Abstract
IL-10 producing regulatory type 1 (TR1) T cells are instrumental in the prevention of tissue inflammation, autoimmunity and graft-versus-host disease. The transcription factor c-Maf is essential for TR1 induction of IL-10, but the molecular mechanisms leading to the development of these cells remain incompletely understood. We demonstrate that the ligand–activated transcription factor aryl hydrocarbon receptor (AhR) induced by IL-27, synergizes with c-Maf to promote the development of TR1 cells. Upon T cell activation under TR1-skewing conditions, the AhR binds to c-Maf and promotes the transactivation of both Il10 and Il21 promoters, resulting in the generation of TR1 cells and amelioration of experimental autoimmune encephalomyelitis. Manipulation of AhR signaling could therefore be beneficial in the resolution of excessive inflammatory responses.
Keywords: AhR, c-Maf, TR1 cell differentiation, experimental autoimmune encephalomyelitis
Regulatory type 1 (TR1) cells have emerged as an important subset of CD4+ T cells that is instrumental in the control of excessive inflammatory responses1. The anti-inflammatory effects of TR1 cells rely on their secretion of interleukin-10 (IL-10), which has been shown to dampen function of antigen presenting cells and antigen-specific effector T cells to suppress tissue inflammation and autoimmunity. However, progress in molecular analysis and biological functions of TR1 cells has been hampered due to the lack of appropriate methods to generate IL-10-producing T cells in large numbers in vitro.
IL-27, a heterodimeric cytokine of the IL-12 family, was initially suggested to induce the expansion of proinflammatory T helper 1 (TH1) cells by activating the transcription factors STAT-1 and T-bet in a manner similar to IL-122. However, it was later found that IL-27 receptor-deficient (Il27ra−/−) mice developed exaggerated proinflammatory T cell responses3 and autoimmunity4, suggesting that IL-27 might be directly involved in inhibiting tissue inflammation. Indeed, we5 and others6, 7 reported that IL-27 is a growth and differentiation factor for TR1 cells. The activation of naïve CD4+ cells in the presence of IL-27 or transforming growth factor (TGF)-β plus IL-27 results in the differentiation of IL-10-producing TR1 cells with potent suppressive activity. In addition to its effects on the differentiation of TR1 cells, IL-27 directly inhibits the differentiation of TH17 cells4, 8 and TGF-β-induced Foxp3+ T regulatory (Treg) cells5.
IL-27 drives the expansion of TR1 cells by inducing the expression of IL-21, a member of the IL-2 family of cytokines, which acts as an autocrine growth factor for TR1 cells9–11. Like IL-10, IL-21 expression was initially reported to be TH2 specific12, but subsequent studies demonstrated that IL-21 was also produced by TR19, TH1713 and T follicular helper (TFH)14 cells. All these cell types produce IL-10, albeit at different levels, suggesting a possible link between IL-21 and IL-10 production. Even though IL-21 promotes the expansion of TH17 cells15, Il21r−/− mice, like Il27ra−/− mice, exhibit an increased susceptibility to the autoimmune disease, experimental autoimmune encephalomyelitis (EAE), thus suggesting a major regulatory role of IL-21 in vivo16.
In an effort to unravel the molecular mechanisms by which IL-27 induces TR1 cells, we found that IL-27 directly induces the transcription factor c-Maf, which is crucial for TR1 cell differentiation9. In the absence of c-Maf, TR1 cell generation and expansion is compromised. Indeed, c-Maf directly transactivates the Il10 and Il21 promoters9, 17. Although these findings highlight the importance of c-Maf and IL-21 for the biology of TR1 cells, the addition of recombinant IL-21 to naïve CD4+ T cells alone failed to generate TR1 cells, suggesting that additional IL-27-driven molecular signals contribute to TR1 cell differentiation.
To explore the molecular mechanisms accounting for the effects of IL-27 in TR1 cell differentiation, we have performed gene expression analysis of IL-27 induced TR1 cells and found that the ligand-activated transcription factor Aryl hydrocarbon receptor (AhR) is induced by IL-27 in TR1 cells. Furthermore, we show that during TR1 cell differentiation, AhR physically associates with c-Maf and transactivates the Il10 and Il21 promoters. Mice with impaired AhR signaling showed decreased production of IL-10 and resistance to IL-27-mediated inhibition of EAE. Taken together, our study demonstrates that AhR and c-Maf synergize to induce IL-27-mediated TR1 cell differentiation.
Results
High expression of Ahr in IL-27-induced-TR1 cells
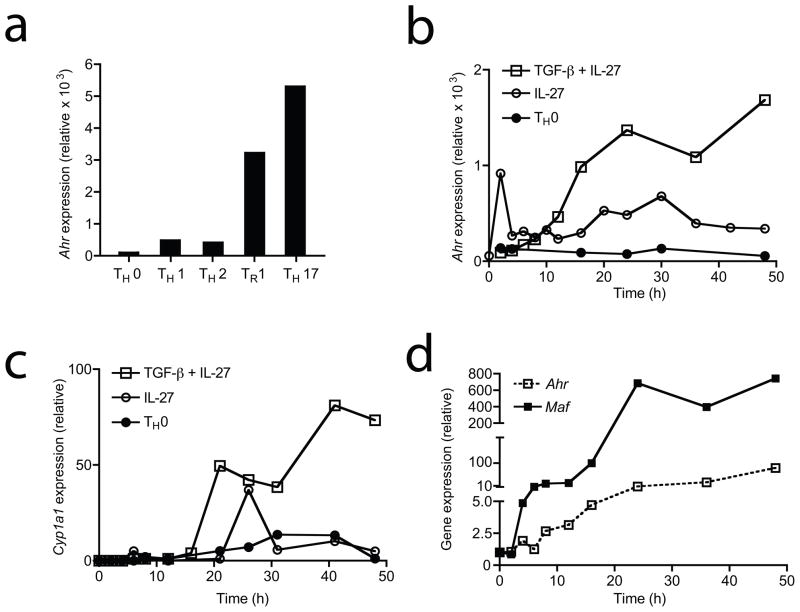
We first studied Ahr expression by real-time PCR (RT-PCR) in different CD4+ T cell subsets. While Ahr expression levels were modest in TH1 or TH2 cells differentiated from naïve CD4+CD25−CD62L+CD44lo T cells, Ahr was expressed at very high levels in TR1 cells induced with TGF-β plus IL-27 (Fig. 1a). Interestingly, Ahr expression in TR1 cells was similar to that found in TH17 cells, where AhR controls the production of IL-2218, 19.
Figure 1. IL-27 upregulates AhR in TR1 cells.
RNA isolated from naïve CD4+CD44loCD62LhiCD25− cells differentiated into indicated populations in the presence of anti-CD3 and anti-CD28 antibodies was subjected to quantitative real-time PCR (RT-PCR) relative to the expression of mRNA encoding β-actin (2−ΔCT x100000) to examine expression of Ahr at different time points following activation. a) RT-PCR analysis of Ahr expression at 48 hours in TH0, TH1, TH2, TH17 and TR1 cells differentiated with either no cytokines, IL-12, IL-4, TGF-β plus IL-6 or TGF-β plus IL-27 respectively. RT-PCR kinetic analysis of b) Ahr and c) xenobiotic metabolizing cytochrome P450 enzyme Cyp1a1 expression in TH0 or TR1 cells differentiated with IL-27 or TGF-β and IL-27. d) RT-PCR kinetic analysis of Ahr and Maf expression in TR1 cells. Gene expression relative to TH0 cells is depicted. Representative data from one of three experiments are shown.
