Abstract
Purpose
Fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET)/computed tomography (CT) has been approved for imaging in many malignancies but not for bladder cancer. This study investigated the value of FDG-PET/CT imaging in the management of patients with advanced bladder cancer.
Patients and Methods
Between May 2006 and February 2008, 57 patients with bladder cancer at our center underwent FDG-PET/CT after CT (n = 52) or magnetic resonance imaging (MRI; n = 5). The accuracy of FDG-PET/CT was assessed using both organ-based and patient-based analyses. FDG-PET/CT findings were validated by either biopsy or serial CT/MRI. Clinician questionnaires performed before and after FDG-PET/CT assessed whether those scan results affected management.
Results
One hundred thirty-five individual lesions were evaluable in 47 patients for the organ-based analysis. Overall sensitivity and specificity were 87% (95% CI, 76% to 94%) and 88% (95% CI, 78% to 95%), respectively. In the patient-based analysis, malignant disease was correctly diagnosed in 25 of 31 patients, resulting in a sensitivity of 81% (95% CI, 63% to 93%). FDG-PET/CT was negative in 15 of 16 patients without malignant lesions for a specificity of 94% (95% CI, 71% to 100%). Pre- and post-PET surveys revealed that FDG-PET/CT detected more malignant disease than conventional CT/MRI in 40% of patients. Post-PET surveys showed that clinicians changed their planned management in 68% of patients based on the FDG-PET/CT results.
Conclusion
FDG-PET/CT has excellent sensitivity and specificity in the detection of metastatic bladder cancer and provides additional diagnostic information that enhances clinical management more than CT/MRI alone. FDG-PET/CT scans may provide better accuracy in clinical information for directing therapy.
INTRODUCTION
Imaging studies are frequently performed for staging and re-evaluation in muscle-invasive and more advanced bladder cancer because of the aggressive biology and high incidence of metastases. Computed tomography (CT) and/or magnetic resonance imaging (MRI) are generally used, but they have limitations in distinguishing between benign and malignant lesions,1–3 making tumor tissue biopsies necessary to confirm suspicious findings.
Fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET)/CT provides anatomic and metabolic information for staging and restaging and has been incorporated into the management of many malignancies.4–6 The use of FDG-PET/CT in patients with bladder cancer may also help to characterize lesions that are indeterminate by CT and/or MRI. PET imaging in bladder cancer has not been fully explored, in part because the urinary excretion of FDG interferes with visualization of the primary bladder tumor and regional nodes. However, evaluation for metastatic lesions, including local lymph nodes, using FDG-PET/CT can potentially aid in staging,7 treatment planning, and assessment of overall prognosis. This study sought to determine the accuracy of FDG-PET/CT in detecting metastatic disease using both a patient-based and an organ-based analysis and to identify the extent to which FDG-PET/CT results affect clinical decisions in patients with bladder cancer.
PATIENTS AND METHODS
Patient Population
Patients eligible for this study were prospectively registered in the National Oncology PET Registry (NOPR) at Memorial Sloan-Kettering Cancer Center between May 2006 and February 2008. All patients had initial anatomic imaging with either CT or MRI followed by FDG-PET/CT.
