Abstract
Purpose
Sentinel lymph node resection (SNR) may reduce morbidity while providing the same clinical utility as conventional axillary dissection (AD). National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 is a randomized phase III trial comparing SNR immediately followed by AD (SNAD) to SNR and subsequent AD if SN is positive. We report the definitive patient-reported outcomes (PRO) comparisons.
Patients and Methods
Eligible patients had clinically node-negative, operable invasive breast cancer. The PRO substudy included all SN-negative participants enrolled May 2001 to February 2004 at community institutions in the United States (n = 749; 78% age ≥ 50; 87% clinical tumor size ≤ 2.0 cm; 84% lumpectomy; 87% white). They completed questionnaires presurgery, 1 and 2 to 3 weeks postoperatively, and every 6 months through year 3. Arm symptoms, arm use avoidance, activity limitations, and quality of life (QOL) were compared with intent-to-treat two-sample t-tests and repeated measures analyses.
Results
Arm symptoms were significantly more bothersome for SNAD compared with SNR patients at 6 months (mean, 4.8 v 3.0; P < .001) and at 12 months (3.6 v 2.5; P = .006). Longitudinally, SNAD patients were more likely to experience ipsilateral arm and breast symptoms, restricted work and social activity, and impaired QOL (P ≤ .002 all items). From 12 to 36 months, fewer than 15% of either SNAD or SNR patients reported moderate or greater severity of any given symptom or activity limitation.
Conclusion
Arm morbidity was greater with SNAD than with SNR. Despite considerable fears about complications from AD for breast cancer, this study demonstrates that initial problems with either surgery resolve over time.
INTRODUCTION
National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-32 is a randomized phase III trial, launched in 1999, designed to determine whether sentinel node resection (SNR) alone in patients with early-stage breast cancer provides reduction in morbidity, with the same prognostic information, regional control, and survival, as conventional axillary dissection (AD). Important secondary outcomes of B-32 were patient-reported outcome (PRO) measures of morbidity as well as observer-rated arm edema and function. The American Society for Clinical Oncology recommendations note that decreased morbidity is a motivation for avoiding AD.1
This report presents our definitive, planned comparison of treatment groups with respect to PROs. We hypothesized that PRO end points would indicate greater morbidity in the sentinel node resection followed by axillary dissection (SNAD) group. Our study differs from other published phase III studies (which were conducted in other countries) in several respects.2–5 We provide data in the early postoperative period (1 and 2 to 3 weeks after surgery) and the long term (36 months). We assessed symptoms in the breast (in addition to the arm), ability to perform specific tasks, daily activities, and overall quality of life (QOL). We also report results separately for patients who received lumpectomy or mastectomy. Our study was conducted in community settings, providing broad representation across the United States. Finally, our study is restricted to patients who were sentinel node negative, hence SNR group patients were not expected to receive a secondary AD.
PATIENTS AND METHODS
Participants
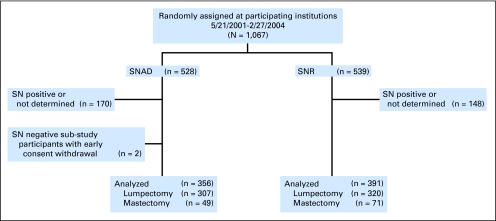
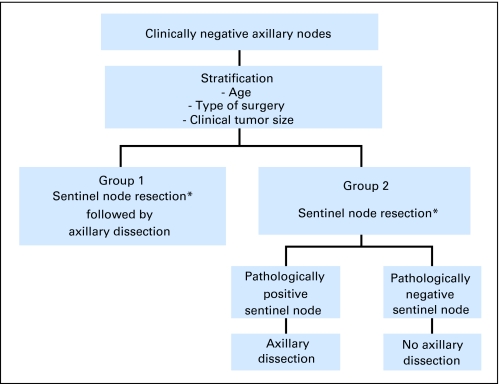
Eligible patients had clinically node-negative operable invasive breast cancer. Primary surgery (mastectomy or breast-conserving) and systemic therapy were given at the discretion of the treating physician. Patients were stratified by age (≤ 49 years, ≥ 50 years), clinical tumor size (eg, ≤ 2.0, 2.1 to 4.0, ≥ 4.1 cm), and surgical treatment plan (eg, mastectomy, lumpectomy), and randomly assigned to either SNR immediately followed by conventional AD (SNAD), or SNR alone (Fig 1). Patients found to be SN-positive intraoperatively or during pathology review, or for whom a SN could not be identified, subsequently underwent AD (Fig 2). The primary comparison groups for all analyses are SN-negative patients. The random assignment is preserved because the determination of SN status was made the same way in both treatment groups. More information about B-32, including the SN surgical training, has been reported elsewhere.6–9
Fig 1.
CONSORT diagram. SN, sentinel node; SNAD, SN resection followed by axillary dissection; SNR, sentinel node resection.
Fig 2.
National Surgical Adjuvant Breast and Bowel Project B-32 schema. (*) Patients in whom a sentinel lymph node is not identified will go on for axillary dissection.
The B-32 trial required a large sample size for the primary clinical outcomes. The PRO substudy required a much smaller sample size and thus accrual to the PRO substudy began 2 years after B-32 opened. By design, the substudy included all SN-negative patients randomly assigned at participating institutions designated as members of the Community Clinical Oncology Program, a National Cancer Institute program that encourages clinical trial participation by community-based physicians. The protocol and consent form were approved by the National Cancer Institute and the institutional review boards of all participating institutions. All participants provided written informed consent.