We investigated the kinetics of Ahr expression during the differentiation of TR1 cells with TGF-β and IL-27 and found that Ahr expression was significantly up-regulated 12 hours after the initiation of the culture and was sustained at high levels throughout TR1 cell differentiation (Fig. 1b). Given that we have previously shown that TR1 cells can also be differentiated by IL-27 without TGF-β9, we analyzed the kinetics of Ahr expression during the differentiation of TR1 cells with IL-27 alone. Ahr expression was also induced by treatment with IL-27 alone, albeit at lower expression levels (Fig. 1b). T cells activated without any polarizing cytokines (TH0 condition) only expressed marginal levels of Ahr.
The expression of the xenobiotic metabolizing enzyme cytochrome P450 encoded by Cyp1a1 is directly controlled by the AhR which transactivates the Cyp1a1 promoter20. To test whether the AhR is activated during TR1 cell differentiation, we analyzed the expression of Cyp1a1 in naïve CD4+ T cells treated with IL-27, with or without TGF-β. We found that Cyp1a1 was expressed in CD4+ cells as early as 20 hours after activation (Fig. 1c). TR1 cells differentiated with IL-27 alone showed a transient expression of Cyp1a1, whereas Cyp1a1 expression was sustained at high levels in TR1 cells differentiated with TGF-β plus IL-27 (Fig. 1c).
We have recently shown that the transcription factor c-Maf plays a major role in TR1 cell differentiation9. Thus, we analyzed Ahr and Maf expression during the differentiation of TR1 cells with TGF-β and IL-27. Maf expression was detectable as early as 6 hours after the initiation of differentiation, while Ahr expression was first detected 8 hours after differentiation and was expressed at lower levels than Maf. Ahr and Maf expression were sustained at high levels after 24 hours (Fig. 1d). Overall, these studies demonstrate that Ahr, like Maf, is highly expressed and active during IL-27 triggered differentiation of TR1 cells.
AhR controls the development of TR1 cells
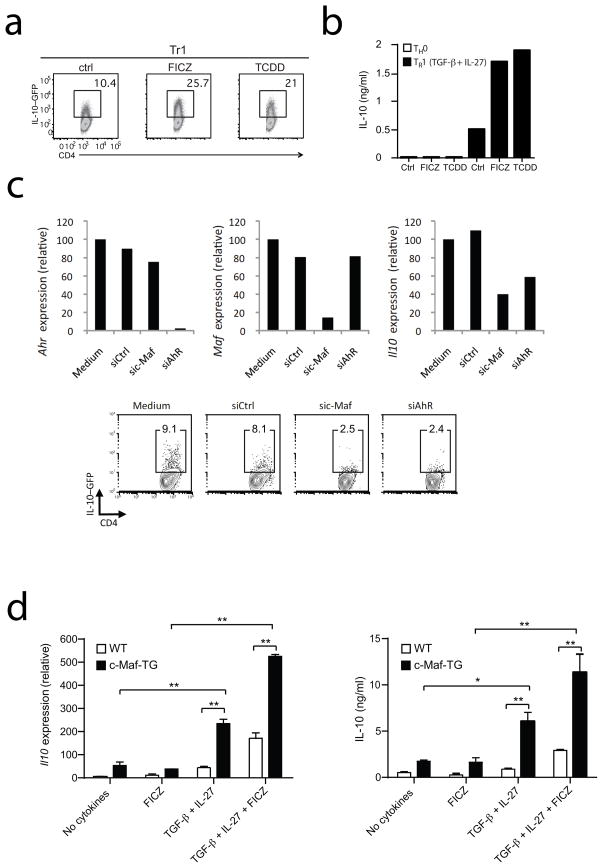
Different AhR ligands such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the prototypic environmental AhR agonist, or the putative endogenous ligand 6-formylindolo [3,2-b] carbazole (FICZ) regulate Treg cell and TH17 differentiation18, 19. Based on the high expression levels of Ahr during TR1 cell differentiation, we hypothesized that AhR ligands might affect TR1 cell differentiation as well. To investigate the effect of AhR signaling on TR1 cell development, we differentiated naïve CD4+ cells from IL-10.eGFP reporter mice (Vert-X)21 with TGF-β and IL-27 in the presence of either of the AhR ligands TCDD and FICZ. Both TCDD and FICZ doubled the frequency of IL-10- producing T cells (Fig. 2a) and increased the secretion of IL-10 by more than three-fold over controls (Fig. 2b), suggesting that AhR activation promotes TR1 cell differentiation. Similar results were obtained with IL-27 alone (Supplementary Fig. 1). Although TCDD has been proposed to support Foxp3+ Treg cell development19, 22, the addition of AhR ligands together with IL-27 during TR1 cell differentiation did not induce Foxp3 expression (Supplementary Fig. 2 and data not shown), thus ruling out any possible involvement of Foxp3+ regulatory T cells in the enhanced IL-10 expression. These results are reminiscent of a previously published study demonstrating that FoxP3 is not induced in regulatory T cells generated by AhR ligands during graft versus host (GVH) responses23. To further characterize the contribution of AhR to TR1 cell differentiation, we knocked-down Ahr expression using siRNA. TR1 cells were differentiated with TGF-β and IL-27 in the presence of an siRNA specific for AhR (siAhR) or control siRNA and IL-10 expression was analyzed by RT-PCR and by flow cytometry. We found that naïve T cells, in which Ahr expression had been downregulated by siRNA, had a decreased ability to produce IL-10 (Fig. 2c). Similar results were obtained when Maf was knocked down as a positive control. In agreement with our previous results, the addition of the AhR antagonist CH-223191 during the differentiation of TR1 cells with TGF-β plus IL-27 decreased IL-10 production (Supplementary Fig. 3a). Similarly, the ability of AhR deficient T cells to differentiate into TR1 cells in the presence of TGF-β plus IL-27 was impaired, confirming that AhR is essential in the differentiation of TR1 cells (Supplementary Fig. 3b). Therefore the modulation of AhR signaling either with the agonists FICZ or TCDD, the antagonist CH-223191, with siRNA mediated downregulation or genetic deletion of the AhR gene profoundly affects the development of IL-10-producing TR1 cells.
Figure 2. AhR regulates IL-10-production in TR1 cells induced by TGF-β and IL-27.
