Abstract
Purpose
Previous studies suggest that some common medications alter prostate-specific antigen (PSA) levels. It remains unclear whether these reported medication effects are due to clinicodemographic factors or concurrent use of other medications. We investigated the impact of individual and combinations of common medications on PSA in a large cross-sectional study of the United States population.
Patients and Methods
The study included men ≥ 40 years old without prostate cancer from the 2003 to 2004 and 2005 to 2006 cycles of the National Health and Nutrition Examination Survey (NHANES). Men with recent prostate manipulation, prostatitis, and those on hormone therapy were excluded. Weighted multivariate linear regression was performed on log-transformed total PSA to determine the effect of the 10 most commonly prescribed medication classes, adjusting for potential confounders including demographics, clinical characteristics, physical examination, laboratory studies, and duration of medication use.
Results
In total, 1,864 men met inclusion criteria. Nonsteroidal anti-inflammatory drug (NSAID; P = .03), statin (P = .01), and thiazide diuretic (P = .025) intake was inversely related to PSA levels. Five years of NSAID, statin, and thiazide diuretic use was associated with PSA levels lower by 6%, 13%, and 26%, respectively. The combination of statins and thiazide diuretics showed the greatest reduction in PSA levels: 36% after 5 years. Concurrent calcium channel blocker use minimizes or negates the inverse relationship of statin use and PSA level.
Conclusion
We found that men using NSAIDs, statins, and thiazide diuretics have reduced PSA levels by clinically relevant amounts. The impact of regularly consuming these common medications on prostate cancer screening is unknown.
INTRODUCTION
In the United States, prostate cancer screening with serum prostate-specific antigen (PSA) is common.1 Prostate cancer is now most frequently diagnosed following the detection of an elevated PSA.2 Therefore, factors that change PSA levels can potentially compromise the identification of prostate cancer.
It is recognized that age, prostatitis, and prostate size can influence PSA. Additionally, medications including 5-alpha reductase inhibitors (5ARIs) can dramatically and quickly decrease PSA.3 Recent studies suggest that other medications, including statins4–6 and nonsteroidal anti-inflammatory drugs (NSAIDs),7 may also lower PSA levels. While provocative, these previous studies were limited because they did not comprehensively control for relevant confounders, examine duration of medication use, or consider combinations of medications.
We investigated the impact of common medications on PSA levels in a cohort of men participating in the National Health and Nutrition Examination Survey (NHANES). With this rich data set to control for a vast array of clinical and sociodemographic factors, we sought to clarify the association between medication use and PSA levels among men typically screened for prostate cancer.
PATIENTS AND METHODS
Study Population
We accessed public use data files from the 2003 to 2004 and 2005 to 2006 cycles of NHANES, an ongoing cross-sectional observational study that collects health-related information from nationally representative samples of the civilian, noninstitutionalized population of the United States. The institutional review board of the National Center for Health Statistics approved the protocol for NHANES. Informed consent was obtained for all participants. Further details on the survey design can be found elsewhere.8,9
We restricted our analysis to men 40 years of age and older who provided a blood sample for PSA evaluation as part of NHANES. Serum total PSA level was measured with the Access Hybritech PSA assay (Beckman Coulter, Fullerton, CA). Because of the cross-sectional nature of this survey, only a single PSA value was available for each man in our cohort. Men with prostate cancer, prostatitis, or recent prostate manipulation (ie, rectal examination within 1 week, and prostate biopsy, surgery, or cystoscopy within 1 month) were ineligible. We also excluded men using 5ARIs or other forms of hormone therapy (ie, testosterone replacement or medical castration) and those with incomplete medication, clinical, or sociodemographic data.
Common Medications
We analyzed the most common outpatient prescription medication classes: metabolic agents, cardiovascular agents, CNS agents including pain medications, psychotherapeutic agents, and GI agents.10 Given the high prevalence of lower urinary tract symptoms among the older male population, we also included genitourinary agents. These medication classes were further subclassified on the basis of mechanisms of action. The 10 most commonly used medication subclasses were included in our analysis. For men using a medication, the duration of use was equal to the years since initiating therapy. For men not using a given medication, the duration of use was zero. Data on specific dosage or previously discontinued prescription medications were not available.