FDG-PET/CT
All patients were imaged on dedicated PET/CT scanners, including Discovery LS, Discovery ST, Discovery STE (all GE Healthcare, Waukesha, WI), or Biograph LSO-16 (Siemens Medical Solutions, Malvern, PA). Patients were asked to fast for 6 hours before the PET/CT scan. Blood glucose was measured on patient arrival in the nuclear medicine clinic and was less than 200 mg/dL (our institutional cutoff) in all patients; 12 to 15 mCi of FDG was then injected intravenously. After an approximately 60- to 90-minute uptake period when patients drank diluted oral contrast, they were asked to void and were then positioned on the scanner table. After scout view and low-dose CT (120 to 140 kV, 80 mA), which was used for attenuation correction and anatomic localization, PET emission images were obtained for 3 minutes per bed position from the skull base to the upper thigh. All PET/CT studies were reviewed by board-certified nuclear medicine physicians using picture archiving and communication systems workstations that allow for the display of CT, PET emission, and PET/CT fusion image sets in various orthogonal planes. These studies were interpreted as part of the daily clinical practice, and so reviewing physicians were aware of the clinical history and findings in other concurrent or prior imaging studies. PET/CT findings were characterized as normal or abnormal/suspicious for malignancy. Maximum standardized uptake values (SUVs), normalized to patient body weight, were recorded using a three-dimensional tool placed over sites of abnormal FDG uptake. All findings and SUVs were catalogued. For the purpose of this retrospective analysis, we used an arbitrary SUV cutoff of 4.0 to define malignancy. (Note, however, that this particular threshold is not applied in daily clinical practice in our institution. Instead, PET/CT interpretation rests primarily on the visual assessment of findings, and SUV numbers are recorded for future reference.) A PET lesion was deemed positive if the SUV was ≥ 4 or if the staff physician characterized the lesion as suspicious for malignancy despite an SUV of less than 4. A PET lesion was deemed negative if the SUV was less than 4 or if the SUV was more than 4 but considered benign (eg, associated with bowel uptake or fractures).
Data Analysis
The first goal was to investigate the sensitivity and specificity of FDG-PET/CT in identifying metastatic lesions in patients with bladder cancer. Lesions recorded in FDG-PET/CT reports were catalogued and assessed further using histopathology from biopsies or serial subsequent imaging studies as the standard of reference. Biopsies were obtained at the discretion of the referring oncologist. Two types of correlations were performed, an organ-based analysis and a patient-based analysis. All FDG-PET/CT findings were classified as true positive, true negative, false positive, or false negative in both the organ-specific lesion analysis and the patient-based analysis.
A lesion was considered to be true positive if it was detected on PET/CT and subsequently confirmed to be cancerous by either biopsy or serial imaging with CT or MRI. A lesion seen on initial CT or MRI was considered a true negative if it was not detected on PET/CT and validated as benign by biopsy or serial imaging with CT or MRI. A finding was considered false positive if suspicious FDG uptake was described on PET/CT but the biopsy was negative or subsequent serial imaging studies did not show evidence for malignancy, such as increase in size. A lesion was considered a false negative if it was not detected by PET/CT but was initially seen on CT or MRI and subsequent imaging studies showed increase in size or if biopsy findings showed malignancy.
Patient-Based Analysis
The patient-based analysis was performed in a manner previously published for other studies.8 In brief, all lesions were classified as true positive, true negative, false positive, or false negative. In the event of a discordant finding, a true-positive lesion superseded all other lesions including false negative, true negative, and false positive. Therefore, if a patient had at least one true-positive lesion, the PET/CT scan was considered true positive. In the absence of a true-positive lesion, a false-negative lesion superseded a true-negative or a false-positive lesion. Therefore, if the PET/CT was false negative in at least one disease site, it was considered to be a false negative overall. This approach reflects the primary question in the management of these patients: Is recurrent/metastatic disease present?
Clinical Impact Analysis
NOPR questionnaires9 on intended patient management, completed by urologic oncologists in our institution, were collected before and after FDG-PET/CT to determine how the findings affected patient management. The pre–FDG-PET/CT survey collected information regarding the indication for the scan and the clinician's management plan if FDG-PET/CT was not available. The post–FDG-PET/CT survey collected information on the clinician's planned management with the available scan results and whether the FDG-PET/CT intervention avoided further testing. The management categories included observation, additional imaging, tissue biopsy, surgical treatment with curative intent, chemotherapy treatment with curative intent, chemotherapy treatment with palliative intent, radiation therapy, and supportive care. The data were collected prospectively, and consent was obtained from patients. The physicians' answers on the surveys were confirmed by medical record review. If a discrepancy was found between the planned and the actual treatment, only the actual treatment was included for analysis.