Instruments
The PRO assessments were primarily focused on arm-related morbidity. There was, at the time of study design, no generally accepted measure of QOL related to axillary node dissection. We adapted items from previous studies and from the validated Disabilities of Arm, Shoulder and Hand Scale,10–13 and used a validated single-item health-related QOL rating scale (0 to 10) adapted from previous NSABP studies.14–16 Twenty-six items (response scale 0 to 4) assessed how bothered patients were by the following symptoms: (1) tenderness, (2) swelling, (3) discomfort or pain, (4) numbness and “pins and needles,” (5) skin sensitivity, (6) tightness, pulling or stretching, and (7) weakness. Symptoms 1 to 6 were assessed for the right and left arms (where arms included underarms, arms, hands, and fingers) and the right and left breast and chest; weakness was assessed only for arms. It also included items (response scale 0 to 3) regarding avoidance of arm use and the difficulty of pushing large objects, lifting objects, and reaching. Social and occupational activity limitations were assessed with two items (response scale 0 to 4; see full questionnaire in Appendix Fig A1 [online only]). The questionnaire is not a psychometric instrument in that it does not use multiple items to measure the same attribute. Rather, it is a clinimetric instrument: most items measure distinct symptoms, and these are summed to form indexes.17,18 The questionnaire also collected the most recent surgical procedure (eg, biopsy, mastectomy, or breast reconstruction).
Questionnaires were given before surgery, 1 week postoperatively, 2 to 3 weeks postoperatively, and every 6 months through year 3. Questionnaires were completed in the physician's office when possible; otherwise by telephone or mail. Patients were expected to complete the questionnaires on schedule until they were diagnosed as sentinel node-positive, had a documented breast cancer recurrence or second primary cancer, died, or withdrew consent from the parent B-32 study. Institution staff completed a QOL missing data form when efforts to administer the questionnaire failed. The QOL missing data form provided reasons that the questionnaire was not done.
Statistical Methods
All analyses were intent-to-treat, grouping patients according to their random assignment, and were carried out separately on patients who received mastectomy versus lumpectomy. Questionnaire compliance (submission of expected forms) was compared between treatment groups with logistic mixed effects modeling.19 All available data were used for all analyses.
In patients who received lumpectomy, we compared the ipsilateral arm symptom subscale (change from baseline) between SNAD and SNR at 6 months and 1 year using two-sample t-tests. This subscale was formed by summing ipsilateral arm symptom scores (Cronbach's α .90). These two tests, along with six other comparisons between dominant and nondominant sides (not presented here), were defined in the protocol as primary analyses, and were to be one-sided at significance level 0.05/8 = 0.00625 (Bonferroni correction). However, two-sided P values are presented in this report per Journal recommendations.
Longitudinal analyses: for the QOL rating scale, we performed a repeated measures mixed effects regression to compare SNAD with SNR through 36 months of follow-up. For every other item (severity > 0 v 0), we performed a separate repeated-measures logistic mixed effects regression. Effects included time as a three level factor (baseline, postoperative = 1 to 3 weeks, long-term = 6 to 36 months), baseline severity, treatment group, systemic therapy (chemotherapy or hormone therapy, as a time-varying effect), a factor indicating whether the surgical procedure was on the dominant or nondominant side, the interaction between time and treatment group, and, for analyses involving mastectomy patients, breast reconstruction. Secondary analyses were performed at a two-sided significance level of .05.
We estimated that 663 lumpectomy patients and 156 mastectomy patients would be SN negative and enroll in the substudy, based on the number expected to enroll in the parent B-32 study. That was estimated to provide 87% power for the comparison of means of the ipsilateral arm symptom scale at 6 months, assuming a true mean difference of 2 points (standard deviation [SD], 7).
Analyses were carried out in SAS version 9.1.3 (SAS Institute, Cary, NC), including PROC GLIMMIX (SAS Institute) for the repeated measures analyses.
RESULTS
Participants and Assessments
The PRO study enrolled 749 participants from May 2001 to February 2004 (Fig 1). Two patients withdrew consent for B-32 early in the study, leaving 747 participants with follow-up (356 SNAD v 391 SNR). Eleven patients in the SNR group with negative nodes underwent elective AD. The mean number of nodes removed was 14.3 (SD, 5.8) in the SNAD group and 3.1 (SD, 2.9) in the SNR group. There were differences between PRO substudy participants and other B-32 participants, reflecting differences between the participating Community Clinical Oncology Programs and other institutions (Table 1). There were no significant differences between treatment groups. Among patients with PRO data at baseline and the 6-month time point, 550 had received only lumpectomy or excisional biopsy as the most extensive breast surgery, 116 had received mastectomy, and seven had received neither. Radiation therapy was administered to 91% of lumpectomy-treated patients and to 11% of mastectomy-treated patients. Among 116 patients who received mastectomy by the 6-month time point, 67 (29 SNAD, 38 SNR) had not received breast reconstruction. The most extensive breast surgery was on the dominant side for 370 patients and on the nondominant side for 379 patients.
Table 1.