Medication Combinations
To assess the association of medication combinations and PSA level, we analyzed interaction variables in our multivariate model. We selected a priori only clinically relevant combinations for evaluation, including widely available fixed-dose combination (FDC) medications (ie, two or more medications in a single formulation): calcium channel blockers (CCBs) with statins, CCBs with angiotensin-converting enzyme (ACE) inhibitors, thiazide diuretics with ACE inhibitors, and thiazide diuretics with beta blockers. We also evaluated interaction variables for medications routinely prescribed concurrently: oral hypoglycemics (ie, sulfonylureas) with ACE inhibitors, which are often indicated for renoprotection in diabetics11 and statins with antihypertensive medications, given the high prevalence of dyslipidemia and hypertension in the general population.12
Explanatory Variables
We controlled for clinical and sociodemographic factors that potentially alter prostate cancer risk or PSA level. We assessed age (continuous), family history for prostate cancer (categorical: yes or no), race (categorical: white, black, Hispanic, other), country of birth (categorical: United States, Mexico, other), education level (categorical: less than high school, high school, more than high school), marital status (categorical: married, widowed, divorced, separated, never married, living with partner), household income (categorical: < $15,000, $15,000 to $34,999, $35,000 to $54,999, $55,000 to $74,999, ≥ $75,000), current health insurance status (categorical: yes or no), smoking (continuous: pack-years of tobacco), and alcohol intake (continuous: alcoholic drinks per week).13,14
We also controlled for clinical conditions and laboratory values possibly influencing PSA. Obesity has been linked to lower PSA levels among patients with prostate cancer15 and was controlled in our analysis by measuring body mass index, (kg/m2). Diabetes has been reported to decrease the risk of developing prostate cancer16 and was adjusted for by including serum hemoglobin A1c (%) levels in our model. Vitamin D status may alter prostate cancer risk.17 We thus controlled for serum vitamin D (ng/mL) levels derived from the Diasorin 25-hydroxycholecalciferol [25-(OH)D3] assay, albumin-adjusted calcium (mmol/L) levels,18 and parathyroid hormone (pg/mL) levels. To evaluate the actions of statins and NSAIDs, we obtained serum levels for total cholesterol (mg/dL) and C-reactive protein (CRP, mg/L), respectively. Patients with cardiovascular disease are frequently managed with a range of medications, including statins, antiplatelets, anticoagulants, and antihypertensives. To measure the risk for cardiovascular disease in our patients, we analyzed mean arterial pressures and serum homocysteine (μmol/L) levels in addition to total cholesterol and CRP levels. Serum testosterone levels were unavailable for analysis. All serum values and body measurements were analyzed as continuous variables.
Statistical Analysis
After accounting for the NHANES survey design with sampling weights, we evaluated the clinical and sociodemographic characteristics of the study population with descriptive statistics. To determine the relationship between PSA levels and medication use, we performed a multivariate linear regression and controlled for the aforementioned explanatory variables by modeling medications as continuous variables based on duration of use. The outcome variable in our model was log-transformed total PSA; to determine the difference in PSA levels between medication users and nonusers, we exponentiated the beta coefficients derived from the multivariate model. We also performed secondary analyses using subpopulations of the study cohort with differing PSA values. The linear relationship, normality, and homoscedasticity between predictor and outcome variables were examined with residual plots. Multicollinearity was also tested. All statistical testing was two-sided, and a P value of ≤ .05 was considered statistically significant. STATA 10.1 software (STATA, College Station, TX) was used for all statistical analyses.
RESULTS
Study Population
The pooled sample from the 2003 to 2004 and 2005 to 2006 NHANES survey cycles included 20,470 participants with 3,151 men at least 40 years of age. Among these men, 508 men did not give consent for PSA evaluation and two men did not provide medication information. We excluded 20 men with a history of prostate cancer, prostatitis, or recent prostate manipulation, 48 men on 5ARIs or hormone therapy, and 709 men with incomplete clinical or sociodemographic information for a final sample size of 1,864.
The median age of the study population was 53 years. The men were predominantly white, born in the United States, married, and insured (Table 1). Most men had at least a high school education and were in the lower or middle socioeconomic classes. The most frequently reported medical conditions were hypercholesterolemia and hypertension (Table 1). Approximately 12% of men reported a family history of prostate cancer. The men in our study population typically drank less than one alcoholic drink per day and smoked 7 pack-years of tobacco. The median mean arterial pressure was within normal limits and the median body mass index was in the overweight category. The median serum levels for total PSA, homocysteine, CRP, hemoglobin A1c, albumin-adjusted calcium, vitamin D, and parathyroid hormone were all within normal limits, while the median serum total cholesterol was in the borderline high range (Table 1).
Table 1.
