Abstract
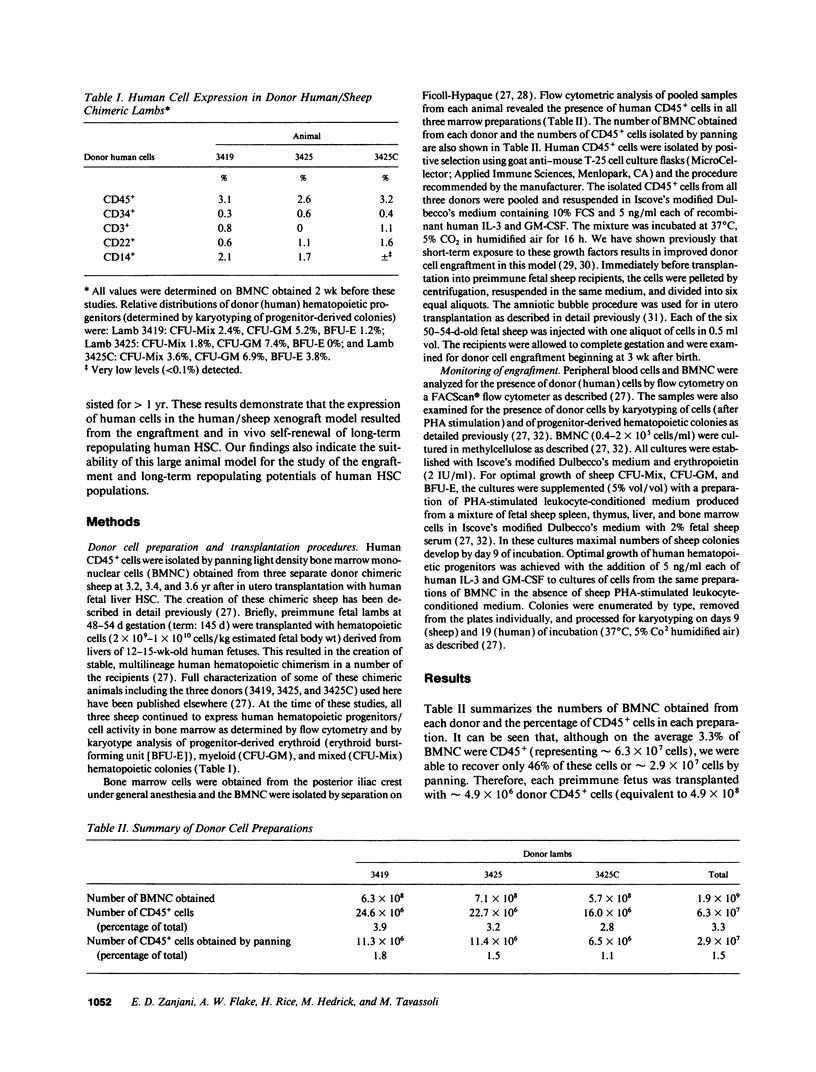
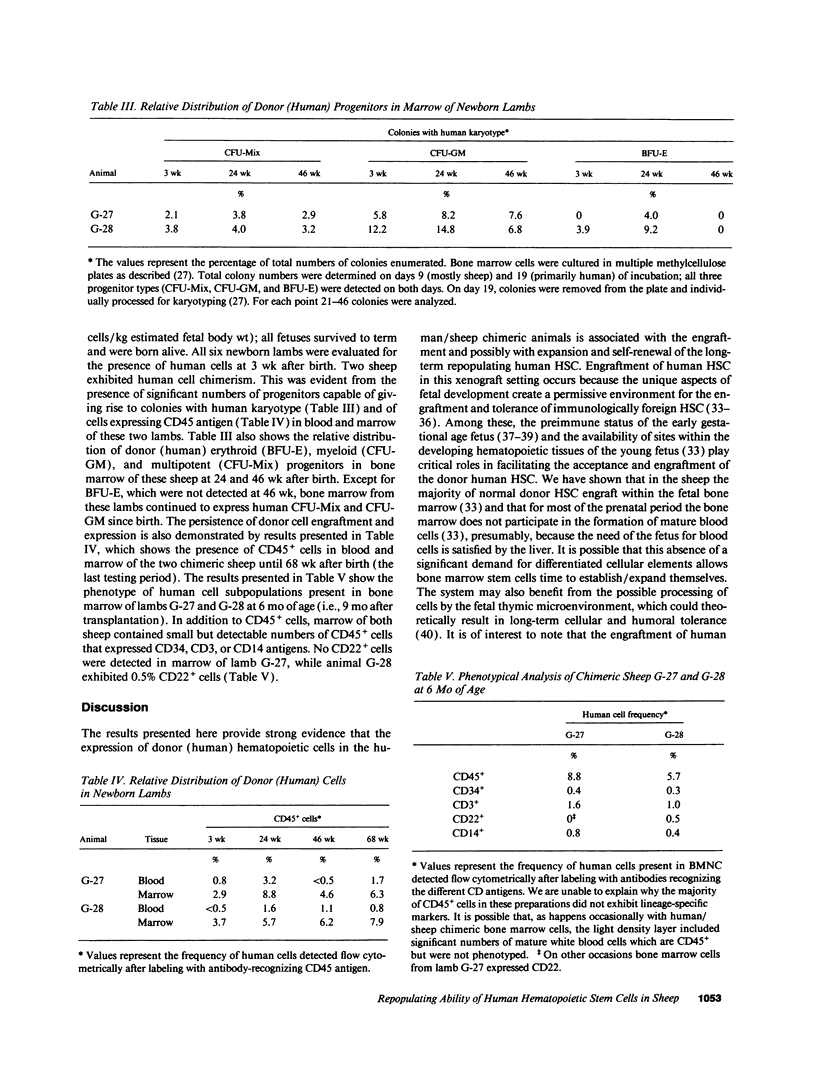
We previously reported on the successful engraftment and long-term multilineage expression (erythroid, myeloid, lymphoid) of human fetal liver hematopoietic stem cells in sheep after transplantation in utero. That the engraftment of long-term repopulating pluripotent stem cells occurred in these animals was shown here by the fact that transplantation of human CD45+ cells isolated from bone marrow of these chimeric animals into preimmune fetal sheep resulted in engraftment and expression of human cells. Marrow cells were obtained from three chimeric sheep at 3.2-3.6 yr after transplant. The relative percentage of human CD45+ cells present in these marrows was 3.3 +/- 0.32%. A total of 29 x 10(6) CD45+ cells were isolated by panning, pooled, and transplanted into six preimmune sheep fetuses (4.8 x 10(6) cells/fetus). All six recipients were born alive. Hematopoietic progenitors exhibiting human karyotype were detected in marrows of two lambs soon after birth. Cells expressing human CD45 antigen were also detected in blood and marrow of both lambs. Human cell expression has been multilineage and has persisted for > 1 yr. These results demonstrate that the expression of human cells in this large animal model resulted from engraftment of long-term repopulating pluripotent human stem cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Barnes R. D., Pottinger B. E., Marston J., Flecknell P., Ward R. H., Kalter S., Heberling R. L. Immunological tolerance induced by in utero injection. J Med Genet. 1983 Feb;20(1):41–45. doi: 10.1136/jmg.20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry T. S., Jones D. M., Richter C. B., Haynes B. F. Successful engraftment of human postnatal thymus in severe combined immune deficient (SCID) mice: differential engraftment of thymic components with irradiation versus anti-asialo GM-1 immunosuppressive regimens. J Exp Med. 1991 Jan 1;173(1):167–180. doi: 10.1084/jem.173.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncello I., Bradley T. R., Hodgson G. S., Dunlop J. M. The resolution, enrichment, and organization of normal bone marrow high proliferative potential colony-forming cell subsets on the basis of rhodamine-123 fluorescence. Exp Hematol. 1991 Mar;19(3):174–178. [PubMed] [Google Scholar]