Naïve CD4+ T-cells from IL-10 reporter mice (Vert-X mice) were cultured with IL-27 and TGF-β in the absence (Control: Ctrl) or presence of the AhR agonists FICZ (100nM) or TCDD (100nM) a) IL-10.GFP expression was analyzed by flow cytometry after 72 hours of culture. b) IL-10 protein was measured by cytokine bead array analysis at 48 hours. c) Naïve cells from IL-10 reporter mice were cultivated with IL-27 plus TGF-β and transfected with either an irrelevant control siRNA or an siRNA against AhR or c- Maf. Ahr, Maf and IL-10 mRNA expression in TR1 cells were assessed after 24 hours of culture by quantitative PCR (top) and IL-10.GFP expression was analyzed by flow cytometry after 48 hours (bottom). d) Naïve T cells isolated from wildtype (WT) or c- Maf transgenic (c-Maf-TG) mice were differentiated into TH0 or TR1 cells with TGF-β and IL-27 in the absence or presence of FICZ (100nM). After 48 hours of culture, Il10 mRNA expression was assessed by quantitative PCR (left panel) and IL-10 secretion was analyzed by ELISA at 72 hours (right panel). (*p<0.05; **p<0.01)
To further demonstrate a role for Ahr in Il10 expression, we retrovirally transduced primary CD4+ T cells with an Ahr-GFP (green fluorescent protein) or a control-GFP overexpression vector and monitored Il10 expression on GFP+ cells. We observed that Il10 (but not Ifnγ) expression was significantly induced when primary CD4+ T cells overexpressed AhR (Supplementary Fig. 4). These results suggest that the IL-27-driven Ahr expression is responsible for the enhanced IL-10 secretion during TR1 cell differentiation. Given that c-Maf was shown to be essential for TR1 cell generation9, we decided to evaluate the relative contribution of c-Maf and AhR to the development of TR1 cells. For this, we differentiated naïve T cells from mice overexpressing the Maf transgene (c-Maf-TG) under the control of the CD4 promoter without any cytokine or with TGF-β plus IL-27. In addition, to activate AhR signaling, we added AhR agonist FICZ to c-Maf transgenic T cells. In line with previous reports17, the overexpression of c- Maf was sufficient to drive IL-10 secretion from TH0 cells (Fig. 2d). Since TGF-β plus IL-27 is required for AhR expression, FICZ alone did not alter the IL-10 secretion from c-Maf-TG cells in the absence of TGF-β and IL-27. However, we found that c-Maf overexpressing T cells dramatically increased IL-10 secretion upon differentiation with TGF-β plus IL-27 and FICZ (Fig. 2d), suggesting that the two transcription factors AhR and c-Maf cooperate to enhance IL-10 secretion from TR1 cells.
AhR regulates IL-21 expression in TR1 cells
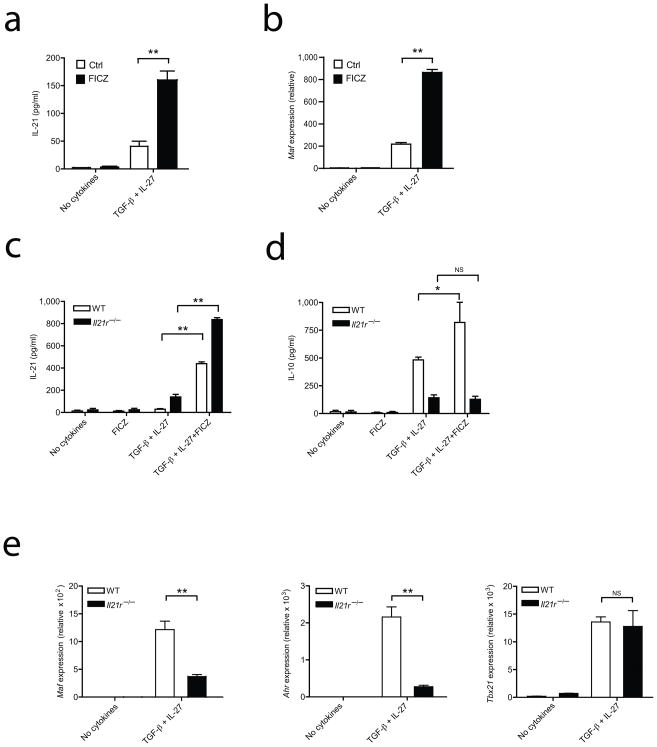
We have previously shown that IL-27 acts on TR1 cells to trigger the production of IL-21, which acts as an autocrine growth factor for TR1 cells9. Therefore we examined the effect of AhR signaling on IL-21 production by TR1 cells. We first differentiated naïve CD4+ T cells with TGF-β plus IL-27 in the presence of the AhR ligand FICZ and found that treatment with FICZ led to a four-fold increase in IL-21 production by TR1 cells (Fig. 3a). Similar results were obtained when we investigated the effect of another AhR ligand TCDD (Supplementary Fig. 5).
Figure 3. AhR signaling dictates IL-21 secretion in TR1 cells.
Naïve T cells were differentiated into TR1 cells without (Ctrl) or with FICZ (100nM) and a) IL-21 cytokine production was assessed by cytokine bead array analysis after 72 hours of culture; b) The transcription factor Maf was quantified by RT-PCR at 48 hours c) and d) Naïve T cells from wild type and Il21r−/− mice were differentiated into TR1 cells and IL-21 and IL-10 production were analyzed by cytokine bead array analysis after 48 hours of culture e) mRNA for Maf, Ahr and Tbx21 in the cells described in c) was quantified by RT-PCR relative to the expression of mRNA encoding β-actin. Data are from one of three experiments with similar results. (*p<0.05; **p<0.01)
We have previously shown that c-Maf regulates IL-21 production9, 14, thus we examined Maf expression in TR1 cells differentiated in the presence of AhR ligands. We found that in addition to enhancing IL-21 expression, AhR activation by FICZ during TR1 cell differentiation led to a significant up-regulation of Maf (Fig. 3b). Importantly, treatment with FICZ or TCDD in the absence of differentiating cytokines had no effect on Maf expression, indicating that AhR activation is not sufficient for up-regulation of Maf (Fig.3b and Supplementary Fig. 5). Thus, AhR activation potentiates the expression of c-Maf and IL-21 during the differentiation of TR1 cells triggered by IL-27.
Naïve CD4+ T cells from IL-21R-deficient mice have an impaired capacity to differentiate into TR1 cells9. To test whether the effects of the AhR activation on TR1 cell differentiation were mediated by IL-21, we differentiated naïve CD4+ T cells from Il21r−/− deficient mice with TGF-β plus IL-27, with or without FICZ. IL-21 secretion was enhanced in FICZ-treated TR1 cells from either wildtype or Il21r−/− mice (Fig. 3c). However, IL-10 production was drastically impaired in TR1 cells derived from Il21r−/− mice (Fig. 3d). These results suggest that the effects of AhR activation on TR1 cell differentiation are at least partly mediated by IL-21.
IL-21 is an autocrine TR1 cell growth factor that enhances IL-10 and c-Maf expression in TR1 cells9. Thus, we examined the effect of IL-21 on the mRNA expression of Maf and Ahr during TR1 cell differentiation. In agreement with our previous findings, Maf expression in TR1 cells was controlled by IL-21 signaling (Fig. 3e). Strikingly, there was a dramatic decrease in Ahr expression in TR1 cells derived from Il21r−/− mice, while the mRNA expression level of the transcription factor T-bet, known to be induced by IL-27 during TR1 cell differentiation24, was unaffected (Fig. 3e). This suggests that, in addition to controlling c-Maf, IL-21 further modulates TR1 cell development by inducing and/or maintaining AhR expression. Collectively, these results demonstrate that AhR signaling controls TR1 cell differentiation partly by regulating IL-21 production, which in turn contributes to TR1 cell development as a positive feedback mechanism, likely enhancing AhR mRNA expression, IL-21 and IL-10 production.