Clinical and Sociodemographic Characteristics of the Study Population
| Characteristic | Men Age ≥ 40 Years (N = 1,864) |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 53 | |
| IQR | 46-62 | |
| Race/ethnicity | ||
| White | 1,503 | 80.7 |
| Black | 146 | 7.8 |
| Hispanic | 150 | 8.0 |
| Other | 65 | 3.5 |
| Country of birth | ||
| United States | 1,650 | 88.5 |
| Mexico | 63 | 3.4 |
| Other | 151 | 8.1 |
| Marital status | ||
| Married | 1,355 | 72.7 |
| Widowed | 62 | 3.3 |
| Divorced | 209 | 11.2 |
| Separated | 44 | 2.4 |
| Never married | 98 | 5.3 |
| Living with partner | 97 | 5.2 |
| Education level | ||
| Less than high school | 121 | 6.5 |
| High school or GED | 680 | 36.5 |
| More than high school | 1,062 | 57 |
| Household income, $ | ||
| 0-14,999 | 146 | 7.8 |
| 15,000-34,999 | 381 | 20.4 |
| 35,000-54,999 | 395 | 21.2 |
| 55,000-74,999 | 280 | 15.0 |
| 75,000+ | 622 | 35.5 |
| Health insurance | ||
| Yes | 1,631 | 87.5 |
| No | 233 | 12.5 |
| Medical history | ||
| Cardiovascular disease | ||
| Hypertension | 732 | 39.3 |
| Heart disease* | 264 | 14.2 |
| Metabolic disease | ||
| Hypercholesterolemia | 958 | 51.4 |
| Diabetes | 202 | 10.8 |
| Urologic disease | ||
| Enlarged prostate | 253 | 13.6 |
| Family history | ||
| Prostate cancer | 233 | 12.5 |
| Habits | ||
| Alcoholic drinks per week | ||
| Median | 0.92 | |
| IQR | 0.02-6 | |
| Pack-years of tobacco | ||
| Median | 7 | |
| IQR | 0-32 | |
| Physical examination | ||
| Mean arterial pressure (mmHg) | ||
| Median | 91 | |
| IQR | 84-98 | |
| Body mass index, kg/m2 | ||
| Median | 28.3 | |
| IQR | 25.5-31.6 | |
| Serum levels | ||
| Total PSA, ng/mL | ||
| Median | 0.8 | |
| IQR | 0.5-1.6 | |
| Total cholesterol, mg/dL | ||
| Median | 202 | |
| IQR | 178-230 | |
| Homocysteine, μmol/L | ||
| Median | 9.2 | |
| IQR | 7.9-11.3 | |
| C-reactive protein, mg/L | ||
| Median | 0.19 | |
| IQR | 0.08-0.39 | |
| Hemoglobin A1c, % | ||
| Median | 5.4 | |
| IQR | 5.2-5.7 | |
| Albumin-adjusted calcium, mmol/L† | ||
| Median | 2.34 | |
| IQR | 2.29-2.39 | |
| Vitamin D, ng/mL | ||
| Median | 23 | |
| IQR | 18-29 | |
| Parathyroid hormone, pg/mL | ||
| Median | 41 | |
| IQR | 32-53 | |
NOTE. Descriptive statistics are adjusted for sampling weights.
Abbreviations: IQR, interquartile range; GED, general equivalency diploma; PSA, prostate-specific antigen.
Includes a history of congestive heart failure, coronary artery disease, angina, myocardial infarction, and cerebral vascular accident.
Albumin-adjusted calcium (mmol/L) = serum total calcium (mmol/L) + 0.012 (39.9–serum albumin [g/L]).18
The most commonly consumed medications were statins followed by beta blockers and ACE inhibitors (Table 2). Men who used common medications typically had a long-term duration of regular use ranging from 2 to 5 years.
Table 2.
Ten Most Commonly Used Medication Classes and Subclasses Among the Study Population
| Medication | % Using Medication | Mean Duration of Use (years) |
|---|---|---|
| Metabolic agents | ||
| Statins | 19.8 | 4.0 |
| Sulfonylureas | 4.2 | 5.5 |
| Cardiovascular agents | ||
| Beta blockers | 12.6 | 5.4 |
| Calcium channel blockers | 5.7 | 5.8 |
| ACE inhibitors | 11.7 | 5.2 |
| Thiazide diuretics | 5.0 | 4.6 |
| GI agents | ||
| Proton pump inhibitors | 8.5 | 3.4 |
| CNS agents | ||
| NSAIDs | 8.9 | 4.0 |
| Psychiatric agents | ||
| SSRIs | 6.0 | 3.6 |
| Genitourinary agents | ||
| Alpha blockers | 4.4 | 3.8 |
NOTE. The percentage and duration of use was adjusted for sampling weights.
Abbreviations: ACE, angiotensin-converting enzyme; NSAID, non-steroidal anti-inflammatory drug; SSRI, selective serotonin reuptake inhibitor.
Multivariate Linear Regression Analysis
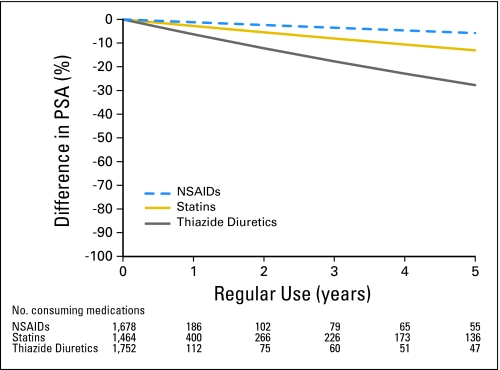
We found an inverse relationship between PSA levels and regular NSAID (P = .03), statin (P = .01), and thiazide diuretic (P = .025) intake. Our multivariate model predicted that longer consumption of these medications accentuated the relative difference in PSA between users and nonusers of NSAIDs, statins, and thiazide diuretics. PSA levels after 1 year of therapy on NSAIDs, statins, and thiazide diuretics were less than the serum PSA levels in men not using these medications by 1%, 3%, and 6%, respectively (Table 3). After 5 years, men using NSAIDs, statins, and thiazide diuretics were associated with 6%, 13%, and 26% lower total PSA levels, respectively (Fig 1). After 5 years, the standard errors for model estimates increased substantially, given the limited numbers of men on therapy with these medications beyond that duration of time. Secondary analyses demonstrated that the relationship between statins and PSA is stable in men across a wide range of PSA values; in contrast, our findings for NSAIDs and thiazide diuretics are primarily driven by men with PSA values of ≥ 2.5 ng/mL (data not shown). There were no other significant associations between other individual medications and serum PSA.
