murphy, R. Levis and G. M. Rubin, 1984. DNA sequence of the white locus of Drosophila melanogaster. J. Mol. Biol. 180 437–455. [DOI] [PubMed] [Google Scholar]
- Ohmi, K., L. C. Kudo, S. Ryazantsev, H. Z. Zhao, S. L. Karsten et al., 2009. Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc. Natl. Acad. Sci. USA 106 8332–8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel, H. K., J. Lew, Z. Ali and J. H. Wang, 1993. Brain proline-directed protein kinase phosphorylates tau on sites that are abnormally phosphorylated in tau associated with Alzheimer's paired helical filaments. J. Biol. Chem. 268 23512–23518. [PubMed] [Google Scholar]
- Reaume, A. G., D. A. Knecht and A. Chovnick, 1991. The rosy locus in Drosophila melanogaster: xanthine dehydrogenase and eye pigments. Genetics 129 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1983. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 11 6341–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela, M., R. Lapatto and K. O. Raivio, 1998. Xanthine oxidoreductase gene expression and enzyme activity in developing human tissues. Biol. Neonate 74 274–280. [DOI] [PubMed] [Google Scholar]
- Schröder, H. C., A. Bernd, R. K. Zahn and W. E. Muller, 1984. Binding of polyribonucleotides and polydeoxyribonucleotides to bovine brain microtubule protein: age-dependent modulation via phosphorylation of high-molecular-weight microtubule-associated proteins and tau proteins. Mech. Aging Dev. 24 101–117. [DOI] [PubMed] [Google Scholar]
- Shoup, J. R., 1966. The development of pigment granules in the eyes of wild type and mutant Drosophila melanogaster. J. Cell Biol. 29 223–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman, J. M., and M. B. Feany, 2003. Genetic modifiers of tauopathy in Drosophila. Genetics 165 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg, M. K., E. Shestakova, Z. Mansuroglu, R. B. Maccioni and E. Bonnefoy, 2006. Tau protein binds to pericentromeric DNA: a putative role for nuclear tau in nucleolar organization. J. Cell. Sci. 119 2025–2034. [DOI] [PubMed] [Google Scholar]
- Sokol, J., J. Blanchette-Mackie, H. S. Kruth, N. K. Dwyer, L. M. Amende et al., 1988. Type C Niemann–Pick disease: lLysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J. Biol. Chem. 263 3411–3417. [PubMed] [Google Scholar]
- Stark, W. S., and R. Sapp, 1988. Eye color pigment granules in wild-type and mutant Drosophila melanogaster. Can. J. Zool. 66 1301–1308. [Google Scholar]
- Steinhilb, M. L., D. Dias-Santagata, T. A. Fulga, D. L. Felch and M. B. Feany, 2007. Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Mol. Biol. Cell 18 5060–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, C., I. A. Leighton and P. Cohen, 1993. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem. J. 296 (Pt 1):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K., C. C. Parker, P. G. Pentchev, D. Katz, B. Ghetti et al., 1995. Neurofibrillary tangles in Niemann–Pick disease type C. Acta Neuropathol. 89 227–238. [DOI] [PubMed] [Google Scholar]
- Tearle, R. G., J. M. Belote, M. Mckeown, B. S. Baker and A. J. Howells, 1989. Cloning and characterization of the scarlet gene of Drosophila melanogaster. Genetics 122 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies, E., and E. M. Mandelkow, 2007. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J. Neurosci. 27 2896–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm, T., K. Balusamy, X. Li, J. Biernat, E. Mandelkow et al., 2008. Glycogen synthase kinase (GSK) 3beta directly phosphorylates serine 212 in the regulatory loop and inhibits microtubule affinity-regulating kinase (MARK) 2. J. Biol. Chem. 283 18873–18882. [DOI] [PubMed] [Google Scholar]
- Wada, Y., K. Ishiguro, T. J. Itoh, T. Uchida, H. Hotani et al., 1998. Microtubule-stimulated phosphorylation of tau at Ser202 and Thr205 by cdk5 decreases its microtubule nucleation activity. J. Biochem. 124 738–746. [DOI] [PubMed] [Google Scholar]
- Wang, X. S., D. L. Wang, J. Zhao, M. H. Qu, X. H. Zhou et al., 2006. The proline-rich domain and the microtubule binding domain of protein tau acting as rna binding domains. Protein Peptide Lett. 13 679–685. [DOI] [PubMed] [Google Scholar]
- Wang, Y., A. P. Mcmahon and B. L. Allen, 2007. Shifting paradigms in Hedgehog signaling. Curr. Opin. Cell. Biol. 19 159–165. [DOI] [PubMed] [Google Scholar]
- Wray, S., M. Saxton, B. H. Anderton and D. P. Hanger, 2008. Direct analysis of tau from PSP brain identifies new phosphorylation sites and a major fragment of N-terminally cleaved tau containing four microtubule-binding repeats. J. Neurochem. 105 2343–2352. [DOI] [PubMed] [Google Scholar]
- Xu, P., T. P. Huecksteadt, R. Harrison and J. R. Hoidal, 1994. Molecular cloning, tissue expression of human xanthine dehydrogenase. Biochem. Biophys. Res. Commun. 199 998–1004. [DOI] [PubMed] [Google Scholar]
- Zhang, S. D., and W. F. Odenwald, 1995. Misexpression of the white (w) gene triggers male-male courtship in Drosophila. Proc. Natl. Acad. Sci. USA 92 5525–5529. [DOI] [PMC free article] [PubMed] [Google Scholar]