- Binns R. M. Bone marrow and lymphoid cell injection of the pig foetus resulting in transplantation tolerance or immunity, and immunoglobulin production. Nature. 1967 Apr 8;214(5084):179–180. doi: 10.1038/214179a0. [DOI] [PubMed] [Google Scholar]
- Boggs D. R., Boggs S. S., Saxe D. F., Gress L. A., Canfield D. R. Hematopoietic stem cells with high proliferative potential. Assay of their concentration in marrow by the frequency and duration of cure of W/Wv mice. J Clin Invest. 1982 Aug;70(2):242–253. doi: 10.1172/JCI110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher G., Neben S., Yee M., Bullis J., Cronkite E. P. Pluripotent stem cells with normal or reduced self renewal survive lethal irradiation. Exp Hematol. 1988 Aug;16(7):627–630. [PubMed] [Google Scholar]
- Civin C. I., Banquerigo M. L., Strauss L. C., Loken M. R. Antigenic analysis of hematopoiesis. VI. Flow cytometric characterization of My-10-positive progenitor cells in normal human bone marrow. Exp Hematol. 1987 Jan;15(1):10–17. [PubMed] [Google Scholar]
- Dick J. E. Establishment of assays for human hematopoietic cells in immune deficient mice. Curr Top Microbiol Immunol. 1989;152:219–224. doi: 10.1007/978-3-642-74974-2_26. [DOI] [PubMed] [Google Scholar]
- Flake A. W., Harrison M. R., Adzick N. S., Zanjani E. D. Transplantation of fetal hematopoietic stem cells in utero: the creation of hematopoietic chimeras. Science. 1986 Aug 15;233(4765):776–778. doi: 10.1126/science.2874611. [DOI] [PubMed] [Google Scholar]
- Huang S., Terstappen L. W. Formation of haematopoietic microenvironment and haematopoietic stem cells from single human bone marrow stem cells. Nature. 1992 Dec 24;360(6406):745–749. doi: 10.1038/360745a0. [DOI] [PubMed] [Google Scholar]
- Jotereau F., Heuze F., Salomon-Vie V., Gascan H. Cell kinetics in the fetal mouse thymus: precursor cell input, proliferation, and emigration. J Immunol. 1987 Feb 15;138(4):1026–1030. [PubMed] [Google Scholar]
- Kamel-Reid S., Dick J. E. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988 Dec 23;242(4886):1706–1709. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- Kyewski B. A. Seeding of thymic microenvironments defined by distinct thymocyte-stromal cell interactions is developmentally controlled. J Exp Med. 1987 Aug 1;166(2):520–538. doi: 10.1084/jem.166.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary A. G., Ogawa M. Blast cell colony assay for umbilical cord blood and adult bone marrow progenitors. Blood. 1987 Mar;69(3):953–956. [PubMed] [Google Scholar]