Table 3.
Multivariate Linear Regression Analysis of the Main Effects of Common Medications on the Serum PSA Level
| Variable | Relative PSA Level* | Coefficient (β) | SE† | 95% CI | P |
|---|---|---|---|---|---|
| Reference | 1.00 | — | — | — | — |
| Metabolic agents | |||||
| Statins | 0.972 | −.028 | 0.010 | −0.049 to −0.0074 | .01 |
| Sulfonylureas | 0.993 | −.0074 | 0.019 | −0.047 to 0.032 | .71 |
| Cardiovascular agents | |||||
| Beta blockers | 0.995 | −.0050 | 0.0098 | −0.025 to 0.015 | .61 |
| Calcium channel blockers | 1.003 | .0033 | 0.018 | −0.034 to 0.04 | .86 |
| ACE inhibitors | 0.99 | −.0086 | 0.010 | −0.029 to 0.012 | .40 |
| Thiazide diuretic | 0.94 | −.065 | 0.028 | −0.12 to −0.0087 | .025 |
| GI agents | |||||
| Proton pump inhibitors | 0.997 | −.003 | 0.026 | −0.056 to 0.05 | .91 |
| CNS agents | |||||
| NSAIDs | 0.988 | −.012 | 0.0053 | −0.023 to −0.0011 | .03 |
| Psychotherapeutic agents | |||||
| SSRIs | 0.975 | −.026 | 0.027 | −0.081 to 0.03 | .35 |
| Genitourinary agents | |||||
| Alpha blockers | 1.002 | .0025 | 0.020 | −0.039 to 0.044 | .90 |
NOTE. Medications modeled as continuous variables based on duration of consumption, adjusting for age, race, education level, country of birth, marital status, household income, health insurance status, body mass index, mean arterial pressure, tobacco use, alcohol use, prostate enlargement, family history of prostate cancer, serum levels for total cholesterol, log-transformed homocysteine, albumin-adjusted calcium, vitamin D, log-transformed C-reactive protein, hemoglobin A1c, and log-transformed parathyroid hormone.
Abbreviations: PSA, prostate-specific antigen; ACE, angiotensin-converting enzyme; NSAID, non-steroidal anti-inflammatory drug; SSRI, selective serotonin reuptake inhibitor.
Relative PSA change after 1 year of regular use compared with no use; relative PSA level = eβ.
Based on jackknife estimation.
Fig 1.
Predicted difference in prostate-specific antigen (PSA) levels over time in men who regularly use nonsteroidal anti-inflammatory drugs (NSAIDs), statins, and thiazide diuretics based on a multivariate model adjusting for explanatory variables. Percent difference in PSA = (1 – e(β × Years)) × 100.
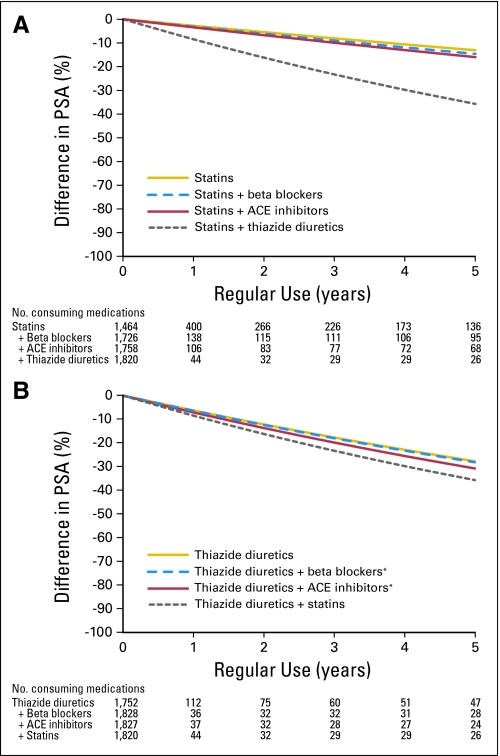
We found that only medication combinations involving statins or thiazide diuretics were also inversely related to PSA levels (Table 4). The main effect of statins on PSA persisted unchanged when combined with either beta blockers or ACE inhibitors, but was minimized and no longer statistically significant when CCBs were concurrently administered (Fig 2A; Table 4). The main effect of thiazide diuretics on PSA was unaffected when taken in combination with beta blockers or ACE inhibitors (Fig 2B). Our model predicted that the combination of statins and thiazide diuretics was associated with the greatest relative decrease in PSA levels: 8% after 1 year increasing to 36% by 5 years (Fig 2). Other medication combinations incorporating sulfonylureas or CCBs demonstrated no statistically significant association with PSA levels.
Table 4.