National Surgical Adjuvant Breast and Bowel Project B-32 Patient Characteristics
| Characteristic | PRO Substudy Patients With Follow-Up Treatment |
All (N = 747) |
Other B-32 Patients With Follow-Up, Enrolled During Substudy Accrual Period (n = 2,000) |
P for Substudy v Parent Study Participant Characteristics* | |||||
|---|---|---|---|---|---|---|---|---|---|
| SNAD (n = 356) |
SNR (n = 391) |
||||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Age, years | .14 | ||||||||
| ≤ 49 | 73 | 20.5 | 90 | 23 | 163 | 21.8 | 490 | 24.5 | |
| 50+ | 283 | 79.5 | 301 | 77 | 584 | 78.2 | 1,510 | 75.5 | |
| Clinical tumor size, cm | .004 | ||||||||
| ≤ 2.0 | 308 | 86.5 | 343 | 87.7 | 651 | 87.1 | 1,645 | 82.3 | |
| 2.1-4.0 | 46 | 12.9 | 45 | 11.5 | 91 | 12.2 | 320 | 16.0 | |
| ≥ 4.1 | 2 | 0.6 | 3 | 0.8 | 5 | 0.7 | 35 | 1.8 | |
| Surgery plan | < .001 | ||||||||
| Lumpectomy | 307 | 86.2 | 320 | 81.8 | 627 | 83.9 | 1,778 | 88.9 | |
| Mastectomy | 49 | 13.8 | 71 | 18.2 | 120 | 16.1 | 222 | 11.1 | |
| Race | < .001 | ||||||||
| White | 314 | 88.2 | 338 | 86.4 | 652 | 87.3 | 1,827 | 91.4 | |
| Black | 30 | 8.4 | 40 | 10.2 | 70 | 9.4 | 62 | 3.1 | |
| Other | 12 | 3.4 | 13 | 3.3 | 25 | 3.3 | 111 | 5.6 | |
| Systemic adjuvant therapy† | .36 | ||||||||
| Yes | 308 | 87.8 | 336 | 87.1 | 644 | 87.4 | 1,666 | 86.0 | |
| No | 43 | 12.3 | 50 | 13.0 | 93 | 12.6 | 271 | 14.0 | |
| Radiation therapy | < .001 | ||||||||
| Yes | 276 | 77.5 | 309 | 79.0 | 585 | 78.3 | 1,683 | 84.2 | |
| No | 80 | 22.5 | 82 | 21.0 | 162 | 21.7 | 317 | 15.9 | |
Abbreviations: PRO, patient-related outcomes; SNAD, sentinel node resection followed by axillary dissection; SNR, sentinel node resection.
P value represents comparison of substudy participants with other B-32 participants enrolled during the substudy accrual period.
Use of systemic adjuvant therapy is unknown for some of the participants.
Submission of expected questionnaires ranged from 99% at baseline (742 of 749) and 92% at 6 months (676 of 738 forms expected) to 84% at 36 months (584 of 699 expected). In longitudinal analyses, the compliance rate differed significantly between treatment groups (P = .006). However, the differences were of small magnitude (91% SNAD v 92% SNR at 6 months; 90% SNAD v 93% SNR at 1 year). Furthermore, 46% of missed forms were attributed to staff oversight or understaffing, reasons unrelated to PROs. Compliance was reported in detail previously.19
Treatment Group Comparisons at 6 Months and 1 Year
Among patients who received lumpectomy, symptoms in the ipsilateral arm at 6 months were significantly greater with SNAD (mean change from baseline 3.6; SD, 5.7, on a 0-28 scale) than SNR (mean 1.7; SD, 4.3), with a mean treatment difference of 1.9 (P < .001). Differences were reduced by 1 year, with a mean change of 2.3 (SD, 5.0) with SNAD versus 1.2 (SD, 4.0) with SNR, but remained significant according to the protocol-defined Bonferroni-adjusted criterion (mean difference 1.1; P = .006). Other cross-sectional comparisons are presented in Appendix and in Table 2.
Table 2.
Symptoms, Arm Use Avoidance, and Social/Occupational Limitations at 6 and 12 Months in National Surgical Adjuvant Breast and Bowel Project B-32 Patient-Reported Outcomes Substudy
| Parameter | Response at Follow-up |
Change From Baseline |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline* |
6 Months† |
12 Months† |
Baseline to 6 Months |
Baseline to 12 Months |
||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Ipsilateral arm symptoms (scale 0-28) | ||||||||||
| SNAD | 1.9 | 1.3 to 2.5 | 4.8 | 4.1 to 5.4 | 3.6 | 3 to 4.2 | 3.6 | 2.9 to 4.3 | 2.3 | 1.6 to 2.9 |
| SNR | 2.1 | 1.3 to 2.8 | 3 | 2.5 to 3.5 | 2.5 | 2 to 3 | 1.7 | 1.2 to 2.2 | 1.2 | 0.7 to 1.7 |
| SNAD v SNR | −0.2 | −1.1 to 0.8 | 1.7 | 0.9 to 2.6 | 1.1 | 0.4 to 1.9 | 1.9 | 1 to 2.7 | 1.1 | 0.3 to 1.8 |
| P | < .001 | .006 | ||||||||
| Ipsilateral breast symptoms (scale 0-24) | ||||||||||
| SNAD | 3.1 | 2.4 to 3.7 | 3.5 | 3 to 4 | 2.8 | 2.3 to 3.2 | 1.2 | 0.6 to 1.7 | 0.4 | −0.1 to 0.9 |
| SNR | 3.2 | 2.4 to 3.9 | 2.6 | 2.2 to 3.1 | 2.1 | 1.7 to 2.5 | 0.5 | 0 to 0.9 | −0.2 | −0.6 to 0.3 |
| SNAD v SNR | −0.1 | −1 to 0.9 | 0.8 | 0.1 to 1.5 | 0.7 | 0.1 to 1.3 | 0.7 | 0 to 1.4 | 0.5 | −0.1 to 1.2 |
| P | .06 | .10 | ||||||||
| Ipsilateral arm avoidance (scale 0-3) | ||||||||||
| SNAD | 0.5 | 0.3 to 0.6 | 0.5 | 0.4 to 0.6 | 0.4 | 0.4 to 0.5 | 0.2 | 0.1 to 0.3 | 0.2 | 0.1 to 0.2 |
| SNR | 0.4 | 0.3 to 0.5 | 0.3 | 0.2 to 0.4 | 0.3 | 0.2 to 0.3 | 0.1 | 0 to 0.2 | 0 | −0.1 to 0.1 |
| SNAD v SNR | 0.1 | −0.1 to 0.2 | 0.2 | 0.1 to 0.3 | 0.2 | 0.1 to 0.3 | 0.1 | 0 to 0.3 | 0.2 | 0 to 0.3 |
| P | .09 | .021 | ||||||||
| Social/occupational limitations (scale 0-8) | ||||||||||
| SNAD | 0.7 | 0.5 to 1 | 0.7 | 0.6 to 0.9 | 0.4 | 0.2 to 0.5 | 0.4 | 0.2 to 0.6 | 0 | −0.2 to 0.2 |
| SNR | 0.6 | 0.3 to 0.8 | 0.4 | 0.3 to 0.5 | 0.4 | 0.3 to 0.5 | 0.0 | −0.1 to 0.2 | 0 | −0.2 to 0.2 |
| SNAD v SNR | 0.2 | −0.2 to 0.5 | 0.3 | 0.1 to 0.5 | 0 | −0.2 to 0.2 | 0.4 | 0.1 to 0.6 | 0 | −0.2 to 0.2 |
| P | .004 | 1.0 | ||||||||
Abbreviations: SNAD, sentinel node resection followed by axillary dissection; SNR, sentinel node resection.
