Abstract
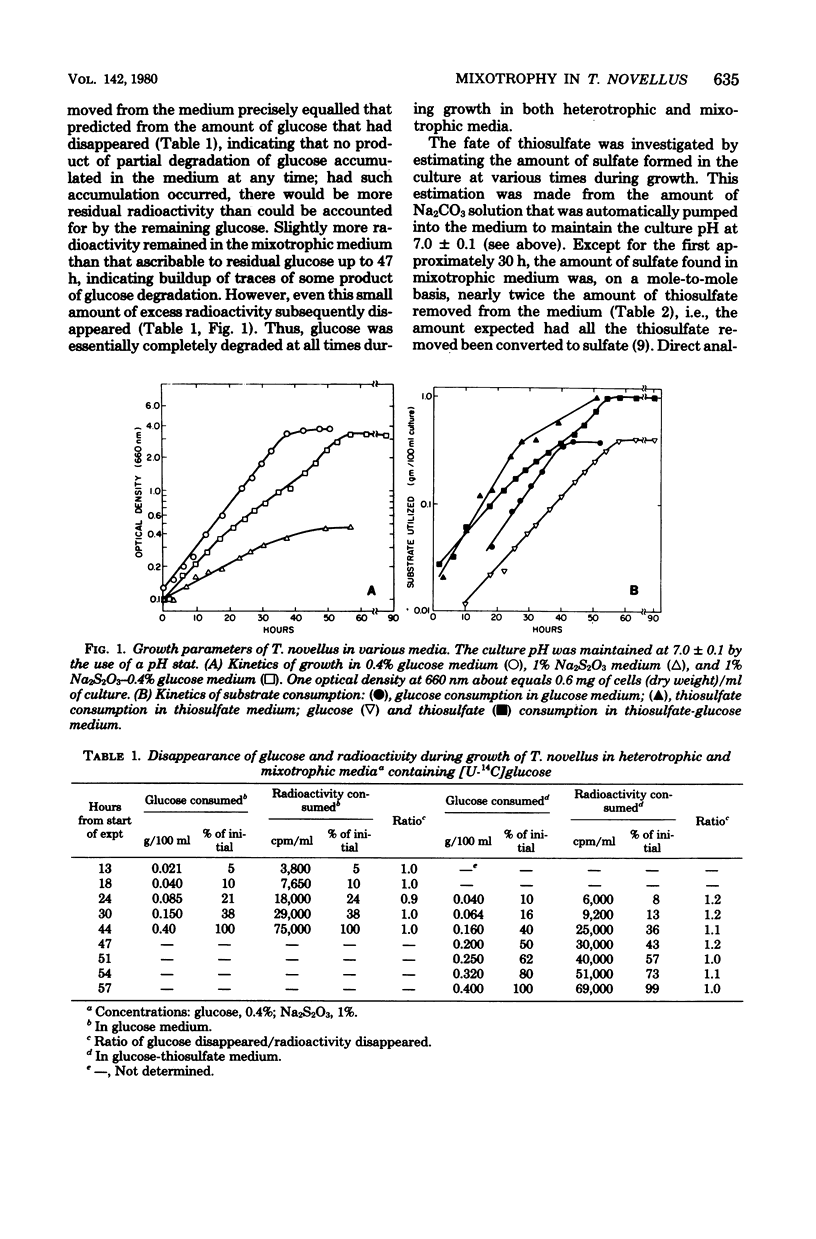
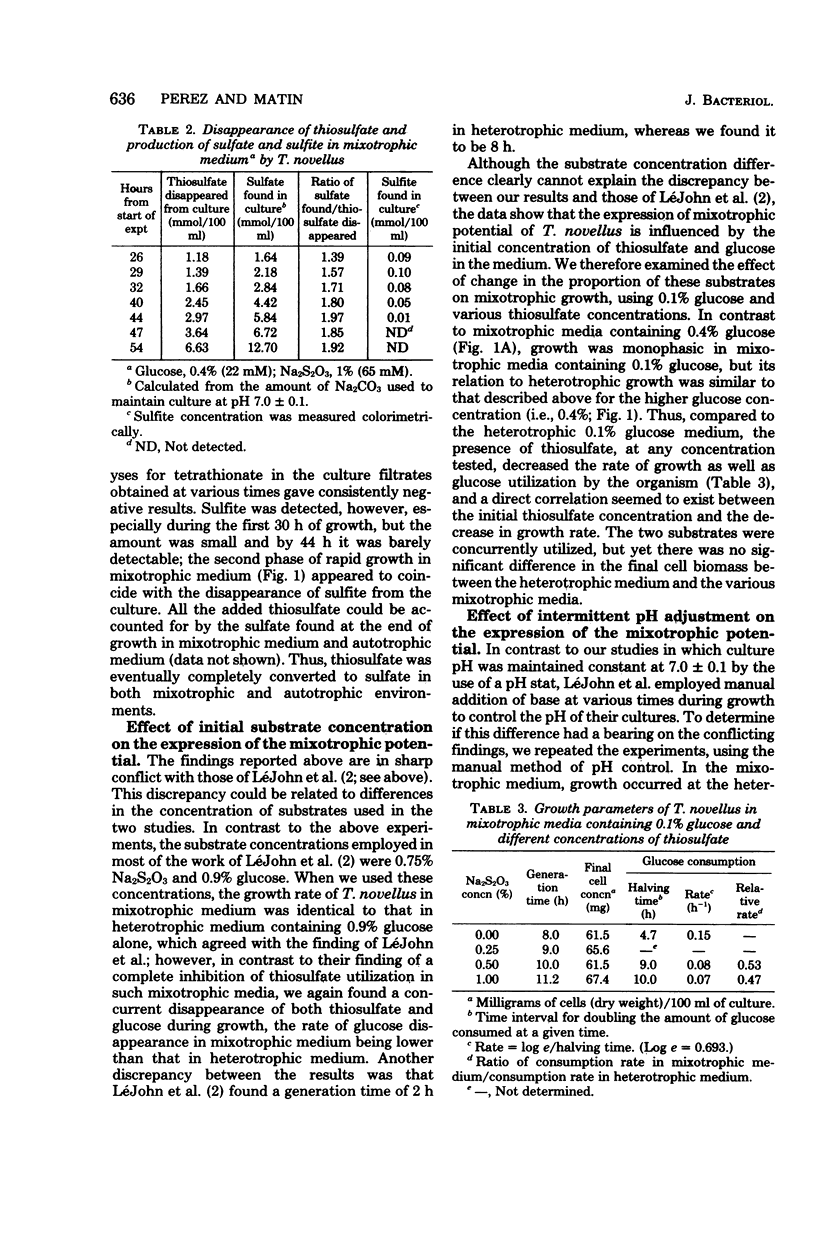
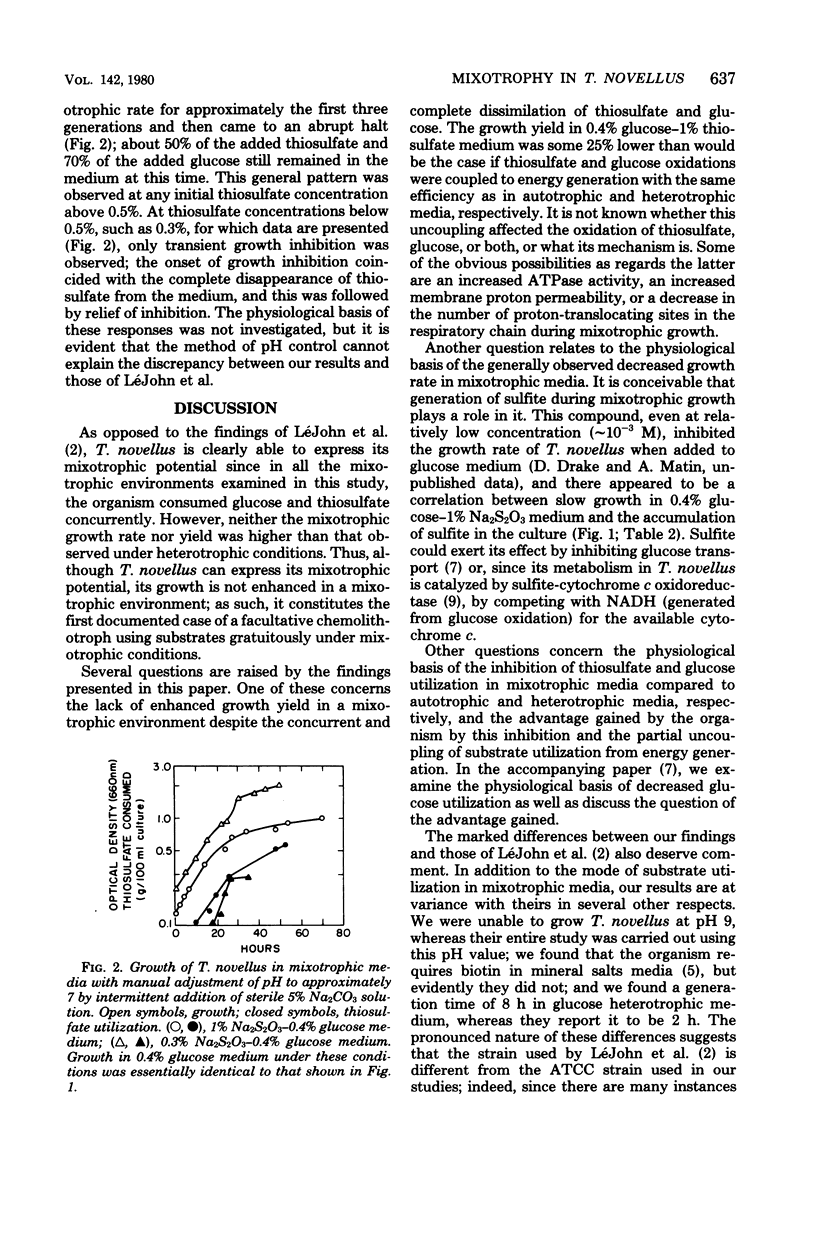
In a mixotrophic environment, Thiobacillus novellus concurrently utilized glucose and thiosulfate but showed no stimulation of growth rate or yield. In most mixotrophic environments examined, the growth rate was lower than the heterotrophic growth rate, the extent of the decrease depending on the concentration and relative proportion of thiosulfate and glucose in the medium. Both thiosulfate and glucose were degraded to their most oxidized products in mixotrophic medium, yet the biomass production in this medium was comparable to that found in heterotrophic medium containing glucose alone at the corresponding concentration. It was postulated that in mixotrophic medium the oxidation of thiosulfate, glucose, or partially that of both was uncoupled from energy generation. These results differ in many respects from those reported earlier by LeJohn et al. (J. Bacteriol. 