Multivariate Linear Regression Analysis Assessing the Interaction Effects of Fixed-Dose Combination Medications and Medications Commonly Prescribed Concurrently on the Serum PSA Level
| Interaction Terms | % Using Medication | Relative PSA Level* | Coefficient (β) | Wald χ2 | P |
|---|---|---|---|---|---|
| Fixed-dose combination medications | |||||
| Calcium channel blockers + statins | 3.5 | 0.98 | −.023 | 1.34 | .26 |
| Calcium channel blockers + ACE inhibitors | 2.7 | 0.99 | −.008 | 0.15 | .7 |
| Beta blockers + thiazide diuretics | 1.9 | 0.94 | −.066 | 5.52 | .026 |
| ACE inhibitors + thiazide diuretics | 2.0 | 0.93 | −.074 | 6.54 | .016 |
| Medications prescribed concurrently | |||||
| Sulfonylureas + ACE inhibitors | 2.6 | 0.98 | −.018 | 0.89 | .35 |
| Statins + beta blockers | 7.4 | 0.97 | −.032 | 5.05 | .032 |
| Statins + ACE inhibitors | 5.7 | 0.97 | −.035 | 4.69 | .039 |
| Statins + thiazide diuretics | 2.4 | 0.92 | −.09 | 11 | .002 |
NOTE. Medications modeled as continuous variables based on duration of consumption, adjusting for age, race, education level, country of birth, marital status, household income, health insurance status, body mass index, mean arterial pressure, tobacco use, alcohol use, prostate enlargement, family history of prostate cancer, serum levels for total cholesterol, log-transformed homocysteine, albumin-adjusted calcium, vitamin D, log-transformed C-reactive protein, hemoglobin A1c, and log-transformed parathyroid hormone. Post-estimation null hypothesis: drug A + drug B + drug A × drug B = 0.
Abbreviations: PSA, prostate-specific antigen; ACE, angiotensin-converting enzyme.
Relative PSA change after 1 year of regular use; relative change = eβ.
Fig 2.
Predicted difference in prostate-specific antigen (PSA) levels over time in men regularly using (A) statins and medication combinations involving statins and (B) thiaizide diuretics and medication combinations involving thiazide diuretics based on a multivariate model adjusting for explanatory variables. Percent difference in PSA = (1 – e(β × Years)) × 100. ACE, angiotensin-converting enzyme. (*) Fixed-dose combination medication.
DISCUSSION
Prostate cancer screening with PSA is prevalent among men older than age 50 years,1 a population that also has a particularly high rate of medication use because of the increased risk of developing chronic medical conditions with age.19 Among elderly Americans, > 80% report regular use of at least one prescription medication and nearly 30% use at least five prescription medications.20 Common among these prescription medications are NSAIDs, statins, and thiazide diuretics, which we found to be associated with decreased PSA levels. This study adds support to the body of evidence suggesting that NSAID and statin use may reduce PSA. In addition, we show that men regularly consuming thiazide diuretics also have significantly lower PSA levels.
NSAIDs
Although statistically significant, the overall magnitude in PSA differences associated with NSAID use is small. However, our secondary analysis suggests that PSA differences may be greater among men with PSA values higher than 2.5 ng/mL. This observation may have profound implications on prostate cancer screening.
Singer et al7 reported an inverse relationship between NSAID use and serum PSA with older data from NHANES but did not consider duration of NSAID use in their analysis. Similarly dichotomizing NSAID consumption into “regular use” or “no use” for our analysis, we find the same result as Singer et al: men regularly using NSAIDs have, on average, a 10% decrease in total PSA (data not shown). Additionally, after controlling for serum CRP in our analysis, we continued to find lower PSA levels in men on NSAIDs. While CRP may be an imperfect marker of prostatic inflammation,21 it nevertheless raises the possibility that the inverse relationship between NSAID use and PSA may in part be explained by noninflammatory actions of NSAIDs, including the inhibition of cyclooxygenase activity in both benign22 and malignant23 prostate tissue resulting in apoptosis,24 induction of cell cycle arrest, and reduction in angiogenesis.25
Statins
Our finding that statins are associated with decreased PSA levels is in general agreement with previous reports. In a study of 15 men started on statins, Cyrus-David et al4 reported that men on statins had a 79.6% lower serum PSA compared with men not on statins after 5 years. This study was limited by its small sample size and univariate analysis. We posit that the substantially smaller difference in PSA between statin users and statin nonusers after 5 years in our study (13%) is explained by our comprehensive control for potential confounders. Hamilton et al5 followed 1,214 men from the Veterans Affairs Health Care System who initiated statin therapy; after adjusting for serum cholesterol levels, a 4.1% reduction in serum PSA was reported after 1 year of statin therapy. The results from this controlled, longitudinal study complement our findings of 3% derived from cross-sectional data.
The mechanism by which statins may alter PSA levels is unclear. Hamilton et al5 found that the PSA-lowering effect of statins was strongly linked to cholesterol parameters. In this study, we controlled for total cholesterol levels and nevertheless found that statin users had significantly lower total PSA levels, suggesting that additional noncholesterol-mediated mechanism(s) may also play an important role in serum PSA levels. Possible mechanisms include directly inhibiting angiogenesis,26,27 induction of apoptosis,28 and reduction in cellular proliferation29 through several intracellular signaling pathways.
