Abstract
Purpose
To assess the role of the recombinant bacterial enzyme, glucarpidase (carboxypeptidase-G2), leucovorin, and thymidine in the management and outcome of patients with high-dose methotrexate (HDMTX) –induced nephrotoxicity.
Methods
Patients with HDMTX-induced nephrotoxicity received one to three doses of intravenous (IV) glucarpidase and leucovorin rescue. The initial cohort (n = 35) also received thymidine by continuous IV infusion. Subsequently, thymidine was restricted to patients with prolonged exposure (> 96 hours) to methotrexate (MTX) or with substantial MTX toxicity at study entry. Plasma MTX, leucovorin, and 5-methyltetrahydrofolate (5-mTHF) concentrations were measured pre- and postglucarpidase. Toxicities were monitored, and logistic regression analysis was used to assess the relationship of baseline characteristics to the development of severe toxicity and death.
Results
Glucarpidase was administered at a median of 96 hours (receiving thymidine, n = 44) and 66 hours (not receiving thymidine, n = 56) after the start of the MTX infusion. Plasma MTX concentrations decreased within 15 minutes of glucarpidase by 98.7%. Plasma 5-mTHF concentrations also decreased more than 98% after administration of glucarpidase. Of 12 deaths, six were directly attributed to irreversible MTX toxicity. Presence of grade 4 toxicity before administration of glucarpidase, inadequate initial increase in leucovorin dosing, and administration of glucarpidase more than 96 hours after the start of the MTX infusion were associated with development of grade 4 and 5 toxicity.
Conclusion
Early intervention with the combination of leucovorin and glucarpidase is highly effective in patients who develop HDMTX-induced renal dysfunction. Severe toxicity and mortality occurred in patients in whom glucarpidase rescue was delayed and occurred despite thymidine administration.
INTRODUCTION
High-dose methotrexate (HDMTX) –induced renal dysfunction leads to delayed renal excretion of methotrexate (MTX) and sustained elevated plasma MTX concentrations that can markedly enhance the toxicities of MTX. Pharmacologic interventions to treat this emergency include administration of high- dose leucovorin,1,2 glucarpidase (carboxypeptidase-G2),3–5 or thymidine.5,6 We previously reported our experience with 21 patients with HDMTX-induced nephrotoxicity treated with glucarpidase, which rapidly hydrolyzes MTX into inactive metabolites,5,7 a finding that was confirmed in two European studies3,4 and multiple case reports.8–20
We initially administered thymidine, an investigational agent that effectively circumvents MTX toxicity in patients with normal renal function,6 with glucarpidase and leucovorin, because thymidine does not compete with MTX for transport into cells and because leucovorin and its metabolites are also substrates for glucarpidase. We now report our experience with the use of glucarpidase and leucovorin with and without thymidine in patients who develop HDMTX-induced renal dysfunction and include an analysis of factors associated with the development of severe (grade 4) or fatal toxicity in a series of 100 consecutive patients. The initial 21 patients from our prior report5,7 are included to allow for more meaningful analysis of toxicities experienced with and without thymidine. In addition, the effect of glucarpidase on plasma concentrations of MTX, leucovorin, and leucovorin's active metabolite, 5-methyltetrahydrofolate (5-mTHF), are described.
METHODS
Patient Eligibility
Patients with MTX-induced nephrotoxicity and delayed MTX excretion were eligible for treatment with glucarpidase if either of the following two conditions were met: plasma MTX concentration was ≥ 10 μmol/L ≥ 42 hours after the start of HDMTX infusion; or serum creatinine was ≥ 1.5 times the upper limit of normal or creatinine clearance was ≤ 60 mL/min/m2 and the plasma MTX concentration was ≥ 2 standard deviations above the mean ≥ 12 hours after MTX administration.
Emergency review of this single-patient, special-exception protocol was performed by local institutional review boards, and written informed consent was obtained from the patient or guardian before treatment.
Glucarpidase, Thymidine, and Leucovorin Administration
Recombinant glucarpidase (Centre for Applied Microbiology and Research, Porton Down, Salisbury, England) and thymidine were provided by the Cancer Therapy Evaluation Program of the National Cancer Institute and shipped by express carrier to the treating institution.