AhR and c-Maf transactivate Il10 and Il21
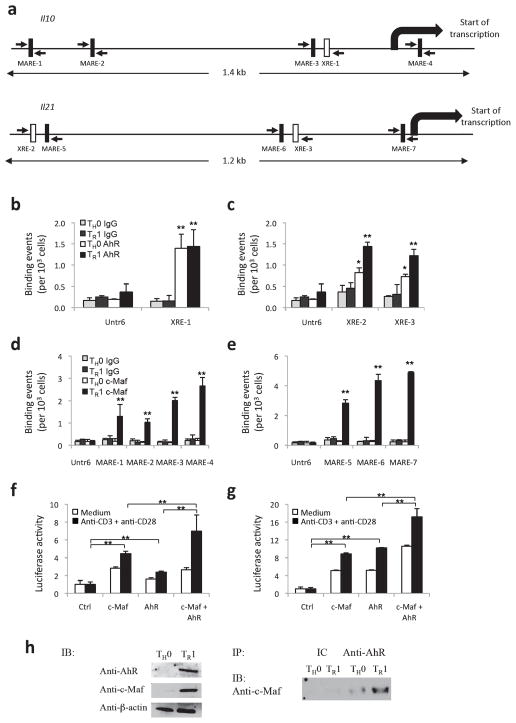
c-Maf transactivates the Il109, 17 and Il219 promoters. Based on our findings pointing to a key role for the AhR in the regulation of IL-10 and IL-21 during TR1 cell differentiation, we hypothesized that the AhR might transactivate the Il10 and Il21 promoters in TR1 cells as well. We first searched the Il10 and Il21 promoters for AhR and c-Maf binding sites. We found one putative AhR binding site (xenobiotic response element, XRE-1) and four putative c-Maf binding sites (Maf recognition element, MARE-1, MARE-2, MARE-3 and MARE-4) in the Il10 promoter (Fig. 4a and Supplementary Fig. 6). Similarly, we identified two putative AhR binding sites (XRE-2 and XRE-3) and three putative c-Maf binding sites (MARE-5, MARE-6, and MARE-7) in the Il21 promoter (Fig. 4a and Supplementary Fig. 6).
Figure 4. AhR and c-Maf transactivate the Il10 and Il21 promoters in TR1 cells.
a) AhR and c-Maf binding sites in the Il10 and the Il21 promoters. Schematic representation of the Il10 and the Il21 promoters, AhR binding sites (XRE) are depicted as open boxes and c-Maf binding sites (MARE) are depicted as filled boxes. b) ChIP analysis of the interaction of AhR or isotype control antibody (IgG) to the XRE in the Il10 and c) the Il21 promoter in in vitro differentiated TR1 or control TH0 cells. (*p<0.01; **p<0.001 between AhR vs IgG) d) ChIP analysis of the interaction of c-Maf or isotype control antibody (IgG) to the MARE in the Il10 and e) the Il21 promoter in in vitro differentiated TR1 or control TH0 cells. (**p<0.001 between c-Maf vs IgG) f) and g) Transactivation of the Il10 or Il21 promoters by c-Maf or AhR. Reporter constructs for the Il10 f) or Il21 g) promoters (Il10-Luc and Il21-Luc, respectively) were co-transfected in EL-4 T cells with vectors coding for AhR and/or c-Maf, and firefly luciferase activity was determined 24 hours later and normalized to the renilla luciferase activity of a co-transfected control. (**p<0.001) h) In vitro differentiated TH0 or TR1 cell expression of AhR and c-Maf (immunoblot; IB, left panel). AhR was immunoprecipitated from nuclear extracts with a specific antibody (IP, right panel), c-Maf and AhR complexes immunoblotted (right panel) using an anti-c-Maf antibody.
To study whether AhR binds to IL-10 and IL-21 XRE promoter sequences, we monitored whether in vitro-translated AhR protein would interact with an oligonucleotide containing the putative AhR binding site located in the Il10 or the Il21 promoter. Since binding of AhR with AhR nuclear translocator (Arnt) transforms AhR into its high affinity DNA binding form25, we studied the binding of AhR complexed with Arnt to XRE 1, 2 and 3. The AhR-Arnt complex was incubated with a radiolabeled oligomer containing the putative AhR binding site located in the Il10 or Il21 promoter. The AhR-Arnt-DNA protein complex was visualized by electrophoretic mobility shift assay (EMSA) (Supplementary Figure 7, lanes 1, 3 and 5). Importantly, binding of AhR to IL-10 and IL-21 XRE promoter sequences was inhibited by inclusion of a competitor oligo containing Cyp1a1 XRE3 AhR DNA binding site26 (Supplementary Figure 7, lanes 2, 4 and 6). To confirm that AhR can also interact with its target sequences in the Il10 and Il21 promoters under physiological conditions, we undertook chromatin immunoprecipitation (ChIP) assays with differentiated TR1 cells in vitro with IL-27 and TGF-β. AhR interacted with XRE-1 in the Il10 promoter both in TR1 and TH0 cells, and with XRE-2 and XRE-3 in the Il21 promoter in TR1 cells (Fig. 4b, c and Supplementary Figure 6). Similarly, ChIP assays revealed a clear interaction of c-Maf with MARE 1–4 and MARE 5–7 on the Il10 and Il21 promoters, respectively, but only in TR1 cells (Fig. 4d, e and Supplementary Figure 6). No interaction with the XRE or MARE sequences in either the Il10 or Il21 promoter was detected when we used isotype control antibodies (IgG), and no significant AhR or c-Maf binding was detected in the control sequence Untr6 (Fig. 4b-e). These data suggest that c-Maf controls the cell specificity of the Il10 and Il21 gene transcription.
To determine the relevance of AhR and c-Maf binding their target sequences in Il10 and Il21, we studied the ability of the AhR and c-Maf to transactivate the Il10 and Il21 promoters in reporter assays. We used reporter constructs containing the firefly luciferase gene under the control of the Il10 promoter (Il10-luc) or the Il21 promoter (Il21-luc). Cotransfection of Il10-luc or Il21-luc with a construct coding for mouse c-Maf resulted in a slight up-regulation of the transcription of the Il10 and the Il21 gene (Fig. 4f, g). A similar upregulation was observed when Il10-luc or Il21-luc were cotransfected with a construct coding for mouse AhR (Fig. 4f, g). Notably, cotransfection of both c-Maf and AhR resulted in an additive transactivation of Il10 as well as Il21 expression (Fig. 4f, g), suggesting that AhR and c-Maf cooperate to control the transcriptional activity of both promoters.
The concomitant upregulation of c-Maf and AhR during TR1 cell differentiation (Fig. 1d), their ability to bind to Il10 promoter elements to induce IL-10 secretion (Fig. 4) and the proximity of the putative binding sites for both c-Maf and AhR on the Il10 or Il21 promoters led us to test whether c-Maf and AhR physically interact with each other. Indeed, AhR has been shown to interact with diverse transcription factors including NF-κB27 and the estrogen receptor28. To address this issue, we differentiated naïve CD4+ T cells into either TH0 or TR1 cells and performed immunoprecipitation followed by immunoblotting. We first found that AhR and c-Maf proteins were upregulated in TR1 cells (Fig. 4h, left panel). Moreover, we could co-precipitate c-Maf and AhR in a TR1 cell nuclear extract by using an antibody against AhR to immunoprecipitate the complex, followed by immunoblotting with an anti-c-Maf antibody (Fig. 4h, right panel), suggesting that AhR physically interacts with c-Maf. Taken together, our results demonstrate that AhR and c-Maf interact in TR1 cells to transactivate the Il10 and Il21 promoters.