RESULTS
Fifty-seven patients (median age, 76 years) with urothelial cancer were included in this study (Table 1). Forty-seven of the 57 patients enrolled were evaluable for analysis. Ten patients did not have further imaging studies after the FDG-PET/CT and were excluded; four of these patients went on to hospice care, two patients died soon after the PET scan was obtained, and four patients returned to their primary physicians or were lost to follow-up. The study population involved patients with superficial (12%), muscle-invasive (44%), and metastatic (44%) disease. FDG-PET/CT was performed only when findings by standard diagnostic imaging were uncertain. The clinical indication for obtaining the FDG-PET/CT was restaging or suspected recurrence in 72% of patients, initial staging in 21%, and monitoring treatment with either chemotherapy or radiation therapy in the remaining 7%. Forty-seven percent of patients had received prior chemotherapy, and 18% had received prior radiation therapy. Eighty-five percent of findings considered suspicious for cancer on FDG-PET/CT had an SUV ≥ 4. In 15% of malignant sites, the SUV was less than 4, but the radiologist stated the lesion was suspicious for malignancy; these lesions had a mean SUV of 3.3. Ninety-seven percent of negative lesions (structural abnormalities that were noncancerous) had an SUV of less than 4; 3% had an SUV of more than 4 and were associated with bowel uptake or bone healing.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients (N = 57) | % |
|---|---|---|
| Age, years | ||
| Median | 76 | |
| Range | 54-91 | |
| Sex | ||
| Male | 38 | 67 |
| Female | 19 | 33 |
| Primary site | ||
| Bladder | 51 | 90 |
| Renal pelvis | 6 | 10 |
| Histology | ||
| TCC | 50 | 87 |
| SCC/adenocarcinoma/neuroendocrine | 7 | 13 |
| Stage* | ||
| 0cis/0a/1 | 7 | 12 |
| 2/3 | 25 | 44 |
| 4 | 25 | 44 |
| Prior treatment | ||
| Chemotherapy | 27 | 47 |
| Radiation therapy | 10 | 18 |
Abbreviations: TCC, transitional cell carcinoma; SCC, squamous cell carcinoma.
Stages are defined as follows: 0cis = carcinoma in situ; 0a = noninvasive papillary carcinoma; 1 = tumor invades subepithelial connective tissue; 2/3 = invasive tumor, muscle/perivesicular tissue; and 4 = metastatic disease.
One hundred fifty-three sites of suspicious FDG uptake were identified in the 47 evaluable patients. FDG-avid lesions were seen in lymph nodes, lung, bone, liver, soft tissue, adrenal, kidney, thyroid, and primary bladder sites. FDG-avid lesions in the thyroid and bladder were excluded from analysis. Bladder lesions were excluded because FDG is excreted in the bladder, making lesion assessment difficult. Thyroid lesions were excluded because every lesion detected in the thyroid was benign on follow-up ultrasound, and these findings were of little relevance to bladder cancer biology. Thus, the remaining 135 lesions were evaluable. Of these 135 lesions, 22 were verified by biopsy, and 113 were validated by serial imaging with CT or MRI within a mean of 3.5 months.
Organ-Based Analysis
Organ-based analysis was performed on 135 lesions in the 47 evaluable patients (Table 2). The predominant site of disease was lymph nodes (38%), followed by lung (23%), bone (17%), soft tissue (8%), liver (7%), adrenal (4%), and kidney (3%). For the organ-based analysis, the overall FDG-PET/CT sensitivity was 87% (95% CI, 76% to 94%), and specificity was 88% (95% CI, 78% to 95%).
Table 2.
Organ-Specific Lesion-Based Analysis of Suspicious FDG-PET/CT Uptake
| Site of Disease | Sites |
Sensitivity |
Specificity |
|||
|---|---|---|---|---|---|---|
| No. | % | % | 95% CI (%) | % | 95% CI (%) | |
| Lymph node | 51 | 38 | 92 | 74 to 99 | 81 | 61 to 93 |
| Lung | 31 | 23 | 88 | 62 to 98 | 87 | 60 to 98 |
| Bone | 22 | 17 | 93 | 66 to 100 | 100 | 63 to 100 |
| Liver | 10 | 7 | 50 | 12 to 88 | 100 | 40 to 100 |
| Soft tissue | 11 | 8 | 100 | 40 to 100 | 86 | 42 to 100 |
| Adrenal | 6 | 4 | 100 | 25 to 100 | 100 | 48 to 100 |
| Kidney | 4 | 3 | 100 | 3 to 100 | 100 | 29 to 100 |
| Total sites | 135 | 100 | 87 | 76 to 94 | 88 | 78 to 95 |
Abbreviations: FDG, fluorine-18 2-fluoro-2-deoxy-D-glucose; PET, positron emission tomography; CT, computed tomography.