- Leary A. G., Ogawa M., Strauss L. C., Civin C. I. Single cell origin of multilineage colonies in culture. Evidence that differentiation of multipotent progenitors and restriction of proliferative potential of monopotent progenitors are stochastic processes. J Clin Invest. 1984 Dec;74(6):2193–2197. doi: 10.1172/JCI111645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Magli M. C., Iscove N. N., Odartchenko N. Transient nature of early haematopoietic spleen colonies. Nature. 1982 Feb 11;295(5849):527–529. doi: 10.1038/295527a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Lo D., Brinster R., Palmiter R., Burkly L., Flavell R. H., Kappler J. The effect of thymus environment on T cell development and tolerance. Cell. 1988 May 20;53(4):627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Verfaillie C., McGlave P. The generation of human natural killer cells from CD34+/DR- primitive progenitors in long-term bone marrow culture. Blood. 1992 Nov 1;80(9):2182–2187. [PubMed] [Google Scholar]
- Owen J. J., Jenkinson E. J. Embryology of the lymphoid system. Prog Allergy. 1981;29:1–34. [PubMed] [Google Scholar]
- Schofield R., Dexter T. M., Lord B. I., Testa N. G. Comparison of haemopoiesis in young and old mice. Mech Ageing Dev. 1986 Mar;34(1):1–12. doi: 10.1016/0047-6374(86)90100-4. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Srour E. F., Brandt J. E., Briddell R. A., Grigsby S., Leemhuis T., Hoffman R. Long-term generation and expansion of human primitive hematopoietic progenitor cells in vitro. Blood. 1993 Feb 1;81(3):661–669. [PubMed] [Google Scholar]
- Srour E. F., Brandt J. E., Briddell R. A., Leemhuis T., van Besien K., Hoffman R. Human CD34+ HLA-DR- bone marrow cells contain progenitor cells capable of self-renewal, multilineage differentiation, and long-term in vitro hematopoiesis. Blood Cells. 1991;17(2):287–295. [PubMed] [Google Scholar]
- Srour E. F., Hoffman R., Zanjani D. Animal models for human hematopoiesis. J Hematother. 1992 Summer;1(2):143–153. doi: 10.1089/scd.1.1992.1.143. [DOI] [PubMed] [Google Scholar]