After reconstitution of glucarpidase, a single dose, two doses 24 hours apart, or three doses every 4 hours of 50 U/kg/dose were administered intravenously for 5 minutes. Different dosage schedules were used to evaluate if second and third doses could achieve additional decreases in plasma MTX concentration. A delayed dose of glucarpidase could be administered to patients who experienced a decrease of ≥ 90% in plasma MTX concentration with the initial dose(s) of glucarpidase but who had persistent plasma MTX concentration ≥ 1 μmol/L.
Thymidine (8 g/m2/d) was administered as continuous intravenous (IV) infusion for ≥ 48 hours after the last dose of glucarpidase and until the plasma MTX concentration was less than 1 μmol/L combined with leucovorin and glucarpidase in the first 35 patients treated. Subsequently, thymidine was restricted to patients with prolonged exposure to MTX (> 96 hours after the start of infusion) before glucarpidase administration or with substantial MTX toxicity, such as grade ≥ 3 mucositis, platelets ≤ 50,000/μL, or neutrophils ≤ 900/μL at the time of the request for glucarpidase.
Leucovorin was administered at a dose of 1 g/m2 IV every 6 hours or per institutional guidelines before administration of glucarpidase and at a dose of 250 mg/m2 IV every 6 hours for 48 hours after administration of the last dose of glucarpidase. The dose and duration of subsequent leucovorin rescue was based on plasma MTX concentration1,21,22 determined by reverse phase high-performance liquid chromatography (patients 1 to 83), or by commercial MTX assay (patients 84 to 100). Pharmacokinetic results in this article only include patients 1 to 83, as analyses with commercially available assays after glucarpidase result in overestimation of MTX as a result of 4-deoxy-4-amino-N10-methylpteroic acid (DAMPA), an interfering metabolite.
Pharmacokinetics
Blood samples for MTX and DAMPA were obtained immediately before administration of glucarpidase as well as 15, 30, 60, and 120 minutes after administration of glucarpidase; samples were then obtained once daily, before repeat doses of glucarpidase, and 60 minutes after repeat doses of glucarpidase and quantified as described.5,7 The elimination half-lives of MTX (after glucarpidase effect) and DAMPA were estimated using least squares regression analysis. Light-protected, ascorbic acid–containing plasma samples were obtained in a subset of patients for quantification of leucovorin and 5-mTHF pre- and postglucarpidase using high-performance liquid chromatography. After solid-phase extraction using C18 cartridges, samples were injected onto a 5-μm, C18 Nova-Pak analytic column (Waters Association, Milford, MA) with a C18 5-μm guard column and eluted at a flow rate of 1.5 mL/min with a linear gradient for 30 minutes from 100% 0.1 M sodium phosphate (pH 6.0) to 80% 0.1 M sodium phosphate (pH 6.0):20% methanol (v:v). Eluant was monitored at wavelengths of 303 and 313 nm using a Waters 996 photodiode array detector (Waters, Milford, MA). Retention times for leucovorin and 5-mTHF were 16.5 and 21 minutes, respectively, and the lower limit of quantification for leucovorin and 5-mTHF was 0.1 μmol/L.
Glucarpidase Antibody Assay
Plasma samples for quantification of anti–glucarpidase antibodies were obtained 3, 7, and 14 days after the first glucarpidase dose and analyzed as described.23,24
Toxicity Grading and Analysis
Treatment-related toxicities were graded using National Cancer Institute Common Toxicity Criteria version 2.0. Because of differences in dose and schedule of MTX and the concomitant administration of other cytotoxic agents to some patients with diagnoses other than osteosarcoma, hematologic toxicity was evaluated separately for patients with osteosarcoma and those with other cancers.
Analysis of Characteristics Associated With Outcome
Single-factor logistic regression analysis was initially used to screen for the relationship of baseline characteristics (Table 1) to the development of grade ≥ 4 toxicity postglucarpidase. On the basis of this initial screening, multiple-factor logistic regression analysis was performed including all variables that were statistically significant (P ≤ .10) in the univariate analysis. Parameters with perfect association with outcome could not be included. The final model was determined using forward and backward selection of variables. Statistical analyses were performed using Stata version 9.0 (Stata, College Station, TX).