Patient-Based Analysis
Study participants had evaluable PET/CT scans that were followed by either a biopsy (n = 22) or follow-up scan (n = 25). The sensitivity was 75% in those with a follow-up scan and 84% in those having a biopsy (Table 3). Malignant disease was correctly diagnosed in 25 of 31 patients, resulting in a sensitivity of 81% (95% CI, 63% to 93%). FDG-PET/CT was negative in 15 of 16 patients without malignant lesions for a specificity of 94% (95% CI, 71% to 100%). On the basis of these findings the positive predictive value is 96%, and the negative predictive value is 71%.
Table 3.
Patient-Based Analysis of Suspicious Lesions Reported by FDG-PET/CT
| Validation | No. of Lesions |
Sensitivity (%) | Specificity (%) | ||||
|---|---|---|---|---|---|---|---|
| Total | True Positive | False Positive | False Negative | True Negative | |||
| Follow-up scans | 25 | 9 | 1 | 3 | 12 | 75 | 92 |
| Biopsy | 22 | 16 | 0 | 3 | 3 | 84 | 100 |
| Total | 47 | 25 | 1 | 6 | 15 | 81 | 94 |
Abbreviations: FDG, fluorine-18 2-fluoro-2-deoxy-D-glucose; PET, positron emission tomography; CT, computed tomography.
Of the 12 patients who had a PET/CT performed for initial staging, 10 patients were evaluable with either a biopsy (n = 4) or a follow-up scan (n = 6). In this group of patients with early disease, there were five true-positive and five true-negative results.
Clinical Impact Analysis
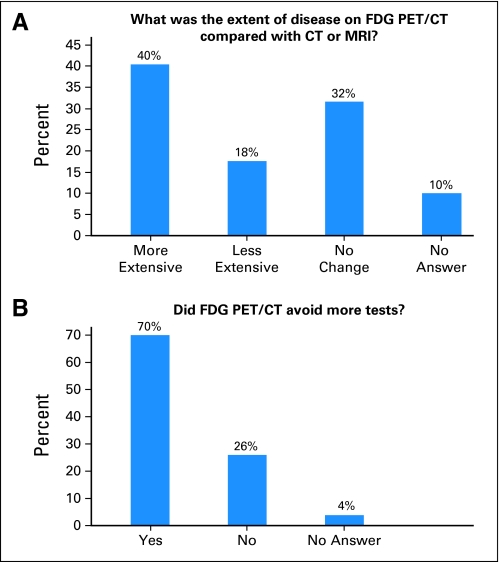
The pre- and post-PET questionnaires identified the impact of the FDG-PET/CT on clinical management in 57 patients. Fifty-three questionnaires were available for interpretation. More patients were included in this analysis because patients who had not received post–FDG-PET/CT imaging or biopsy, either because of death or hospice care, had questionnaires appropriate for inclusion. Surveyed physicians stated that more disease was found on FDG-PET/CT compared with standard CT or MRI in 40% of patients and less disease in 18% of patients. In 70% of patients, physicians stated that PET/CT avoided more tests (Fig 1). Most patients (n = 52) had a baseline CT, and a small number (n = 5) had an MRI. The additional test planned by physicians was an MRI in all but one patient, in whom a bone scan was planned.
Fig 1.