Patients with baseline regardless of having 6 months or 1 year follow-up.
Among patients with baseline data.
Longitudinal Analysis
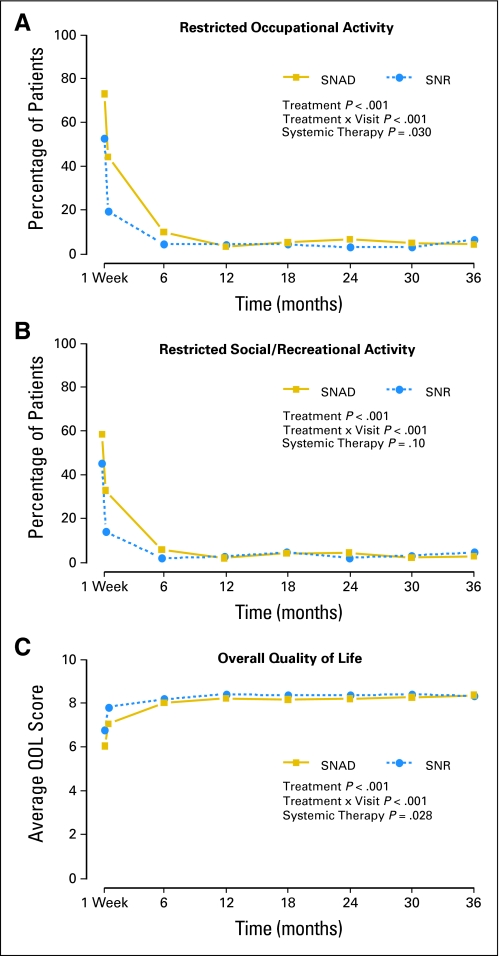
Among patients whose intended surgery was lumpectomy, SNAD patients were significantly more likely to experience difficulty over time on study (through 3 years) in the following end points: pushing large objects, lifting objects, reaching, and conducting social and work activities (P < .001). They were more likely to avoid ipsilateral arm use and more likely to experience all the symptoms assessed on the ipsilateral side (P = .002 breast tenderness; P < .001 all other items). For some items, the treatment difference diminished significantly over time (moving or lifting objects; reaching; arm: pain, numbness, skin sensitivity, tightness, or weakness; social or work activities; Fig 3A and Table 3). For other items, the difference persisted throughout the study (avoidance of arm use; arm or breast tenderness or swelling; breast: pain, numbness, skin sensitivity, or tightness; Fig 3B; other data not shown). All symptoms were significantly associated with the baseline response and diminished over time (P < .001 all items). Patients who received systemic therapy reported significantly more arm symptoms: tenderness, pain, skin sensitivity, tightness (all P < .001), and weakness (P = .007); breast symptoms: tenderness, swelling, pain, numbness, skin sensitivity (all P < .001), tightness (P = .004), and occupational limitations (P = .030) than those who did not receive systemic therapy. Overall QOL, adjusted for baseline levels, was significantly better among SNR patients (P < .001), lower among patients who received systemic therapy (P = .028) and improved significantly over time (P < .001); the difference between treatment groups diminished significantly over time (P < .001). The time course of activity limitations and overall QOL indicate that the impact of the procedures was seen mainly in the few weeks after surgery (Figs 3A to 3C).
Fig 3.
(A) Longitudinal graph of the proportion of patients with any restriction in recreational or social activity, among those with no restriction at baseline. P values from repeated measures logistic regression are shown. (B) Longitudinal graph of the percentage of patients with any restriction in occupational activity, among those with no restriction at baseline. P values from repeated measures logistic regression are shown. (C) Longitudinal graph of the mean quality of life score. P values from repeated measures linear regression are shown. SNAD, sentinel node resection followed by axillary dissection; SNR, sentinel node resection; QOL, quality of life.
Table 3.