Table 1.
Baseline Characteristics Included in the Single-Factor Logistic Regression Analysis to Assess the Relationship to the Development of Grade 4 Toxicity and Death
| Characteristic | No. of Patients (n = 100) | |
|---|---|---|
| Age, years | ||
| Median | 17 | |
| Range | ≤ 1-82 | |
| Sex | ||
| Male | 63 | |
| Female | 37 | |
| Diagnosis | ||
| Osteosarcoma | 42 | |
| Other | 58 | |
| Thymidine administration | ||
| No | 56 | |
| Yes | 44 | |
| MTX dose, g/m2 | ||
| Median | 7.7 | |
| Range | 0.4-12 | |
| Infusion duration, hours | ||
| Median | 4 | |
| Range | 1-36 | |
| Plasma MTX at 36-48 hours, μM | ||
| < 50 | 45 | |
| ≥ 50 | 52 | |
| Unavailable | 3 | |
| Plasma MTX prior to glucarpidase, μM | ||
| Median | 17 | |
| Range | 0.37-849 | |
| Serum creatinine, mg/dL | ||
| Median | 3.1 | |
| Range | 0.8-10.2 | |
| Diuretic use | ||
| No | 19 | |
| Yes | 65 | |
| Unavailable | 16 | |
| Dialysis use | ||
| No | 67 | |
| Yes | 27 | |
| Unavailable | 6 | |
| Appropriate leucovorin increase* | ||
| No | 12 | |
| Yes | 86 | |
| Unavailable | 2 | |
| Grade 4 toxicity† prior to glucarpidase | ||
| No | 81 | |
| Yes | 14 | |
| Unavailable | 5 | |
| Glucarpidase doses | ||
| 1 | 62 | |
| 2 every 24 hours | 27 | |
| 3 every 4 hours | 5 | |
| Delayed dose | 6 | |
| Initial plus delayed glucarpidase dose (hours after start of MTX) | ||
| ≤ 96 | 71 | |
| > 96 | 27 | |
| Unavailable | 2 |
Abbreviation: MTX, methotrexate.
Increased leucovorin dose to 100-1,000 mg/m2 every 3 to 6 hours within 3 days of the start of the MTX infusion.
Grade 4 toxicity defined as mucositis, neutropenia, febrile neutropenia/sepsis, skin toxicity, diarrhea, and nausea/vomiting.
RESULTS
One hundred patients (median age, 17 years; range, 0.3 to 82 years) from institutions within and outside of the United States were consecutively enrolled onto our glucarpidase protocol during a seven-year period (Table 2). Completed case report forms were returned for 93 patients. Of the seven remaining patients, detailed summaries and laboratory data were submitted for four patients, and survival outcome was determined for all patients. The initial 35, as well as nine of the subsequent, patients received glucarpidase, leucovorin, and thymidine, and 56 patients received leucovorin and glucarpidase without thymidine.
Table 2.