Physician answers (n =53) to National Oncology Positron Emission Tomography (PET) Registry questionnaires concerning extent of disease and the impact of fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) PET/computed tomography (CT) on clinical management. Responses were confirmed by medical record review. (A) Responses to extent of disease on FDG-PET/CT compared with CT or magnetic resonance imaging (MRI). (B) Responses to FDG-PET/CT and avoidance of additional tests.
On the basis of the FDG-PET/CT results, physicians reported a change in the treatment plan compared with before scanning in 36 patients (68%). Physicians reported that additional imaging tests were avoided with the use of FDG-PET/CT, and the need for biopsy was negated in 21% of patients, thus avoiding an invasive procedure. The potential biopsy sites in these patients included lymph nodes (n = 4), lung (n = 3), adrenal (n = 1), kidney (n = 1), liver (n = 1), and a pelvic mass (n = 1). In patients planned for treatment of organ-confined muscle-invasive disease, 19% were found to have metastatic disease on FDG-PET/CT and thus required systemic chemotherapy. In some instances, surveillance was changed to treatment, and local treatment with radiation therapy was changed to systemic chemotherapy for more advanced disease in one patient. In summary, 68% of patients had their treatment changed based on the findings on FDG-PET/CT (Table 4). Within the group with a change of treatment, eight patients had a biopsy, and one patient had additional imaging (MRI) because of the findings on PET/CT.
Table 4.
Patient Management Changes Based on FDG-PET/CT Results
| Physician Changes | No. of Patients (n = 53) | % |
|---|---|---|
| Biopsy eliminated | 11 | 21 |
| Additional imaging avoided | 11 | 21 |
| Organ-confined treatment changed to metastatic treatment | 10 | 19 |
| Surveillance changed to treatment | 3 | 6 |
| Local radiotherapy changed to chemotherapy | 1 | 2 |
| Total management changes | 36 | 68* |
Abbreviations: FDG, fluorine-18 2-fluoro-2-deoxy-D-glucose; PET, positron emission tomography; CT, computed tomography.
The total management change with the imaging-adjusted impact is 47% (see Results).
Including patients for whom the plan before PET/CT was another type of imaging (eg, CT or MRI) may have overestimated the impact of PET/CT on the change in patient management. It is possible that if the original imaging test had been performed, then this would have led to the same management strategy. Previous authors have described an imaging-adjusted impact10 to try and account for this concern. Applying the imaging-adjusted impact to our results excludes 11 patients and changes the percentage of patients who had their management changed based on the findings on FDG-PET/CT from 68% to 47%.
The benefit in the change of management caused by FDG-PET/CT was assessed by medical record review. The change in management was appropriate in 34 of 36 patients on follow-up. In two patients, the FDG-PET/CT was a false negative and missed metastatic disease; this led to attempted curative surgery in one patient and a delay in chemotherapy for recurrent disease in another.
DISCUSSION
In this study, FDG-PET/CT showed excellent sensitivity and specificity in the detection of metastases in patients with advanced disease. This approach reflects daily practice in a busy cancer center and may thus be relevant for routine patient management. The majority of patients in this study were undergoing restaging or evaluation for suspected recurrence, a clinical scenario that was ideal for the use of FDG-PET/CT because these patients represent approximately 25% of all patients with bladder cancer but have the highest likelihood of metastases. The sensitivity and specificity of FDG-PET/CT in this disease were similar to the sensitivity and specificity in other epithelial malignancies, such as non–small-cell lung cancer.11
In total, over the last 13 years, eight studies have addressed the use of FDG-PET in 300 patients with bladder cancer (Table 5).7,8,12–17 FDG-PET, rather than FDG-PET/CT, was frequently used, thereby reducing the reliability of the scan and undermining the utility of this type of imaging. This body of literature shows wide variability insensitivity and specificity. Meaningful interpretation of these studies is also limited by the large number of patients for whom FDG-PET was performed in the routine preoperative setting.
Table 5.