Thiazide Diuretics
We found that men who regularly take thiazide diuretics had lower PSA levels than men taking NSAIDs or statins (Fig 1). We hypothesize that the effect of thiazide diuretics on PSA may be mediated through effects on vitamin D metabolism. A placebo-controlled longitudinal study showed that after 1 year of regular thiazide diuretic intake, mean serum 25-(OH)D3 and mean serum calcium levels were unchanged, but there was a decrease in 1,25-dihydroxycholecalciferol [1,25-(OH)2D3].30 Furthermore, several studies have found that 1,25-(OH)2D3 is associated with a dose-dependent secretion of PSA from LNCaP cells in cell culture.31 It is plausible that consumption of thiazide diuretics preferentially reduces 1,25-(OH)2D3 levels, resulting in the observed decrease in total PSA levels. Therefore, the serum 1,25-(OH)2D3 level may represent an unmeasured confounder because the NHANES laboratory data on serum vitamin D is derived from the Diasorin 25-(OH)D3 assay, a laboratory test that does not detect 1,25-(OH)2D3.
Thiazide diuretics may also be associated with lower PSA levels by inducing an androgen-deficient state. While previous investigations have noted that thiazide diuretics do not change serum total testosterone concentrations,32,33 Perry et al34 found that the amount of bioavailable testosterone decreases by approximately 30% in elderly men taking thiazide diuretics compared with controls. The reduction in bioavailable testosterone may be responsible for the documented impairment of male sexual function associated with thiazide diuretics use.32,35,36 Because serum testosterone levels were not available for analysis in this study, we are unable to confirm the previous observations. Nevertheless, these studies suggest that intake of thiazide diuretics may result in functional hypogonadism with consequently lower PSA levels.
Medication Combinations
The lowest levels of serum PSA among men in the study were associated with the medication combination of statins and thiazide diuretics: approximately 36% lower at 5 years (Fig 2). This reduction in total PSA is, however, less than the additive effects of statins and thiazide diuretics alone, indicating that there may be an interaction between these two medication subclasses. Further investigation is necessary to elucidate the underlying mechanism of this drug interaction.
We found that the association between statins and PSA is limited or negated with concurrent CCB use. It is feasible that CCBs antagonize statins by specifically inhibiting statin-induced cell responses. Previous studies demonstrate that CCBs prevent apoptosis mediated by cholesterol-dependent mechanisms.37,38 Therefore, the CCBs may disrupt the cell signaling pathways in prostate epithelial cells by which statins alter PSA production.
Clinical Implications
Recent level I evidence by Schröder et al39 shows that prostate cancer screening with PSA leads to a 20% reduction in prostate cancer-specific mortality. It is, therefore, important to thoroughly understand the consequences of regular NSAID, statin, and thiazide diuretic use on the quality of prostate cancer screening. If the lower PSA levels in men taking these medications result in a delayed diagnosis of prostate cancer, a “medication-adjusted” PSA threshold for prostate cancer screening may need to be defined. Alternatively, if NSAIDs, statins, and thiazide diuretics influence prostate volume, they may actually improve the diagnostic accuracy of prostate cancer screening.40,41
Importantly, this study excluded men with prostate cancer and therefore cannot comment on whether use of NSAIDs, statins, or thiazide diuretics alters prostate cancer risk. Epidemiologic studies do raise the possibility that NSAIDs42,43 and statins44–46 may decrease the risk of prostate cancer. There is currently no empiric evidence to suggest that thiazide diuretics impact prostate carcinogenesis. If future studies demonstrate that thiazide diuretics reduce prostate carcinogenesis, then they could represent a useful form of prostate cancer chemoprevention in addition to their recognized cardiovascular benefits.
Limitations
There are several important limitations to this study. Missing data in the NHANES data set, which limited our study cohort from 3,151 men to 1,864 men, may have been nonrandom, thus potentially resulting in nonresponder bias. The cross-sectional nature of our data precludes determination of a causal relationship between duration of medication use and differences in PSA levels. Furthermore, we are unable to confirm the inverse relationship between PSA levels and NSAID, statin, and thiazide diuretic use substantially beyond 5 years, given the lack of men using the medications for more than 5 years. Despite the detailed NHANES questionnaire, we did not have sufficient dosage information or data regarding medication compliance. In particular, FDC medications generally use lower dosages and therefore our model may overestimate the effects of some FDC medications. Our analysis was not exhaustive because we limited our analysis to the 10 most commonly prescribed medication subclasses; we may have failed to identify medications that possess highly significant associations with serum PSA simply because they are less commonly prescribed.
In conclusion, in men without a history of prostate cancer, use of NSAIDs, statins, or thiazide diuretics is significantly associated with lower PSA levels. This difference in PSA levels is more pronounced with prolonged medication use during at least the initial 5 years of regular use. The concurrent use of CCBs minimizes and potentially negates the inverse relationship between statins and PSA, while the combination of thiazide diuretics and statins is associated with the largest difference in PSA. The overall effect of using NSAIDs, statins, and thiazide diuretics on prostate cancer screening is unknown at this time and deserves further evaluation.