Percentages of Lumpectomy Patients Without Each Symptom Listed at Baseline Who Experienced Each Ipsilateral Symptom at Moderate or Severe Levels, at Selected Time Points
| Parameter | SNAD |
SNR |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Weeks 2-3 | Month 6 | Month 12 | Month 24 | Month 36 | Week 1 | Weeks 2-3 | Month 6 | Month 12 | Month 24 | Month 36 | |
| Move objects* | 57 | 32 | 7 | 7 | 9 | 7 | 43 | 16 | 3 | 3 | 3 | 5 |
| Lift objects* | 64 | 39 | 9 | 6 | 6 | 6 | 46 | 19 | 6 | 4 | 3 | 6 |
| Reach above shoulders* | 39 | 14 | 3 | 3 | 1 | 1 | 14 | 6 | 2 | 1 | 1 | 2 |
| Avoid using arm† | 70 | 38 | 10 | 8 | 8 | 6 | 51 | 20 | 8 | 3 | 4 | 4 |
| Arm tenderness | 74 | 52 | 22 | 10 | 10 | 12 | 58 | 28 | 13 | 11 | 7 | 5 |
| Arm swelling | 39 | 27 | 13 | 8 | 10 | 8 | 30 | 10 | 4 | 4 | 4 | 3 |
| Arm pain | 70 | 44 | 20 | 11 | 10 | 10 | 48 | 22 | 11 | 11 | 8 | 7 |
| Arm numbness | 46 | 36 | 19 | 14 | 13 | 10 | 16 | 10 | 8 | 9 | 7 | 6 |
| Arm skin sensitivity | 45 | 37 | 15 | 8 | 7 | 7 | 26 | 15 | 8 | 5 | 5 | 2 |
| Arm tightness | 66 | 49 | 20 | 11 | 9 | 10 | 40 | 15 | 9 | 7 | 4 | 2 |
| Arm weakness | 49 | 30 | 12 | 9 | 9 | 8 | 28 | 9 | 8 | 8 | 5 | 6 |
| Breast tenderness | 50 | 30 | 13 | 9 | 9 | 9 | 48 | 25 | 14 | 10 | 7 | 5 |
| Breast swelling | 30 | 21 | 9 | 7 | 3 | 5 | 28 | 12 | 5 | 3 | 3 | 3 |
| Breast pain | 52 | 28 | 14 | 10 | 8 | 5 | 38 | 20 | 14 | 9 | 4 | 3 |
| Breast numbness | 25 | 19 | 8 | 7 | 7 | 3 | 13 | 8 | 4 | 5 | 3 | 2 |
| Breast skin sensitivity | 34 | 22 | 12 | 6 | 4 | 4 | 23 | 15 | 10 | 4 | 3 | 3 |
| Breast tightness | 46 | 31 | 11 | 8 | 5 | 7 | 31 | 14 | 8 | 6 | 4 | 1 |
| Restricted social activity | 59 | 33 | 6 | 3 | 5 | 3 | 45 | 15 | 3 | 3 | 3 | 5 |
| Restricted work activity | 73 | 44 | 10 | 3 | 7 | 4 | 53 | 19 | 4 | 4 | 3 | 6 |
Abbreviations: SNAD, sentinel node resection followed by axillary dissection; SNR, sentinel node resection.
Response categories are: ″very difficult″ or more severe (score 2 to 3 of the 0 to 3 range).
Response categories are: ″often avoided″ or more severe ( score 2 to 3 of the 0 to 3 range). All other response categories: ″somewhat bothered″ or more severe (score of 2 to 4 of the 0 to 4 range).
Among patients whose surgical plan was mastectomy, longitudinal analysis revealed that SNAD patients reported significantly greater arm use avoidance (P = .044), arm swelling (P = .021), arm and breast numbness (P < .001; P = .017 respectively), arm skin sensitivity (P = .032), arm tightness (P = .001) and social limitations (P = .026) than SNR patients. Patients with breast reconstruction experienced more breast swelling (P = .015) and tightness (P = .007) over time. Overall QOL did not differ significantly by SNAD versus SNR or reconstruction. The treatment difference in arm swelling diminished over time (P = .009); other differences persisted. Systemic therapy was associated with arm tenderness (P = .005) and tightness (P = .020) in this group. Presurgery symptom severity significantly predicted post-treatment values for nearly all items (data not shown). Scores for all items diminished significantly over time.
DISCUSSION
At the time B-32 was launched, several other randomized comparisons of SNR and AD opened elsewhere20: the Axillary Lymphatic Mapping Against Nodal Axillary Clearance (ALMANAC) trial of the British Association of Surgical Oncology,2,3,21 the Sentinella-Gruppo Interdisciplinare Veneto Oncologia Mammaria (GIVOM) trial,4,22 and the Royal Australasian College of Surgeons (RACS) Sentinel Node Biopsy versus Axillary Clearance (SNAC) trial,5,23–25 (Table 4) as well as two smaller trials.26–28
Table 4.
PROs in Phase III Randomized Trials of SNR v AD
| Trial | PRO Analysis Sample Size | PROs | PRO Time Points Reported |
|---|---|---|---|
| ALMANAC2,3,21 | 829 | QOL, arm symptoms, anxiety (FACT-B, additional questions about arm symptoms and function, and the STAI) | Baseline, 1, 3, 6, 12, 18 months |
| Sentinella-GIVOM4,22 | 697 (pain); 310 (QOL) | Pain, MOS SF-36, and the PGWB Index | Every 6 months (pain); 6, 12, 24 months (SF-36 and PGWB) |
| RACS SNAC5,23–25 | 1,028 | Symptoms, function, disabilities (SNAC Study-Specific Scales) [Other quality-of-life outcomes were assessed but have not yet been reported.] | Baseline, 1, 6, 12 months |
| NSABP B-326–9 | 747 | Symptoms, function, activity limitations, overall QOL | Baseline, 1 and 2-3 weeks post-operative; 6, 12, 18, 24, 30, 36 months |
Abbreviations: PRO, patient-reported outcomes; SNR, sentinel node resection; AD, axillary dissection; ALMANAC, the Axillary Lymphatic Mapping Against Nodal Axillary Clearance trial; QOL, quality of life; FACT-B, Functional Assessment of Cancer Therapy–Breast Cancer; STAI, State/Trait Anxiety Inventory; GIVOM, Gruppo Interdisciplinare Veneto Oncologia Mammaria; MOS SF, Medical Outcomes Study Short Form; PGWB, Psychological General Well Being; RACS, Royal Australasian College of Surgeons; SNAC, Sentinel Node Biopsy Versus Axillary Clearance; NSABP, National Surgical Adjuvant Breast and Bowel Project.