AhR controls TR1 cell generation in vivo
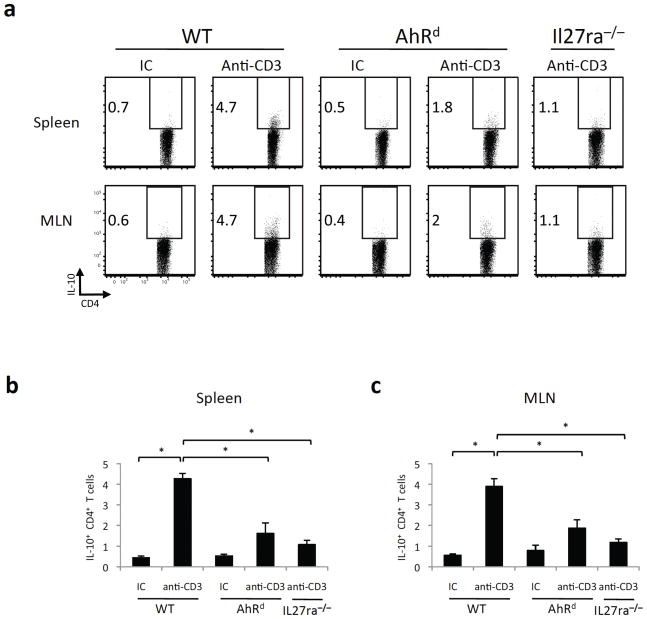
It has previously been shown that repeated in vivo treatment with anti-CD3 induces IL-10+ regulatory T cells29. Given that we have shown that AhR and Maf are induced upon in vitro differentiation of TR1 cells (Fig. 1), we assumed that they might be similarly induced in vivo in IL-10+ T cells elicited by anti-CD3 treatment. To test this, we repeatedly administered anti-CD3 or an isotype control antibody (IC) to IL-10.eGFP reporter mice (Vert-X) and assessed AhR and Maf expression within GFP+ IL-10-producing T cells in the spleen and mesenteric lymph nodes 4 hours after the last injection. In line with our in vitro findings, both Ahr and Maf were significantly induced in IL-10+ T cells (Supplementary Fig. 8). Thus, we used this model to analyze the role of the AhR in the generation of TR1 cells in vivo. We administered anti-CD3 or an isotype control antibody to wild type, mutant AhR (AhRd) and IL-27 receptor deficient mice (Il27ra−/− mice), and studied the frequency of IL-10+ T cells in the spleen and mesenteric lymph nodes (MLN). AhRd mice have point mutations in the AhR ligand-binding pocket and therefore show defective AhR mediated responses in vivo30. Since IL-10 is produced by TH17 cells17, Foxp3+ Treg cells29 and TR1 cells, we analyzed the production of IL-10 by Foxp3−IL-17−CD4+CD3+TCRαβ+ T cells (Supplementary Fig. 9). We found that the administration of anti-CD3 to wild type mice resulted in a significant induction of IL-10+ T cells in the spleen and the mesenteric lymph nodes (MLN; Fig. 5a-c). The induction of IL-10-producing cells in this setting was mediated by IL-27, since it was not observed in anti-CD3 treated Il27ra−/− mice (Fig. 5a-c). In addition, AhRd mice also showed a significant impairment in their ability to produce IL-10+ T cells upon treatment with anti-CD3, both in the spleen and the MLN (Fig. 5a-c). Thus, AhR controls the generation of IL-10+ T cells in vivo.
Figure 5. AhR controls the generation of TR1 cells in vivo.
AhRd, wild type (WT) or Il27ra−/− mice were injected i.p. with 20 μg of antibodies to CD3 or an isotype control (IC) once every 3 days, for a total of 3 times. 4 hours after the last injection, mice were sacrificed and the expression of IL-10 in the spleen and the mesenteric lymph nodes (MLN) was analyzed by flow cytometry. a) Representative plots of IL-10 expression in CD4+ cells in the spleen and the MLN. b) and c) Frequency of IL-10+ CD4+ T cells in anti-CD3 or isotype control (IC) treated mice (mean + s.d. of 3–5 mice) in spleen (b) or MLN (c). (*p<0.01)
AhR controls the IL-27-mediated inhibition of EAE
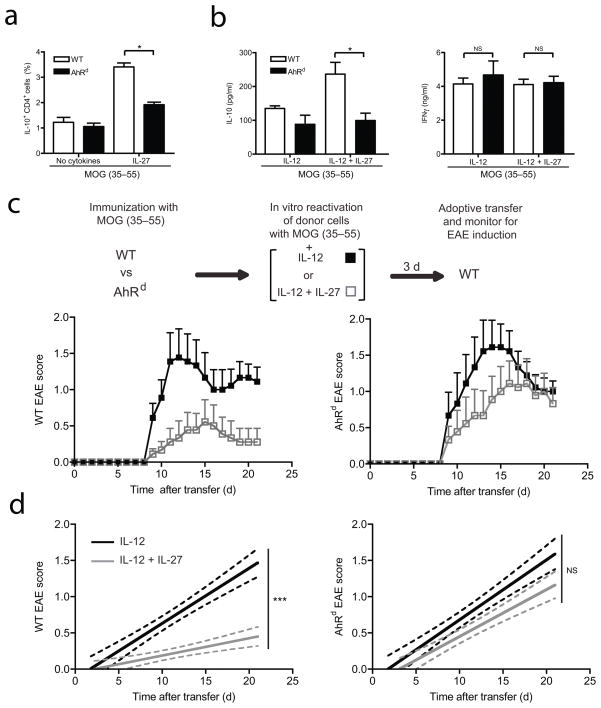
To address the in vivo relevance of AhR in inducing IL-27-driven TR1 cells and their effect on regulating autoimmunity and tissue inflammation, we exploited an adoptive transfer model of EAE in which IL-10, induced by IL-27, regulates EAE induced by adoptive T cell transfer7. We therefore used this model system to investigate the role of the AhR in the in vivo suppressive activity of IL-27-induced IL-10 production. This experimental model allowed us to exclude the effects of AhR on non-TR1 T cells, since IL-27, if given in vivo, can also inhibit TH17 and Treg cell differentiation5, 31. We first immunized wild type or AhRd mice with the myelin oligodendrocyte peptide 35–55 (MOG(35–55)) emulsified in Complete Freund’s Adjuvant (CFA) and tested the secretion of IL-10 by CD4+ T cells reactivated with MOG(35–55) and IL-27. We observed a significant decrease in the production of IL-10 by CD4+ T cells from AhRd mice treated with IL-27 (Fig. 6a).
Figure 6. AhR controls the IL-27-mediated inhibition of EAE.
MOG-specific cells from spleen and lymph nodes from wild type (WT) or AhRd mutant mice were obtained 11 days after immunization and a) the percentage of IL-10-producing CD4+ T cells was assessed by flow cytometry after five days of culture with MOG in the presence or absence of IL-27. b) Cells were restimulated in vitro with MOG in the presence of IL-12 either with or without IL-27 and IL-10 and IFN-γ secretion was analyzed 3 days later by cytokine bead array analysis. (*p<0.05) c) and d) WT or AhRd MOG specific cells prepared as in b) were adoptively transferred into WT mice and recipient animals were monitored for the development of EAE. c) The mean clinical disease score in each group is shown for WT or AhRd donor cells. d) Linear regression curves of the disease for each group are shown for the experiments depicted in c). The disease course differs significantly between the two treatments (IL-12 versus IL-12 plus IL-27) of WT donor cells but not of AhRd donor cells. The 95% confidence intervals for each curve are represented with dashed lines. (***p<0.0001).
In line with previous findings7, wild type CD4+ T cells reactivated solely with MOG(35–55) were poor inducers of EAE (not shown). However, reactivation of T cells in the presence of IL-12 before adoptive transfer generated MOG(35–55)-specific donor cells that induce EAE with high incidence7. Based on these observations, we compared the secretion of IL-10 by donor cells from MOG(35–55)-immunized wild type or AhRd mice, and restimulated in vitro with IL-12 with or without IL-27. We found that IL-27 enhanced IL-10 production in wild type but not in AhRd T cells (Fig. 6b). There was no effect of IL-27 on the production of interferon-γ (IFN-γ) triggered by IL-12 (Fig. 6b), and we failed to detect significant levels of IL-17 upon stimulation of donor cells with IL-12 and IL-27 (not shown). We then adoptively transferred these cells to induce EAE and found that wild type effector cells reactivated in the presence of IL-12 induced disease in the majority of the recipient mice. The incidence of EAE was significantly reduced when the donor cells were reactivated with IL-12 and IL-27 in vitro (Fig. 6c, d and Table 1). AhRd donor T cells activated in the presence of IL-12 also induced EAE upon adoptive transfer. However, the activation of AhRd T cells in the presence of IL-27 resulted in a significantly increased incidence of disease as compared with wild type T cells similarly treated (Fig. 6c, d and Table 1) suggesting that the AhR is essential for the IL-27-mediated inhibition of EAE in vivo. These data show that IL-27 controls the adoptive induction of EAE with wild type, but not AhRd T cells, and that this disease inhibition by IL-27 correlates with the AhR-dependent induction of IL-10 production by IL-27.