Previous FDG-PET Studies in Patients With Bladder Cancer
| Study | Year of Publication | No. of Patients | Prior Therapy (%) | PET (%) | PET/CT (%) | Sensitivity (%) | Specificity (%) | Validation (%) |
|
|---|---|---|---|---|---|---|---|---|---|
| Pathology | Imaging | ||||||||
| Kosuda et al12 | 1997 | 12 | 0 | 100 | 0 | NR | NR | 75 | 25 |
| Heicappell et al13 | 1999 | 8 | 0 | 100 | 0 | NR | NR | 100 | 0 |
| Bachor et al14 | 1999 | 64 | 0 | 100 | 0 | 67 | 86 | 100 | 0 |
| Drieskens et al8 | 2005 | 40 | 0 | 0 | 100 | 60 | 88 | 70 | 30 |
| Liu et al15 | 2006 | 46 | 22 | 100 | 0 | 77 | 97 | 37 | 63 |
| Jadvar et al16 | 2008 | 35 | 69 | 49 | 51 | NR | NR | 4 | 96 |
| Swinnen et al17 | 2009 | 51 | 0 | 0 | 100 | 46 | 97 | 100 | 0 |
| Kibel et al7 | 2009 | 43 | 0 | 0 | 100 | 70 | 94 | 100 | 0 |
Abbreviations: FDG, fluorine-18 2-fluoro-2-deoxy-D-glucose; PET, positron emission tomography; CT, computed tomography; NR, not reported.
Patients with muscle-invasive disease and a normal CT before cystectomy have an approximately 25% chance of lymph node metastasis; in most cases, these lymph node metastases are microscopic. The NOPR allows physicians to order FDG-PET/CT when there is a reasonable risk of nodal involvement or suspicion for lymph node involvement on CT scan. In this study, 10 patients fell into this category, with organ-confined disease diagnosed on CT but more extensive disease noted by FDG-PET/CT. These patients were planned for curative treatment with radical cystectomy but had their surgery cancelled and instead were treated with systemic chemotherapy for more advanced disease as a result of the findings on FDG-PET/CT.
This study includes few patients with superficial disease. Prior literature does not support routine PET/CT in this setting because nodal involvement or metastatic disease to other sites is so rare in superficial disease that FDG-PET/CT would not offer a benefit.
A unique feature in this study is the NOPR questionnaire used to determine physicians' assessment of the clinical utility for PET/CT. Thirty-six of the 57 patients in this study were deemed by treating physicians to have derived direct benefit from FDG-PET/CT over that achievable with routine MRI or CT. Physicians reported that 11 biopsies were avoided. Although pathologic confirmation of suspected metastatic disease remains the gold standard, biopsy is not always possible in this disease because of the risk involved with lesions deep in the pelvis near vascular structures, a medical contraindication to biopsy such as anticoagulation, or patient refusal. In these instances, FDG-PET/CT may serve as an acceptable substitute to assess the extent of disease and to direct treatment.
On the basis of these findings, a cost analysis of FDG-PET/CT in bladder cancer is warranted because FDG-PET/CT could make it possible to avoid further testing, unnecessary invasive procedures, or inadequate therapy, as has been observed in other malignancies.18 Costs can be contained if an FDG-PET/CT is performed only when the findings with standard diagnostic imaging are uncertain, as was the case in this study.19 Although FDG-PET/CT is a high-cost diagnostic study, further testing and/or invasive procedures such as curative surgery in a patient with metastatic disease are potentially more expensive and, more importantly, wrong management.
A limitation of FDG-PET/CT is the detection of disease in lesions less than 1 cm. Eight of the patients had lesions less than 1 cm (four lesions were 9 mm, and four lesions ranged from 5 to 8 mm). A study focusing on small lesions is needed to examine the accuracy of PET in lesions less than 1 cm.
In practice, there is no specific SUV number that distinguishes between benign and malignant FDG uptake. Various authors have suggested numbers ranging from 2.5 to 5.0.20–22 In this study, the SUV value of ≥ 4 was chosen arbitrarily as the cutoff point for a malignant finding, well within the ranges reported in other studies.
Selection bias may have occurred because only patients with a likelihood for recurrent/metastatic disease were referred for a PET/CT scan. However, this reflects appropriate patient management, in that scans are not ordered widely and unselectively but only if and when there is clinical suspicion or abnormality on standard cross-sectional imaging tests such as CT or MRI.