Footnotes
Supported by Stanford National Institutes of Health/National Center for Research Resources Clinical and Translational Science Awards Grant No. KL2 RR025743.
Presented at the 85th Annual Meeting of the American Urological Association, Western Section, October 25-29, 2009, Las Vegas, NV, and the 2010 Genitourinary Cancers Symposium, March 5-7, 2010, San Francisco, CA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Steven L. Chang, Lauren C. Harshman, Joseph C. Presti Jr
Financial support: Steven L. Chang
Administrative support: Steven L. Chang
Collection and assembly of data: Steven L. Chang
Data analysis and interpretation: Steven L. Chang, Lauren C. Harshman, Joseph C. Presti Jr
Manuscript writing: Steven L. Chang, Lauren C. Harshman, Joseph C. Presti Jr
Final approval of manuscript: Steven L. Chang, Lauren C. Harshman, Joseph C. Presti Jr
REFERENCES
- 1.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States: Does practice reflect the evidence? JAMA. 2003;289:1414–1420. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–8151. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 3.Etzioni RD, Howlader N, Shaw PA, et al. Long-term effects of finasteride on prostate specific antigen levels: Results from the prostate cancer prevention trial. J Urol. 2005;174:877–881. doi: 10.1097/01.ju.0000169255.64518.fb. [DOI] [PubMed] [Google Scholar]
- 4.Cyrus-David MS, Weinberg A, Thompson T, et al. The effect of statins on serum prostate specific antigen levels in a cohort of airline pilots: A preliminary report. J Urol. 2005;173:1923–1925. doi: 10.1097/01.ju.0000158044.94188.88. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton R, Goldberg KC, Platz EA, et al. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100:1511–1518. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 6.Mondul AM, Selvin E, De Marzo AM, et al. Statin drugs, serum cholesterol, and prostate-specific antigen in the National Health and Nutrition Examination Survey 2001-2004. Cancer Causes Control. 2010;21:671–678. doi: 10.1007/s10552-009-9494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer EA, Palapattu GS, van Wijngaarden E. Prostate-specific antigen levels in relation to consumption of nonsteroidal anti-inflammatory drugs and acetaminophen: Results from the 2001-2002 National Health and Nutrition Examination Survey. Cancer. 2008;113:2053–2057. doi: 10.1002/cncr.23806. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. NHANES 2003-2004 Public Data General Release File Documentation. http://www.cdc.gov/NCHS/data/nhanes/nhanes_03_04/general_data_release_doc_03-04.pdf.
- 9.Centers for Disease Control and Prevention. NHANES 2005-2006 Public Data General Release File Documentation. http://www.cdc.gov/NCHS/data/nhanes/nhanes_05_06/general_data_release_doc_05_06.pdf.
- 10.Soni A. 2005. Statistical Brief #199: The top five therapeutic classes of outpatient prescription drugs by total expense for the Medicare population age 65 and older in the U.S. civilian noninstitutionalized population. February 2008. [Google Scholar]
- 11.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 12.Muntner P, He J, Roccella EJ, et al. The impact of JNC-VI guidelines on treatment recommendations in the US population. Hypertension. 2002;39:897–902. doi: 10.1161/01.hyp.0000013862.13962.1d. [DOI] [PubMed] [Google Scholar]
- 13.Dall'era MA, Hosang N, Konety B, et al. Sociodemographic predictors of prostate cancer risk category at diagnosis: Unique patterns of significant and insignificant disease. J Urol. 2009;181:1622–1627. doi: 10.1016/j.juro.2008.11.123. [DOI] [PubMed] [Google Scholar]
- 14.Lund Nilsen TI, Johnsen R, Vatten LJ. Socio-economic and lifestyle factors associated with the risk of prostate cancer. Br J Cancer. 2000;82:1358–1363. doi: 10.1054/bjoc.1999.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bañez LL, Hamilton R, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 16.Kasper JS, Liu Y, Giovannucci E. Diabetes mellitus and risk of prostate cancer in the health professionals follow-up study. Int J Cancer. 2009;124:1398–1403. doi: 10.1002/ijc.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James MT, Zhang J, Lyon AW, et al. Derivation and internal validation of an equation for albumin-adjusted calcium. BMC Clin Pathol. 2008;8:12. doi: 10.1186/1472-6890-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurwitz JH, Avorn J. The ambiguous relation between aging and adverse drug reactions. Ann Intern Med. 1991;114:956–966. doi: 10.7326/0003-4819-114-11-956. [DOI] [PubMed] [Google Scholar]
- 20.Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedland SJ, Aronson WJ. Invited commentary: Lower urinary tract symptoms and inflammation—Weighing the evidence. Am J Epidemiol. 2009;169:1291–1293. doi: 10.1093/aje/kwp084. [DOI] [PubMed] [Google Scholar]