Table 1.
EAE incidence after cell transfer
| Group | Disease Incidence | Maximum score | Mean day of onset |
|---|---|---|---|
| WT IL-12 | 8/9 (89%) | 2.l ± 0.2 | 11.5 ± 1.5 |
| WT IL-12 + IL-27 | 3/9 (33%)* | 1.8 ± 0.5 | 10.3 ± l.3 |
| AhRd IL-12 | 8/9 (89%) | 2.4 ± 0.2 | 11.0 ± 0.9 |
| AhRd IL-12 + IL-27 | 8/9 (89%) | 2.0 ± 0.2 | 12.8 ± 1.4 |
P<0.05 (Fisher’s exact test)
Discussion
IL-27 has recently been shown to promote the differentiation of TR1 cells that are instrumental in controlling autoimmunity and tissue inflammation5. In this paper, we report that AhR, like the proto-oncogene c-Maf, is strongly induced during TR1 cell differentiation and that its expression in TR1 cells is as high as that observed in TH17 cells. Besides IL-10, activation of AhR by a putative endogenous ligand FICZ also increases IL-21 production in TR1 cells, which supports their development. Furthermore, the two transcription factors (AhR and c-Maf) associate with each other to transactivate the Il10 and Il21 promoters. The relevance of these findings is underscored by the ability of AhR signaling to control IL-27-driven IL-10 producing T cells in vivo.
TR1 cells are an important regulatory T cell type, which predominantly produce IL-10 and do not express Foxp3 but suppress tissue inflammation, GVH and autoimmunity in an IL-10-dependent manner. Although IL-10 was initially described to be a differentiation factor for TR1 cells, IL-27 additionally generates IL-10-producing TR1 cells5, 8. IL-27 induces both IL-10 and IFN-γ in T cells. These IL-10-IFN-γ double producing T cells have previously been reported to be generated in vivo following treatment with altered peptides ligands32 and regulate autoimmune tissue inflammation. Whether IL-10 and IFN-γ both contribute to the immunosuppressive function is not clear at this stage, but initial data suggest that IFN-γ produced by TR1 cells is a potent inhibitor of TH17 cells. This supports the view that both IFN-γ and IL-10 might be contributing to the immunoregulatory properties of TR1 cells.
While the ability of c-Maf to transactivate the Il10 promoter has already been demonstrated17, previous findings showed that the ability of c-Maf to transactivate Il10 in hepatocytes stimulated by fatty acids needed additional cofactors that were critical for inducing Il10 gene expression33. In addition, c-Maf and AhR have been suggested to cooperate to induce the transcription of β7-integrins34. Here we show that IL-27 induces AhR, which associates with c-Maf for the generation of IL-10-producing TR1 cells. Our results reveal that AhR and c-Maf have the ability to bind to proximal regions in both the Il10 (XRE-1 and MARE-3) and Il21 (XRE-2 and MARE-5; XRE-3 and MARE-6) promoters. This, together with our observation that AhR and c-Maf bind to each other to transactivate the Il10 and Il21 promoters, support a critical role for these two transcription factors in the development of TR1 cells. In addition, our ChIP results show that, while AhR binds to the Il10 and Il21 promoters in both TR1 and TH0 cells, c-Maf associates with Il10 and Il21 promoters only in TR1 cells, suggesting that c-Maf is controlling the tissue specificity of Il10 and Il21 gene transcription.
IL-21 acts as a growth factor for both TR1 and TH17 cells. Interestingly, IL-21 was reported to support IL-17 secretion from TH17 cells through a self-amplifying loop35. Our results similarly suggest that during TR1 cell differentiation, AhR and c-Maf participate in the self-amplifying feed-forward loop driven by IL-21 signaling which is essential for amplification and maintenance of the phenotype of differentiated TR1 cells. However, the actual mechanism by which IL-21 induces and/or maintains AhR and c- Maf expression remains to be determined. IL-21 could mediate this effect by increasing the frequency of IL-10-producing TR1 cells and also strengthen the expression of both c-Maf and AhR in a cell intrinsic manner. Overall, we propose that during the differentiation of TR1 cells with IL-27, AhR is essential for supporting c-Maf in its ability to transactivate the Il10 and Il21 promoters and thus enhances the differentiation of TR1 cells.
In addition to IL-10, AhR is essential for IL-22 production18. IL-22, a TH17 specific cytokine, promotes acanthosis in psoriasis but also protects mice from dextran sulfate-mediated colitis and concanavalin A induced liver damage36. Interestingly, IL-22 is a member of the IL-10-related cytokine family36 and might be similarly regulated. Therefore, our results raise the possibility that the c-Maf and AhR interaction might not only control IL-10 but also IL-22 production. Indeed, motif analysis shows that the Il22 promoter, like the Il10 promoter, contains c-Maf and AhR binding sites in close proximity, suggesting that c-Maf and AhR might also cooperate to induce Il22 gene transcription.
Environmentally ubiquitous polycyclic aromatic and planar halogenated hydrocarbons (PAH and HAH respectively), for which AhR is a cellular receptor, represent two major classes of environmental pollutants to which humans are regularly exposed. Endogenous ligands, although not yet completely classified37, represent an additional category of AhR activators. Notably, exposure to these chemicals can result in contrasting AhR-dependent effects on the immune response. For example, TCDD-driven AhR activation enhances inflammation in rheumatoid arthritis38 and endogenous ligand-driven AhR activation induces production of inflammatory cytokines by TH17 cells18, 19. On the other hand, prototypic PAH and HAH can impair B and T cell proliferative responses, alter antibody isotype switching, block plasma cell differentiation, compromise antibody production, induce apoptosis in developing lymphocytes, inhibit NK activity, modulate cytokine production, decrease cytotoxic T lymphocyte activity, and promote tumor growth39–46. In this vein, our report, together with the study of Gandhi et al. (cosubmitted manuscript), provides evidence suggesting that the interaction of AhR with c-Maf is essential for the generation of mouse and human regulatory IL-10-secreting TR1 cells that suppress inflammatory responses. These contrasting outcomes suggest that the in vivo immunologic effects of AhR activation are tissue- and/or ligand-specific. In the context of autoimmunity, outcomes likely depend on the type of T cell differentiation pathway activated by a given AhR ligand. Since AhR and c-Maf are expressed in TH1717, Treg, and TR19 cells, it is unlikely that AhR alone or in combination with c-Maf acts as a specific “lineage specification” transcription factor for TR1 cells. We would rather postulate that AhR, in combination with c-Maf, controls parts of the TR1 cell transcriptional and differentiation program. Thus, in response to different environmental ligands, AhR can differentially induce opposing T cell subsets resulting in either tissue inflammation or immunosuppression. Therefore, while the molecular basis for the difference in differentiation pathways favored by an AhR ligand(s) is not clear at this stage, one can nevertheless predict that AhR ligands direct the nature of downstream signaling and thus provide specificity and dictate the T cell subset dominance (TR1 vs. TH17) during an immune response.