Another potential limitation of this study is the relatively small number of patients. Nevertheless, this is one of the larger series conducted during the past decade in the use of FDG-PET/CT in patients with bladder cancer.
In summary, this study demonstrated that FDG-PET/CT accurately detects metastatic disease and can be used as a clinical tool in advanced bladder cancer. FDG-PET is already approved for use in nine other malignancies (ie, breast, cervical, colorectal, esophageal, head and neck, non–small-cell lung, and thyroid cancer, lymphoma, and melanoma). We found the accuracy of FDG-PET/CT comparable to that in other malignancies for which it has already been approved.23 On the basis of the results of this study, further research of FDG-PET/CT as a management tool is warranted in bladder cancer.
Acknowledgment
In memory of Mary (Geri) Boyle. We thank Carol Pearce, MFA, Memorial Sloan-Kettering Cancer Center Department of Medicine, for her editorial review of this article.
Footnotes
Supported by National Institutes of Health Training Grant No. T-32CA09207, Bethesda, MD, and The Zena and Michael A. Wiener Research and Therapeutic Program in Bladder Cancer.
Presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium, February 26-28, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Andrea B. Apolo, Dean F. Bajorin
Administrative support: Jamie Riches, Dean F. Bajorin
Provision of study materials or patients: Andrea B. Apolo, Matthew I. Milowsky, Dean F. Bajorin
Collection and assembly of data: Andrea B. Apolo, Jamie Riches, Heiko Schöder, Oguz Akin, Alisa Trout, Matthew I. Milowsky, Dean F. Bajorin
Data analysis and interpretation: Andrea B. Apolo, Jamie Riches, Heiko Schöder, Oguz Akin, Alisa Trout, Matthew I. Milowsky, Dean F. Bajorin
Manuscript writing: Andrea B. Apolo, Jamie Riches, Heiko Schöder, Oguz Akin, Alisa Trout, Matthew I. Milowsky, Dean F. Bajorin
Final approval of manuscript: Andrea B. Apolo, Jamie Riches, Heiko Schöder, Oguz Akin, Alisa Trout, Matthew I. Milowsky, Dean F. Bajorin
REFERENCES
- 1.Yaman O, Baltaci S, Arikan N, et al. Staging with computed tomography, transrectal ultrasonography and transurethral resection of bladder tumour: Comparison with final pathological stage in invasive bladder carcinoma. Br J Urol. 1996;78:197–200. doi: 10.1046/j.1464-410x.1996.01008.x. [DOI] [PubMed] [Google Scholar]
- 2.Paik ML, Scolieri MJ, Brown SL, et al. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000;163:1693–1696. [PubMed] [Google Scholar]
- 3.Buy JN, Moss AA, Guinet C, et al. MR staging of bladder carcinoma: Correlation with pathologic findings. Radiology. 1988;169:695–700. doi: 10.1148/radiology.169.3.3186994. [DOI] [PubMed] [Google Scholar]
- 4.Czernin J, Phelps ME. Positron emission tomography scanning: Current and future applications. Annu Rev Med. 2002;53:89–112. doi: 10.1146/annurev.med.53.082901.104028. [DOI] [PubMed] [Google Scholar]
- 5.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305–332. doi: 10.1148/radiol.2312021185. [DOI] [PubMed] [Google Scholar]
- 6.Podoloff DA, Ball DW, Ben-Josef E, et al. NCCN task force: Clinical utility of PET in a variety of tumor types. J Natl Compr Canc Netw. 2009;7(suppl 2):S1–S26. doi: 10.6004/jnccn.2009.0075. [DOI] [PubMed] [Google Scholar]
- 7.Kibel AS, Dehdashti F, Katz MD, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27:4314–4320. doi: 10.1200/JCO.2008.20.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drieskens O, Oyen R, Van Poppel H, et al. FDG-PET for preoperative staging of bladder cancer. Eur J Nucl Med Mol Imaging. 2005;32:1412–1417. doi: 10.1007/s00259-005-1886-9. [DOI] [PubMed] [Google Scholar]