Our finding of significant differences favoring SNR in arm-related symptoms was consistent with the three other large trials. However, women in our trial reported less severe symptoms. For example, arm numbness at 6 months was at least moderately bothersome for 19.3% of SNAD patients in B-32, compared with 26.5% in ALMANAC. Arm numbness appears to be a sensitive indicator of surgical change in the axilla. This may be related to direct cutaneous nerve injury or subsequent seroma/hematoma formation. For the SNR group, this was considerably lower at 9.3%, reflecting less cutaneous nerve trauma from sentinel node only removal and less surgical trauma. The greater severity in other trials might be because all randomly assigned patients were included in the analysis, whereas in B-32 only sentinel node–negative patients from both treatment groups were included. Therefore, according to the protocol, the SNR group did not receive AD, and the SNAD group received SNR immediately followed by AD. The differences might also be related to how the symptoms were elicited and rated.
In addition to the decreased severity for lumpectomy-treated, node-negative B-32 patients as compared with patients in the other trials, the patterns of symptom differences during early postoperative and long-term follow-up in B-32 were informative. A prospective patient could, for example, conclude from Table 3 that she will have a 73% chance of restricted work activity one week after SNAD, or a 53% chance after SNR; but that by 6 months, those rates will be reduced to 10% and 4%, respectively. The differences between treatment groups during the second year in B-32 were similar to the other randomized trials. For example, the percentages of patients bothered by numbness were about double in the AD versus SNR groups in the second year of GIVOM and ALMANAC. The proportions were similarly about double in B-32 patients through 30 months and remained different (9.6% v 6.4%) at 36 months in B-32. Arm pain differed between treatment groups in the first year but became similar between groups during the second year in ALMANAC, GIVOM, and B-32. However, the third year in B-32 revealed persistent differences in arm pain between groups.
Interestingly, increased morbidity due to AD was not limited to the arms. Breast tenderness, swelling, pain, numbness, sensitivity, and tightness were all significantly increased in the SNAD group, and the differences did not diminish significantly over time. This is important because most concerns have focused on the arm, and breast symptoms have not been reported from other randomized studies. The arm may have better circulation of lymph fluid after AD than the breast because of natural arm movement and because that area has collateral channels that are less compromised during surgery. This is likely further compounded by breast irradiation.
This study also provides new information about social and occupational activity. We found that restrictions in activity were severe in both groups during the postoperative period, and greater for the SNAD group, but diminished by 12 months. Nonetheless, for some patients in both treatment groups, limitations persisted to 36 months.
In our study, we found that overall QOL was significantly better for SNR patients during the postoperative period. This confirms that the symptoms experienced by the women undergoing AD are important for their overall perception of QOL. However, the treatment difference was slight by 6 months, and no difference remained at 36 months. Similarly, there was no negative effect of SNR on mental or emotional function in either GIVOM or ALMANAC. GIVOM also showed better psychological well-being in the SNR group. The authors concluded that SNR was associated with reduced arm morbidity and better QOL, with no increase in anxiety.
Longer-term follow-up is available from small observational studies that have been conducted in recent years.29–36 For example, in one study with a mean follow-up of 6.6 years after AD and of 4.9 years after SNR, AD was associated with significantly greater likelihood of subjective arm numbness, chest or axillary numbness, and arm and hand swelling.37 A Memorial Sloan-Kettering study collected PROs up to 60 months after surgery from 187 patients.38 They found that symptoms were moderate but were greater among patients who received AD, even after 5 years.
A limitation of this study is that no widely used, well-validated instruments to measure arm and breast morbidity in this setting were available for our use. However, the items development was based on prior research, and items demonstrated responsiveness todifferences between treatments and over time. A second potential limitation is that some differences existed between participants in the B-32 substudy and parent study, which was anticipated in the design by conducting this sub-study among community-based institutions. Thus, these results are more representative of the patients treated in community settings rather than tertiary centers, which serves to make the substudy conclusions more generalizable.
Ultimately, the differences in PROs will be weighed against differences in clinical outcomes, including survival, to be reported separately. In forthcoming articles, our group will also report the observer-reported arm function, and a comparison between arm function and PROs.39 In the meantime, SNR is available to many patients. Two studies of preferences suggest that approximately half of women elect SNR as an alternative to AD when presented with a one in 10 rate of arm morbidity with AD and a one in 1,000 rate of increased 5-year mortality with SNR.40,41 B-32 provides critical information regarding the patient experience. A major finding was that symptoms were less severe than expected with AD, which will reassure patients who may require this surgery as part of their cancer treatment.
Supplementary Material
Acknowledgment
We thank the courageous participants, without whom this trial could not have been accomplished; Elaina Boudros, BS, Data Manager; Myoung Keun Lee, MS, Sarah R. Haile, PhD, and Reena S. Cecchini, MS, Data Analysts; Marcie W. Ritter, PhD, Behavioral and Health Outcomes Compliance Officer; Kate Murphy, Patient Advocate; Barbara C. Good, PhD, Director of Scientific Publications for the NSABP, and Wendy L. Rea, Editorial Assistant for the NSABP. None of the acknowledged people was compensated beyond normal salary for this work.
Appendix
Methods
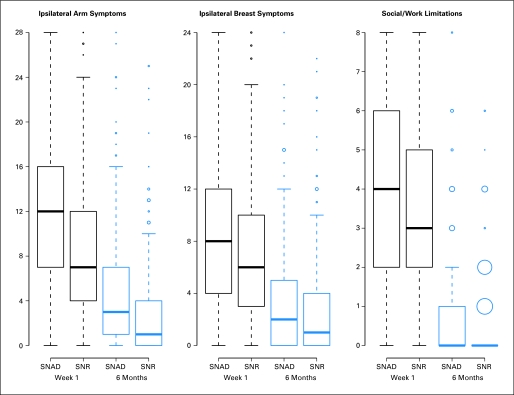
In secondary analysis, we compared ipsilateral breast symptoms, arm use avoidance (single item), and activity limitations among patients who underwent lumpectomy between SNAD and SNR at 6 months and 1 year. Relevant items were summed to form subscales for ipsilateral arm (Cronbach's alpha = 0.90) and ipsilateral breast symptoms (Cronbach's alpha = 0.90). The activity limitations scale was formed by summing two items regarding the limitation of social, recreational, and occupational activities (Cronbach's alpha = 0.81, scale 0-8.). We also performed treatment comparisons at 6 months and 1 year among patients who received mastectomy, using analysis of variance with factors for group and reconstruction surgery.