Baseline Characteristics of 100 Patients Who Received Glucarpidase and Leucovorin With or Without Thymidine
| Characteristic | All Patients |
Patients Categorized by Diagnosis |
||||||
|---|---|---|---|---|---|---|---|---|
| Osteosarcoma |
NHL/ALL |
Other* | ||||||
| Thd+ | Thd− | Thd+ | Thd− | Thd+ | Thd− | Thd+ | Thd− | |
| No. of patients | 44 | 56 | 19 | 23 | 20 | 29 | 5 | 4 |
| Age, years | ||||||||
| Median | 18 | 15 | 15 | 14 | 39 | 17 | 60 | 20 |
| Range | 0.3-82 | 2.5-79 | 11-48 | 7-53 | 0.3-82 | 3-79 | 40-79 | 26-72 |
| Sex | ||||||||
| Female | 17 | 20 | 10 | 13 | 5 | 5 | 2 | 2 |
| Male | 27 | 36 | 9 | 10 | 15 | 24 | 3 | 2 |
| MTX dosing | ||||||||
| Dose, g/m2 | ||||||||
| Median | 7.4 | 8 | 12 | 12 | 4.3 | 5 | 2.9 | 7.3 |
| Range | 0.0025†-12 | 1-12 | 8-12 | 8-12 | 0.4-9.0 | 1-8.4 | 0.0025†-3 | 6.6-12 |
| Infusion duration, hours | ||||||||
| Median | 4 | 4 | 4 | 4 | 24 | 24 | 2.5 | 4 |
| Range | 1-24 | 2-36 | 4-6 | 4-6 | 1-24 | 2-36 | 1-24 | 3-6 |
| Course in which toxicity occurred | ||||||||
| Median | 2 | 1 | 2 | 1 | 1 | 1.5 | 2 | 1 |
| Range | 1-10 | 1-19 | 1-10 | 1-5 | 1-3 | 1-19 | 1-3 | |
| Plasma MTX, μM | ||||||||
| Study entry‡ | ||||||||
| Median | 53 | 51.3 | 480 | 199 | 9.8 | 14 | 51.5 | 57 |
| Range | 0.1-1,290 | 2.0-5,800 | 7.9-1,290 | 12.2-5,800 | 1.9-781 | 2.0-662 | 0.1-79 | 39-96 |
| Preglucarpidase‡ | ||||||||
| Median | 28.2 | 12.3 | 89.1 | 64.8 | 5.3 | 7.0 | 32.1 | 12.3 |
| Range | < 0.37-849§ | 0.8-835§ | 1.0-849 | 4.2-835 | 0.37-321 | 0.8-662 | 0.1-44.8 | 8.7-91 |
| Time from start of MTX infusion, hours | ||||||||
| Study entry‡ | ||||||||
| Median | 48 | 46 | 44 | 38 | 64 | 48 | 123 | 56 |
| Range | 12-292 | 4-120 | 12-192 | 4-101 | 29-192 | 24-120 | 24-192 | 36-96 |
| Preglucarpidase§ | ||||||||
| Median | 96 | 66 | 64 | 57 | 119 | 68 | 144 | 67 |
| Range | 22-294 | 22-192∥ | 22-242 | 22-144∥ | 47-294 | 20-192∥ | 37-216 | 46-84 |
| Serum creatinine, mg/dL | ||||||||
| Prior to MTX infusion | ||||||||
| Median | 0.8 | 0.7 | 0.7 | 0.6 | 0.9 | 0.8 | 1.1 | 0.9 |
| Range | 0.2-4.8 | 0.3-3.7 | 0.5-4.8 | 0.4-2.3 | 0.2-1.7 | 0.3-3.7 | 0.6-1.7 | 0.7-1.0 |
| Study entry‡ | ||||||||
| Median | 4.0 | 2.8 | 3.6 | 2.4 | 4.1 | 2.6 | 4.7 | 2.9 |
| Range | 0.6-11.8 | 1.1-6.1 | 1.2-5.9 | 1.3-5.0 | 0.6-8.6 | 1.1-6.1 | 3.3-11.8 | 1.7-3.9 |
Abbreviations: NHL, non-Hodgkin's lymphoma; ALL, acute lymphoblastic leukemia; Thd+, received thymidine rescue; Thd−, did not receive thymidine rescue; MTX, methotrexate.
Other diagnoses included gastric cancer (n = 3), sarcoma (n = 2), breast cancer (n = 1), rheumatoid arthritis (n = 1), Hodgkin's lymphoma (n = 1), acute myeloid leukemia (n = 1).
One patient with rheumatoid arthritis received 2.5 mg MTX three times per week.
Study entry defined as when National Cancer Institute was contacted to obtain glucarpidase.
Only plasma MTX concentrations immediately before glucarpidase are shown determined by high-performance liquid chromatography for 39 patients receiving thymidine and 36 patients not receiving thymidine.
One patient received delayed glucarpidase at hour 144, and another received it at hour 192.