- 9.Hillner BE, Siegel BA, Liu D, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: Initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155–2161. doi: 10.1200/JCO.2007.14.5631. [DOI] [PubMed] [Google Scholar]
- 10.Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: Findings of the national oncologic PET registry. J Nucl Med. 2008;49:1928–1935. doi: 10.2967/jnumed.108.056713. [DOI] [PubMed] [Google Scholar]
- 11.Hoekstra CJ, Stroobants SG, Smit EF, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:8362–8370. doi: 10.1200/JCO.2005.01.1189. [DOI] [PubMed] [Google Scholar]
- 12.Kosuda S, Kison PV, Greenough R, et al. Preliminary assessment of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with bladder cancer. Eur J Nucl Med. 1997;24:615–620. doi: 10.1007/BF00841398. [DOI] [PubMed] [Google Scholar]
- 13.Heicappell R, Müller-Mattheis V, Reinhardt M, et al. Staging of pelvic lymph nodes in neoplasms of the bladder and prostate by positron emission tomography with 2-[(18)F]-2-deoxy-D-glucose. Eur Urol. 1999;36:582–587. doi: 10.1159/000020052. [DOI] [PubMed] [Google Scholar]
- 14.Bachor R, Kotzerke J, Reske SN, et al. Lymph node staging of bladder neck carcinoma with positron emission tomography. Urologe A. 1999;38:46–50. doi: 10.1007/s001200050244. [DOI] [PubMed] [Google Scholar]
- 15.Liu IJ, Lai YH, Espiritu JI, et al. Evaluation of fluorodeoxyglucose positron emission tomography imaging in metastatic transitional cell carcinoma with and without prior chemotherapy. Urol Int. 2006;77:69–75. doi: 10.1159/000092937. [DOI] [PubMed] [Google Scholar]
- 16.Jadvar H, Quan V, Henderson RW, et al. [F-18]-Fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol. 2008;13:42–47. doi: 10.1007/s10147-007-0720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swinnen G, Maes A, Pottel H, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol. doi: 10.1016/j.eururo.2009.05.014. [epub ahead of print on May 18, 2009] [DOI] [PubMed] [Google Scholar]
- 18.Moulin-Romsee G, Spaepen K, Stroobants S, et al. Non-Hodgkin lymphoma: Retrospective study on the cost-effectiveness of early treatment response assessment by FDG-PET. Eur J Nucl Med Mol Imaging. 2008;35:1074–1080. doi: 10.1007/s00259-007-0690-0. [DOI] [PubMed] [Google Scholar]
- 19.Yen RF, Yen MF, Hong RL, et al. The cost-utility analysis of 18-fluoro-2-deoxyglucose positron emission tomography in the diagnosis of recurrent nasopharyngeal carcinoma. Acad Radiol. 2009;16:54–60. doi: 10.1016/j.acra.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Beggs AD, Hain SF, Curran KM, et al. FDG-PET as a “metabolic biopsy” tool in non-lung lesions with indeterminate biopsy. Eur J Nucl Med Mol Imaging. 2002;29:542–546. doi: 10.1007/s00259-001-0736-7. [DOI] [PubMed] [Google Scholar]
- 21.Stephens AW, Gonin R, Hutchins GD, et al. Positron emission tomography evaluation of residual radiographic abnormalities in postchemotherapy germ cell tumor patients. J Clin Oncol. 1996;14:1637–1641. doi: 10.1200/JCO.1996.14.5.1637. [DOI] [PubMed] [Google Scholar]
- 22.De Santis M, Becherer A, Bokemeyer C, et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: An update of the prospective multicentric SEMPET trial. J Clin Oncol. 2004;22:1034–1039. doi: 10.1200/JCO.2004.07.188. [DOI] [PubMed] [Google Scholar]
- 23.Gambhir SS, Czernin J, Schwimmer J, et al. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42(suppl):1S–93S. [PubMed] [Google Scholar]