- 22.Zha S, Gage WR, Sauvageot J, et al. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- 23.Shappell SB, Manning S, Boeglin WE, et al. Alterations in lipoxygenase and cyclooxygenase-2 catalytic activity and mRNA expression in prostate carcinoma. Neoplasia. 2001;3:287–303. doi: 10.1038/sj.neo.7900166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song X, Lin HP, Johnson AJ, et al. Cyclooxygenase-2, player or spectator in cyclooxygenase-2 inhibitor-induced apoptosis in prostate cancer cells. J Natl Cancer Inst. 2002;94:585–591. doi: 10.1093/jnci/94.8.585. [DOI] [PubMed] [Google Scholar]
- 25.Patel MI, Subbaramaiah K, Du B, et al. Celecoxib inhibits prostate cancer growth: Evidence of a cyclooxygenase-2-independent mechanism. Clin Cancer Res. 2005;11:1999–2007. doi: 10.1158/1078-0432.CCR-04-1877. [DOI] [PubMed] [Google Scholar]
- 26.Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 27.Weis M, Heeschen C, Glassford AJ, et al. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Wong WW, Khosravi F, et al. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 2004;64:6461–6468. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- 29.Park C, Lee I, Kang WK. Lovastatin-induced E2F-1 modulation and its effect on prostate cancer cell death. Carcinogenesis. 2001;22:1727–1731. doi: 10.1093/carcin/22.10.1727. [DOI] [PubMed] [Google Scholar]
- 30.Riis B, Christiansen C. Actions of thiazide on vitamin D metabolism: A controlled therapeutic trial in normal women early in the postmenopause. Metab Clin Exp. 1985;34:421–424. doi: 10.1016/0026-0495(85)90206-9. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh TY, Ng CY, Mallouh C, et al. Regulation of growth, PSA/PAP and androgen receptor expression by 1 alpha,25-dihydroxyvitamin D3 in the androgen-dependent LNCaP cells. Biochem Biophys Res Commun. 1996;223:141–146. doi: 10.1006/bbrc.1996.0859. [DOI] [PubMed] [Google Scholar]
- 32.Scharf MB, Mayleben DW. Comparative effects of prazosin and hydrochlorothiazide on sexual function in hypertensive men. Am J Med. 1989;86:110–112. doi: 10.1016/0002-9343(89)90144-7. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, Tominaga T, Kumagai H, et al. Effects of first-line antihypertensive agents on sexual function and sex hormones. J Hypertens. 1988;6:S649–S651. doi: 10.1097/00004872-198812040-00204. [DOI] [PubMed] [Google Scholar]
- 34.Perry HM, Jensen J, Kaiser FE, et al. The effects of thiazide diuretics on calcium metabolism in the aged. J Am Geriatr Soc. 1993;41:818–822. doi: 10.1111/j.1532-5415.1993.tb06176.x. [DOI] [PubMed] [Google Scholar]
- 35.Croog SH, Levine S, Sudilovsky A, et al. Sexual symptoms in hypertensive patients. A clinical trial of antihypertensive medications. Arch Intern Med. 1988;148:788–794. [PubMed] [Google Scholar]
- 36.Wassertheil-Smoller S, Blaufox MD, Oberman A, et al. Effect of antihypertensives on sexual function and quality of life: The TAIM Study. Ann Intern Med. 1991;114:613–620. doi: 10.7326/0003-4819-114-8-613. [DOI] [PubMed] [Google Scholar]
- 37.Ares MP, Pörn-Ares MI, Thyberg J, et al. Ca2+ channel blockers verapamil and nifedipine inhibit apoptosis induced by 25-hydroxycholesterol in human aortic smooth muscle cells. J Lipid Res. 1997;38:2049–2061. [PubMed] [Google Scholar]
- 38.Sasaki H, Watanabe F, Murano T, et al. Vascular smooth muscle cell apoptosis induced by 7-ketocholesterol was mediated via Ca2+ and inhibited by the calcium channel blocker nifedipine. Metab Clin Exp. 2007;56:357–362. doi: 10.1016/j.metabol.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 39.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 40.Elliott CS, Shinghal R, Presti J. The influence of prostate volume on prostate-specific antigen performance: Implications for the prostate cancer prevention trial outcomes. Clin Cancer Res. 2009;15:4694–4699. doi: 10.1158/1078-0432.CCR-08-2277. [DOI] [PubMed] [Google Scholar]
- 41.Thompson IM, Chi C, Ankerst DP, et al. Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst. 2006;98:1128–1133. doi: 10.1093/jnci/djj307. [DOI] [PubMed] [Google Scholar]
- 42.Dasgupta K, Di Cesar D, Ghosn J, et al. Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J. 2006;12:130–135. [PubMed] [Google Scholar]
- 43.Roberts RO, Jacobson DJ, Girman CJ, et al. A population-based study of daily nonsteroidal anti-inflammatory drug use and prostate cancer. Mayo Clin Proc. 2002;77:219–225. doi: 10.4065/77.3.219. [DOI] [PubMed] [Google Scholar]
- 44.Graaf MR, Beiderbeck AB, Egberts AC, et al. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 46.Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: A case-control study. Am J Epidemiol. 2005;162:318–325. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]