Glucarpidase was administered at a median of 96 hours (range, 22 to 294 hours) after the start of the MTX infusion to patients receiving thymidine and a median of 66 hours (range, 22 to 192 hours) to patients not receiving thymidine. Sixty-five patients received one glucarpidase dose, 28 received two glucarpidase doses 24 hours apart, and seven received three glucarpidase doses every 4 hours. Six of the patients who received a single planned dose also received a delayed (> 24 hours) glucarpidase dose.
Glucarpidase
Glucarpidase was well tolerated. Transient grade 1 adverse events with possible attribution to glucarpidase in seven patients were flushing (n = 2), a feeling of warmth (n = 2), tingling fingers (n = 1), head pressure (n = 1), numbness and burning sensation around the penis (n = 1), minimal burning of face and extremities (n = 1), shaking (n = 1), neck rash 1 day after glucarpidase (n = 1), and erythema and pruritus of face and legs (n = 1). One additional patient receiving warfarin (Coumadin; Bristol-Myers Squibb, New York, NY) experienced a transient increase in prothrombin time from grade 1 to grade 3. Plasma antibody to glucarpidase could not be detected in 28 evaluable patients.
MTX
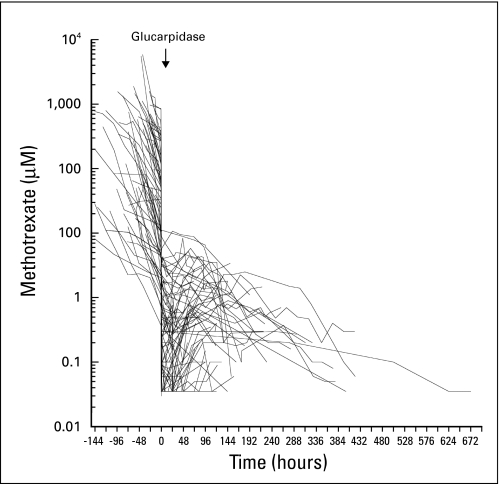
Median plasma MTX concentration preglucarpidase was 28.2 μmol/L (range, 0.37 to 849 μmol/L) in patients receiving thymidine (n = 39) and 12.3 μmol/L (range, 0.76 to 835 μmol/L) in patients not receiving thymidine (n = 36). Plasma MTX concentration decreased within 15 minutes after the first glucarpidase dose by 98.7% (range, 84% to 99.2%) in patients receiving thymidine and by 98.8% (range, 86.1% to 99.5%) in patients not receiving thymidine (Fig 1). Subsequent scheduled second and third glucarpidase doses did not result in additional decreases in plasma MTX concentration, and only two of six patients who received a delayed glucarpidase dose experienced a ≥ 50% decrease in MTX concentration. A small rebound in MTX concentration was observed in 30 of 75 evaluable patients (Fig 2). For the postglucarpidase residual MTX, the median terminal elimination half-life of elimination (n = 16) was more than 72 hours (range, 17.6 to > 72 hours), and the median terminal elimination half-life of DAMPA (n = 23) was 5.5 hours (range, 0.7 to > 72 hours).
Fig 1.
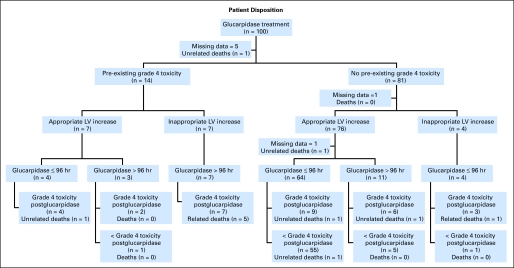
Disposition of 100 patients who received glucarpidase, indicating pre-existence or absence of grade 4 toxicity before administration of glucarpidase; appropriate or inappropriate increase in leucovorin (LV) rescue within 3 days after the start of high-dose methotrexate (HDMTX); timing of administration of glucarpidase at ≤ 96 hours or more than 96 hours after starting HDMTX; presence of grade 4 toxicity after the administration of glucarpidase; and death directly attributed or not directly attributed to MTX toxicity.
Fig 2.
Plasma methotrexate (MTX) concentration/time profiles for 75 patients who had plasma MTX concentration determined by high-performance liquid chromatography. Lines are point to point, and symbols indicating individual measurements are not included. Glucarpidase administration was at hour 0. Plasma MTX concentration before administration of glucarpidase was determined at local institutions with commercially available assays.