94: 1484--1491, 1967); experiments designed to exactly duplicate some of the growth conditions employed by these workers did not resolve the discrepancy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leefeldt R. H., Matin A. Growth and physiology of Thiobacillus novellus under nutrient-limited mixotrophic conditions. J Bacteriol. 1980 May;142(2):645–650. doi: 10.1128/jb.142.2.645-650.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léjohn H. B., Van Caeseele L., Lees H. Catabolite repression in the facultative chemoautotroph Thiobacillus novellus. J Bacteriol. 1967 Nov;94(5):1484–1491. doi: 10.1128/jb.94.5.1484-1491.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Enzymes of carbohydrate metabolism in Thiobacillus species. J Bacteriol. 1971 Jul;107(1):179–186. doi: 10.1128/jb.107.1.179-186.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Schleiss M., Perez R. C. Regulation of glucose transport and metabolism in Thiobacillus novellus. J Bacteriol. 1980 May;142(2):639–644. doi: 10.1128/jb.142.2.639-644.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Veldkamp H. Physiological basis of the selective advantage of a Spirillum sp. in a carbon-limited environment. J Gen Microbiol. 1978 Apr;105(2):187–197. doi: 10.1099/00221287-105-2-187. [DOI] [PubMed] [Google Scholar]
- SANTER M., BOYER J., SANTER U. Thiobacillus novellus. I. Growth on organic and inorganic media. J Bacteriol. 1959 Aug;78:197–202. doi: 10.1128/jb.78.2.197-202.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H. Enzymic microassays for D-mannose, D-glucose, D-galactose, L-fucose, and D-glucosamine. Methods Enzymol. 1975;41:3–10. doi: 10.1016/s0076-6879(75)41003-5. [DOI] [PubMed] [Google Scholar]
- Taylor B. F., Hoare D. S. New facultative Thiobacillus and a reevaluation of the heterotrophic potential of Thiobacillus novellus. J Bacteriol. 1969 Oct;100(1):487–497. doi: 10.1128/jb.100.1.487-497.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]