In summary, we have demonstrated that the AhR, together with c-Maf, regulates the generation of TR1 cells induced by IL-27. Besides unraveling the molecular mechanisms accounting for the generation of TR1 cells, these findings, together with other studies18, 19, suggest that the AhR is not only a receptor for environmental pollutants but an important target for regulating T cell differentiation and the quality of immune responses in vivo.
Online methods
Animals and induction of EAE
IL-10–eGFP reporter mice (Vert-X)21, c-Maf transgenic47 and AhR deficient48 mice have been described. C57BL/6 wild type and AhRd mice were purchased from the Jackson Laboratories.
Adoptive transfer of EAE was performed as described previously7. AhRd or wild type mice were immunized with 100 μg o f MOG (35–55) peptide (MEVGWYRSPFSRVVHLYRNGK) and 500 μg of M. tuberculosis extract H37 Ra (Difco). Draining lymph nodes and spleens were collected 11 days after immunization and cultured for 3 days with MOG(35–55) peptide (20 μg/ml) and carrier-free recombinant IL-12 (10 ng/ml; R&D Systems) with or without carrier-free recombinant mouse IL-27 (25 ng/ml; R&D Systems). 15 × 106 cells were subsequently transferred i.v. into naïve wild-type mice, which were injected i.p. with 200 ng of pertussis toxin on days 0 and 2. All experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee (IACUC) at Harvard Medical School.
In vitro T cell differentiation
Naïve CD4+ T cells (CD4+CD44loCD62LhiCD25−) from C57BL/6 wild type, c-Maf transgenic, Ahr−/− or Il21r−/− mice were activated with plate-bound antibodies against CD3 (145-2C11, 2μg/ml) and CD28 (PV-1, 2 μg/ml). Mouse IL-27 (25ng/ml) and TGF-β (2 ng/ml), were all purchased from R&D Systems. TCDD (Sigma Aldrich) and FICZ (Enzo Life Sciences) were added at the start of the cultures at a final concentration of 100nM. Similarly, the AhR antagonist CH-223191 (Calbiochem) was used at a final concentration of 3μM.
siRNA
AhR- or c-Maf-specific Accell siRNAs were used according to the manufacturer’s instructions (Dharmacon Inc., Lafayette, CO, USA). Naïve CD4+ T cells were differentiated into TR1 cells with anti-CD3, anti-CD28, TGF-β (3 ng/ml) and IL-27 (30 ng/ml) using T cell differentiation medium containing 3 % FBS in the presence of 1 μM siRNA.
Measurement of cytokines
Secreted cytokines were measured after 48 hours by cytometric bead array (BD Biosciences) or ELISA. For intracellular cytokine staining, cells were stimulated for 4 hours at 37 C with PMA (50 ng/ml; Sigma), ionomycin (1 μg/ml; Sigma) and monensin (GolgiStop; 1 μg/ml; BD Biosciences). After staining for surface markers, cells were fixed and permeabilized according to the manufacturer’s instructions (BD Biosciences). All antibodies to cytokines were purchased from Biolegend.
Quantitative RT-PCR
RNA was extracted with RNAeasy minikits (Qiagen) and was analyzed by real-time PCR (RT-PCR) using the GeneAmp 7500 Sequence Detection System (Applied Biosystems). Expression was normalized to the expression of β-actin. Primers-probe mixtures were purchased from Applied Biosystems: IL-10 (Mm00439615-g1); c-Maf (Mm02581355-S1); IL-21 (Mm00517640-m1); AhR (Mm00478930-ml); T-bet (Mm00450960-m1); cyp1a1 (Mm00487217-m1); β-actin (Mm00446968-m1).
Chromatin immunoprecipitation (ChIP)
Cells were differentiated for 5 days into TR1 cells with TGF-β and IL-27, fixed with 1% formaldehyde for 15 min and quenched with 0.125 M glycine. Chromatin was isolated and sheared to an average length of 300–500 bp by sonication. Genomic DNA (Input) was prepared by treating aliquots of chromatin with RNase, proteinase K and heated for de-crosslinking, followed by ethanol precipitation. AhR-bound DNA sequences were immuno-precipitated with an AhR-specific antibody (Biomol SA-210), c-Maf bound sequences were immunoprecipitated using a c-Maf specific antibody (Santa Cruz sc-7866). Crosslinks were reversed by incubation overnight at 65 C, and ChIP DNA was purified by phenol-chloroform extraction and ethanol precipitation. Quantitative PCR (Q-PCR) reactions were carried out in triplicate and experimental Ct values were converted to copy numbers detected by comparison with a DNA standard curve run on the same PCR plates. Copy number values were normalized for primer efficiency using the values obtained with input DNA and the same primer pairs. Error bars represent standard deviations calculated from triplicate determinations.
Electrophoretic mobility shift assay (EMSA)
AhR was in vitro-translated using a TNT-coupled reticulocyte lysate kit (Promega Corporation). To make the probes, the complementary oligonucleotides, containing the AhR binding site of either mouse il10 or il21 promoter were annealed and radiolabeled using [γ-32p]dATP. The oligonucleotides used for making probes are as follows: XRE-1 (5′-ATGACCTGGGAGTGCGTGAATGGAATCCACA-3′ a n d 5′-TGTGGATTCCATTCACGCACTCCCAGGTCAT-3′), X R E-2 (5′-TCTTCACGGAGAGCACGCTGTCTACTTAGT-3′ a n d 5′-ACTAAGTAGACAGCGTGCTCTCCGTGAAGA-3′), X R E-3 (5′-ATCCCTGCCCCCACACGCACACGTACACCT-3′ a n d 5′-AGGTGTACGTGTGCGTGTGGGGGCAGGGAT-3′). In vitro-translated AhR and purified Arnt proteins (OriGene Technologies Inc.) were mixed together and incubated for 60 min at 25°C in transformation buffer (25 mM HEPES (pH 7.5), 1mM EDTA, 10mM Sodium molybdate, 10% glycerol). The transformed proteins were then incubated with radiolabeled DNA probe for 15 min at 25°C in binding buffer (25mM HEPES, 200 mM KCl, 10mM DTT, 5 mM EDTA, 20% glycerol, 75 μg/mL CHAPS, and 25 ng/μL polydI:dC). DNA–protein complexes were fractionated in a 6% nondenaturing polyacrylamide gel. For identification of binding specificity, proteins were preincubated with unlabeled annealed competitor oligo (XRE; 5′-GATCTGGCTCTTCTCACGCAACTCCGGATC-3′ and 5′-GATCCGGAGTTGCGTGAGAAGAGCCAGATC-3′).
Immunoprecipitation and immunoblotting
Purified naïve T cells were differentiated into TH0 cells or TR1 cells for 6 days and lyzed with a lysis buffer [1% Nonidet NP40, 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mM Na2VO4, 0.5 mM DTT and protease inhibitor]. AhR was immunoprecipitated with anti-AhR antibody (Biomol SA-210) and then subjected to SDS-PAGE. The immunocomplexes were analyzed with Western Blotting by using an anti c-Maf antibody (Santa Cruz sc-7866).
Reporter assays
107 EL-4 cells were electroporated as described49, activated in the presence of TGFβ1 (3 ng/ml) and analyzed after 24 h with the dual luciferase assay kit (New England Biolabs).