- Srour E. F., Zanjani E. D., Brandt J. E., Leemhuis T., Briddell R. A., Heerema N. A., Hoffman R. Sustained human hematopoiesis in sheep transplanted in utero during early gestation with fractionated adult human bone marrow cells. Blood. 1992 Mar 15;79(6):1404–1412. [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Lansdorp P. M., Thacker J. D., Hogge D. E. Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood. 1991 Aug 1;78(3):666–672. [PubMed] [Google Scholar]
- Sutherland H. J., Hogge D. E., Cook D., Eaves C. J. Alternative mechanisms with and without steel factor support primitive human hematopoiesis. Blood. 1993 Mar 15;81(6):1465–1470. [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Tavassoli M., Konno M., Shiota Y., Omoto E., Minguell J. J., Zanjani E. D. Enhancement of the grafting efficiency of transplanted marrow cells by preincubation with interleukin-3 and granulocyte-macrophage colony-stimulating factor. Blood. 1991 Apr 1;77(7):1599–1606. [PubMed] [Google Scholar]
- Verfaillie C. M. Direct contact between human primitive hematopoietic progenitors and bone marrow stroma is not required for long-term in vitro hematopoiesis. Blood. 1992 Jun 1;79(11):2821–2826. [PubMed] [Google Scholar]
- Verfaillie C., Blakolmer K., McGlave P. Purified primitive human hematopoietic progenitor cells with long-term in vitro repopulating capacity adhere selectively to irradiated bone marrow stroma. J Exp Med. 1990 Aug 1;172(2):509–502. doi: 10.1084/jem.172.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanjani E. D., Ascensao J. L., Harrison M. R., Tavassoli M. Ex vivo incubation with growth factors enhances the engraftment of fetal hematopoietic cells transplanted in sheep fetuses. Blood. 1992 Jun 1;79(11):3045–3049. [PubMed] [Google Scholar]
- Zanjani E. D., Ascensao J. L., Tavassoli M. Liver-derived fetal hematopoietic stem cells selectively and preferentially home to the fetal bone marrow. Blood. 1993 Jan 15;81(2):399–404. [PubMed] [Google Scholar]
- Zanjani E. D., Mackintosh F. R., Harrison M. R. Hematopoietic chimerism in sheep and nonhuman primates by in utero transplantation of fetal hematopoietic stem cells. Blood Cells. 1991;17(2):349–366. [PubMed] [Google Scholar]
- Zanjani E. D., Pallavicini M. G., Ascensao J. L., Flake A. W., Langlois R. G., Reitsma M., MacKintosh F. R., Stutes D., Harrison M. R., Tavassoli M. Engraftment and long-term expression of human fetal hemopoietic stem cells in sheep following transplantation in utero. J Clin Invest. 1992 Apr;89(4):1178–1188. doi: 10.1172/JCI115701. [DOI] [PMC free article] [PubMed] [Google Scholar]