Results
Distributions of symptom and activity scales are illustrated in Figure 3. Symptoms in the ipsilateral breast were not significantly different at 6 months (P = .06) or 1 year (P = .10; Table 2). Ipsilateral arm use avoidance was not significantly different at 6 months (P = .09), but at 1 year, avoidance was significantly greater for SNAD (mean difference = 0.15, P = .021). Social/occupational limitation was greater for SNAD at 6 months (mean difference = 0.4, P = .004) but not at 1 year (mean difference = 0, P = 1.0). Among patients who underwent mastectomy, symptoms in the ipsilateral underarm, arm, hands, and fingers were significantly greater for SNAD versus SNR at 1 year (P = .029). There were nonsignificant associations of breast reconstruction with arm use avoidance (P = .07) and activity limitations (P = .049) at 6 months. Other comparisons of these end points were also not significant (data not shown).
Fig A1.
Box plots illustrate the distribution of major end point scales. Boxes are drawn from the 25th to the 75th percentile. Heavy horizontal lines within boxes are drawn at the median. Circles are potential outliers; larger circles reflect multiple equal observations. SNAD, sentinel node resection followed by axillary dissection; SNR, sentinel node resection.
Footnotes
Supported by the Grants No. U10-CA-12027, U10-CA-69651, U10-CA-37377, U10-CA-69974, and 5RO1-CA-074137 (D.N.K.) from the National Cancer Institute, Department of Health and Human Services, Public Health Service, and by Grant No. P30 CA22435 from the Vermont Cancer Center.
Presented in part at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003830.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Stephanie R. Land, Jacek A. Kopec, Thomas B. Julian, David N. Krag, Patricia A. Ganz
Administrative support: Stephanie R. Land, Thomas B. Julian, Joseph P. Costantino, Norman Wolmark
Provision of study materials or patients: Thomas B. Julian
Collection and assembly of data: Stephanie R. Land, Ann M. Brown, Stewart J. Anderson, Joseph P. Costantino
Data analysis and interpretation: Stephanie R. Land, David N. Krag, Nicholas J. Christian, Norman Wolmark, Patricia A. Ganz
Manuscript writing: Stephanie R. Land, Jacek A. Kopec, Thomas B. Julian, Ann M. Brown, Stewart J. Anderson, Joseph P. Costantino, Patricia A. Ganz
Final approval of manuscript: Stephanie R. Land, Jacek A. Kopec, Thomas B. Julian, Ann M. Brown, Stewart J. Anderson, David N. Krag, Nicholas J. Christian, Joseph P. Costantino, Norman Wolmark, Patricia A. Ganz
REFERENCES
- 1.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 3.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 4.Zavagno G, De Salvo GL, Scalco G, et al. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: Results of the Sentinella/GIVOM trial. Ann Surg. 2008;247:207–213. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 5.Gill G. Sentinel-lymph-node-based management or routine axillary clearance? One-year outcomes of sentinel node biopsy versus axillary clearance (SNAC): A randomized controlled surgical trial. Ann Surg Oncol. 2009;16:266–275. doi: 10.1245/s10434-008-0229-z. [DOI] [PubMed] [Google Scholar]
- 6.Harlow SP, Krag DN. Sentinel lymph node–why study it: Implications of the B-32 study. Semin Surg Oncol. 2001;20:224–229. doi: 10.1002/ssu.1037. [DOI] [PubMed] [Google Scholar]
- 7.Harlow SP, Krag DN, Julian TB, et al. Prerandomization surgical training for the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial: A randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer. Ann Surg. 2005;241:48–54. doi: 10.1097/01.sla.0000149429.39656.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver DL, Krag DN, Manna EA, et al. Detection of occult sentinel lymph node micrometastases by immunohistochemistry in breast cancer: An NSABP protocol B-32 quality assurance study. Cancer. 2006;107:661–667. doi: 10.1002/cncr.22074. [DOI] [PubMed] [Google Scholar]
- 9.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 10.Ganz PA, Coscarelli A, Fred C, et al. Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 11.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand): The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 12.Shimozuma K, Ganz PA, Petersen L, et al. Quality of life in the first year after breast cancer surgery: Rehabilitation needs and patterns of recovery. Breast Cancer Res Treat. 1999;56:45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- 13.Westrup JL, Lash TL, Thwin SS, et al. Risk of decline in upper-body function and symptoms among older breast cancer patients. J Gen Intern Med. 2006;21:327–333. doi: 10.1111/j.1525-1497.2006.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopec JA, Yothers G, Ganz PA, et al. Quality of life in operable colon cancer patients receiving oral compared with intravenous chemotherapy: Results from National Surgical Adjuvant Breast and Bowel Project trial C-06. J Clin Oncol. 2007;25:424–430. doi: 10.1200/JCO.2005.05.2597. [DOI] [PubMed] [Google Scholar]
- 15.Land SR, Kopec JA, Yothers G, et al. Health-related quality of life in axillary node-negative, estrogen receptor-negative breast cancer patients undergoing AC versus CMF chemotherapy: Findings from the National Surgical Adjuvant Breast and Bowel Project B-23. Breast Cancer Res Treat. 2004;86:153–164. doi: 10.1023/B:BREA.0000032983.87966.4e. [DOI] [PubMed] [Google Scholar]
- 16.de Boer AG, van Lanschot JJ, Stalmeier PF, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13:311–320. doi: 10.1023/B:QURE.0000018499.64574.1f. [DOI] [PubMed] [Google Scholar]