Retroviral infection
Naïve CD62LhiCD25−CD44loCD4+ T cells were transduced with retroviruses as described50. MSCV GFP-RV retroviral DNA plasmids were transfected into the Phoenix packaging cell line and 72 h later the retrovirus-containing supernatants were collected. MACS-purified CD4+ T cells were activated 24 h with plate-bound antibodies to CD3 and CD28, and infected by centrifugation (45 min at 2000 rpm) with retrovirus-containing supernatant supplemented with 8 μg/ml Polybrene (Sigma-Aldrich) and recombinant human IL-2 (25 units/ml).
In vivo treatment with anti-CD3
AhRd, Il27ra−/− and control littermates were treated with 20 μg of antibodies to CD3 (clone 2C11) or isotype control, administered i.p. every 3 days for a total of 3 times. Mice were sacrificed 4 hours after the last treatment, single cell suspensions were prepared from mesenteric lymph nodes and spleens and IL-10 expression was analyzed by intracellular staining.
Statistical analysis
Statistical analysis was performed using Prism software (Graph Pad software, La Jolla, CA, USA). P values<0.05 were considered significant.
Supplementary Material
Acknowledgments
We would like to thank Dr. Shi-Chuen Miaw and Dr. Jiangnan Xu for providing the IL-21 and IL-10 reporter constructs respectively, Dr. I-Cheng Ho for providing c-Maf transgenic mice, Dr. Dan Littman for the AhR-GFP overexpressing vector and Prof. Christopher Karp for IL-10-eGFP reporter mice. We thank Deneen Kozoriz for cell sorting. This work was supported by grants R37NS030843, P01NS038037, P01AI056299 and P01AI039671 from the National Institutes of Health (NIH) to VKK, AI435801 and NS38037 from the NIH to HLW, grants 1K99AI075285 from the NIH and RG4111A1 from the National Multiple Sclerosis Society to FJQ, and PO1-ES11624 to DHS. FJQ is a recipient of the Harvard Medical School Office for Diversity and Community Partnership (DCP) Faculty Fellowship. L.A. is supported by the European Molecular Biology Organization (EMBO), C.P. by the Swiss National Science Foundation (FSBMB) and the Novartis Foundation, N.J. by the Swiss National Science Foundation.
Abbreviations
- AhR
Aryl Hydrocarbon Receptor
- c-Maf
avian musculoaponeurotic fibrosarcoma v-maf
- TR1 cell
regulatory type 1 cell
- EAE
experimental autoimmune encephalomyelitis
Footnotes
Authors’ contribution
L.A, F.J.Q. and C.P. performed in vitro and in vivo experiments and wrote the manuscript, N.J. performed in vivo experiments, S.X., D.K. and E.B. performed in vitro experiments, D.S. provided essential reagents and hints to perform the study, H.L.W. and V.K.K. supervised the study and edited the manuscript.
References
- 1.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 2.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 3.Villarino A, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 4.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 5.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 6.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 8.Murugaiyan G, et al. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pot C, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J Interferon Cytokine Res. 30:381–388. doi: 10.1089/jir.2010.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wurster AL, et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 14.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, et al. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 19.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 20.Jones PB, Galeazzi DR, Fisher JM, Whitlock JP., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985;227:1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerkvliet NI, et al. Activation of aryl hydrocarbon receptor by TCDD prevents diabetes in NOD mice and increases Foxp3 T cells in pancreatic lymph nodes. Immunotherapy. 2009;1:539–547. doi: 10.2217/imt.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funatake CJ, Marshall NB, Steppan LB, Mourich DV, Kerkvliet NI. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J Immunol. 2005;175:4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 24.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 25.Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 26.Chiaro CR, Morales JL, Prabhu KS, Perdew GH. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry. 2008;47:8445–8455. doi: 10.1021/bi800712f. [DOI] [PubMed] [Google Scholar]
- 27.Kim DW, et al. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- 28.Klinge CM, Kaur K, Swanson HI. The aryl hydrocarbon receptor interacts with estrogen receptor alpha and orphan receptors COUP-TFI and ERRalpha1. Arch Biochem Biophys. 2000;373:163–174. doi: 10.1006/abbi.1999.1552. [DOI] [PubMed] [Google Scholar]
- 29.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Okey AB, Vella LM, Harper PA. Detection and characterization of a low affinity form of cytosolic Ah receptor in livers of mice nonresponsive to induction of cytochrome P1-450 by 3-methylcholanthrene. Molecular pharmacology. 1989;35:823–830. [PubMed] [Google Scholar]
- 31.Fitzgerald DC, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson LB, Greer JM, Sobel RA, Lees MB, Kuchroo VK. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 33.Morari J, et al. The role of proliferator-activated receptor gamma coactivator-1alpha in the fatty-acid-dependent transcriptional control of interleukin-10 in hepatic cells of rodents. Metabolism. 59:215–223. doi: 10.1016/j.metabol.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Monteiro P, et al. AhR- and c-maf-dependent induction of beta7-integrin expression in human macrophages in response to environmental polycyclic aromatic hydrocarbons. Biochem Biophys Res Commun. 2007;358:442–448. doi: 10.1016/j.bbrc.2007.04.111. [DOI] [PubMed] [Google Scholar]
- 35.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 32:17–31. doi: 10.1007/s00281-009-0188-x. [DOI] [PubMed] [Google Scholar]
- 37.Heath-Pagliuso S, et al. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37:11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi S, et al. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:1317–1322. doi: 10.1093/rheumatology/ken259. [DOI] [PubMed] [Google Scholar]
- 39.Kong LY, Luster MI, Dixon D, O’Grady J, Rosenthal GJ. Inhibition of lung immunity after intratracheal instillation of benzo(a)pyrene. Am J Respir Crit Care Med. 1994;150:1123–1129. doi: 10.1164/ajrccm.150.4.7921446. [DOI] [PubMed] [Google Scholar]
- 40.Allan L, Sherr D. Suppression of plasma cell differentiation by benzo[a]pyrene, an environmental pollutant. Environ Health J. 2010;9:15. doi: 10.1186/1476-069X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camacho IA, Nagarkatti M, Nagarkatti PS. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces Fas-dependent activation-induced cell death in superantigen-primed T cells. Arch Toxicol. 2002;76:570–580. doi: 10.1007/s00204-002-0390-2. [DOI] [PubMed] [Google Scholar]
- 42.Head JL, Lawrence BP. The aryl hydrocarbon receptor is a modulator of anti-viral immunity. Biochemical pharmacology. 2009;77:642–653. doi: 10.1016/j.bcp.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu HY, et al. Environmental chemical-induced bone marrow B cell apoptosis: death receptor-independent activation of a caspase-3 to caspase-8 pathway. Molecular pharmacology. 2005;68:1087–1096. doi: 10.1124/mol.105.014712. [DOI] [PubMed] [Google Scholar]
- 45.Schneider D, Manzan MA, Yoo BS, Crawford RB, Kaminski N. Involvement of Blimp-1 and AP-1 dysregulation in the 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated suppression of the IgM response by B cells. Toxicol Sci. 2009;108:377–388. doi: 10.1093/toxsci/kfp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh NP, Nagarkatti M, Nagarkatti P. Primary peripheral T cells become susceptible to 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated apoptosis in vitro upon activation and in the presence of dendritic cells. Molecular pharmacology. 2008;73:1722–1735. doi: 10.1124/mol.107.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho IC, Lo D, Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med. 1998;188:1859–1866. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 50.Quintana FJ, et al. Adaptive autoimmunity and Foxp3-based immunoregulation in zebrafish. PLoS One. 2010;5:e9478. doi: 10.1371/journal.pone.0009478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.