- 17.Feinstein AR. New Haven, CT: Yale University Press; 1987. Clinimetrics. [Google Scholar]
- 18.Fayers PM, Machin D. New York, NY: Wiley & Sons; 2000. Quality of Life: Assessment, Analysis and Interpretation. [Google Scholar]
- 19.Land SR, Ritter MW, Costantino JP, et al. Compliance with patient-reported outcomes in multicenter clinical trials: Methodologic and practical approaches. J Clin Oncol. 2007;25:5113–5120. doi: 10.1200/JCO.2007.12.1749. [DOI] [PubMed] [Google Scholar]
- 20.Sato K. Clinical trials for sentinel node biopsy in patients with breast cancer. Breast Cancer. 2007;14:31–36. doi: 10.2325/jbcs.14.31. [DOI] [PubMed] [Google Scholar]
- 21.Goyal A, Newcombe RG, Chhabra A, et al. Morbidity in breast cancer patients with sentinel node metastases undergoing delayed axillary lymph node dissection (ALND) compared with immediate ALND. Ann Surg Oncol. 2008;15:262–267. doi: 10.1245/s10434-007-9593-3. [DOI] [PubMed] [Google Scholar]
- 22.Del Bianco P, Zavagno G, Burelli P, et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: Results of the sentinella-GIVOM Italian randomised clinical trial. Eur J Surg Oncol. 2008;34:508–513. doi: 10.1016/j.ejso.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Ung OA. Australasian experience and trials in sentinel lymph node biopsy: The RACS SNAC trial. Asian J Surg. 2004;27:284–290. doi: 10.1016/S1015-9584(09)60052-X. [DOI] [PubMed] [Google Scholar]
- 24.Gill PG. Sentinel lymph node biopsy versus axillary clearance in operable breast cancer: The RACS SNAC trial, a multicenter randomized trial of the Royal Australian College of Surgeons (RACS) Section of Breast Surgery, in collaboration with the National Health and Medical Research Council Clinical Trials Center. Ann Surg Oncol. 2004;11:216S–21S. doi: 10.1007/BF02523632. [DOI] [PubMed] [Google Scholar]
- 25.Smith MJ, Gill PG, Wetzig N, et al. Comparing patients' and clinicians' assessment of outcomes in a randomised trial of sentinel node biopsy for breast cancer (the RACS SNAC trial) Breast Cancer Res Treat. 2009;117:99–109. doi: 10.1007/s10549-008-0202-3. [DOI] [PubMed] [Google Scholar]
- 26.Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: Update of a randomised controlled study. Lancet Oncol. 2006;7:983–990. doi: 10.1016/S1470-2045(06)70947-0. [DOI] [PubMed] [Google Scholar]
- 27.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 28.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: Results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–4321. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 29.Schijven MP, Vingerhoets AJ, Rutten HJ, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol. 2003;29:341–350. doi: 10.1053/ejso.2002.1385. [DOI] [PubMed] [Google Scholar]
- 30.Armer J, Fu MR, Wainstock JM, et al. Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology. 2004;37:73–91. [PubMed] [Google Scholar]
- 31.Dubernard G, Sideris L, Delaloge S, et al. Quality of life after sentinel lymph node biopsy in early breast cancer. Eur J Surg Oncol. 2004;30:728–734. doi: 10.1016/j.ejso.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Husted Madsen A, Haugaard K, Soerensen J, et al. Arm morbidity following sentinel lymph node biopsy or axillary lymph node dissection: A study from the Danish Breast Cancer Cooperative Group. Breast. 2008;17:138–147. doi: 10.1016/j.breast.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: A prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245:452–461. doi: 10.1097/01.sla.0000245472.47748.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulze T, Mucke J, Markwardt J, et al. Long-term morbidity of patients with early breast cancer after sentinel lymph node biopsy compared to axillary lymph node dissection. J Surg Oncol. 2006;93:109–119. doi: 10.1002/jso.20406. [DOI] [PubMed] [Google Scholar]
- 35.Rietman JS, Geertzen JH, Hoekstra HJ, et al. Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer. Eur J Surg Oncol. 2006;32:148–152. doi: 10.1016/j.ejso.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Rietman JS, Dijkstra PU, Geertzen JH, et al. Treatment-related upper limb morbidity 1 year after sentinel lymph node biopsy or axillary lymph node dissection for stage I or II breast cancer. Ann Surg Oncol. 2004;11:1018–1024. doi: 10.1245/ASO.2004.03.512. [DOI] [PubMed] [Google Scholar]
- 37.Crane-Okada R, Wascher RA, Elashoff D, et al. Long-term morbidity of sentinel node biopsy versus complete axillary dissection for unilateral breast cancer. Ann Surg Oncol. 2008;15:1996–2005. doi: 10.1245/s10434-008-9909-y. [DOI] [PubMed] [Google Scholar]
- 38.Baron RH, Fey JV, Borgen PI, et al. Eighteen sensations after breast cancer surgery: A 5-year comparison of sentinel lymph node biopsy and axillary lymph node dissection. Ann Surg Oncol. 2007;14:1653–1661. doi: 10.1245/s10434-006-9334-z. [DOI] [PubMed] [Google Scholar]
- 39.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. doi: 10.1002/jso.21535. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gan S, Magarey C, Schwartz P, et al. Women's choice between sentinel lymph node biopsy and axillary clearance. ANZ J Surg. 2002;72:110–113. doi: 10.1046/j.1445-2197.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- 41.Asaad M, Tamer M, Mokbel K. British women's choice between sentinel node biopsy and axillary node clearance for breast cancer. Curr Med Res Opin. 2003;19:570–574. doi: 10.1185/030079903125002199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.