Leucovorin and 5-mTHF
In 16 patients studied, the median plasma 5-mTHF and leucovorin concentrations before administration of glucarpidase were 14.1 μM (range, 1.2 to 97 μM) and 354 μM (range, 80.2 to 926.3 μM), respectively. 5-mTHF, an active metabolite of leucovorin, was efficiently hydrolyzed by glucarpidase with a median decrease from baseline of 98.6% (range, 75% to 99.9%) 15 minutes after glucarpidase was administered. Plasma leucovorin concentration, which primarily represents the inactive d-isomer, decreased by a median of 17.7% (range, 0% to 62.4%). Leucovorin was administered for a median of 15 days (range, 4 to 36 days; information missing, n = 26).
Thymidine
Reversible toxicities possibly attributable to thymidine were grade 1 somnolence, tremulousness, and light-headedness (n = 1); grade 2 to 3 confusion for two days (n = 1); and grade 2 to 3 neurocortical toxicity on days 10 and 11 of thymidine (n = 1).
Recovery of Renal Function
Serum creatinine peaked at a median of 4 days (range, 1 to 13 days) after HDMTX administration, and recovery of renal function—defined as serum creatinine within normal limits—occurred at a median of 22 days (range, 5 to 77 days) in 70 patients (no recovery as a result of death, n = 12; additional nephrotoxic chemotherapy before renal recovery, n = 2; recovery by glomerular filtration rate, n = 3; insufficient data, n = 13). The median maximum serum creatinine was 4.0 mg/dL (range, 0.8 to 12.7 mg/dL). Sixty-five patients received diuretics, and 27 patients underwent hemodialysis or hemoperfusion for oliguria, for metabolic reasons, or to decrease plasma MTX before administration of glucarpidase. Of 88 surviving patients, 12 patients received additional HDMTX after recovery of renal function with no reported toxicity.
Treatment-Related Toxicity
All patients with osteosarcoma (n = 42) and 18 patients with acute lymphoblastic leukemia or non-Hodgkin's lymphoma received MTX as single agent. Additional cytotoxic chemotherapy was administered to 14 patients (missing information n = 26) within 2 weeks of MTX administration, and in these patients, toxicities could not be solely attributed to MTX.
Four patients had grade 4 neutropenia (absolute neutrophil count ≤ 500/μL) before HDMTX administration. Before administration of glucarpidase, 14 patients experienced grade 4 toxicity, including neutropenia (n = 10), mucositis (n = 7), febrile neutropenia (n = 7), nausea/vomiting (n = 3), thrombocytopenia (n = 2), encephalopathy (n = 1), and pulmonary (n = 1).
After administration of glucarpidase, 34 patients experienced grade 4 toxicity, including neutropenia (n = 29), mucositis (n = 15), febrile neutropenia (n = 12), nausea/vomiting (n = 4), skin (n = 2), pulmonary (n = 1), and seizure (n = 1). Reversible grade 4 elevation in hepatic transaminases (ALT) was observed in 18 patients, and grade 4 increase in bilirubin was observed in nine patients. Hematologic toxicity was not severe in most cases, but 31 patients received filgrastim at some point (no filgrastim, n = 41; missing information, n = 28). In patients with osteosarcoma, there were no statistically significant differences in pretreatment and nadir absolute neutrophil count and the pretreatment and nadir platelet counts between patients receiving and not receiving thymidine (data not shown).
There were 12 patient deaths (median age, 41 years; range, 12 to 79 years), six of which were considered directly related to MTX toxicity. These patients experienced grade 4 myelosuppression (n = 5), grade 3 (n = 1) or grade 4 (n = 3) mucositis, sepsis (n = 5), and toxic epidermal necrolysis (n = 2). Initial leucovorin rescue was inadequate in five patients, and leucovorin was discontinued prematurely despite a plasma MTX concentration of more than 0.1 μmol/L in one patient. All six patients had received thymidine. The other six deaths (median age, 60.5 years; range, 42 to 72 years) were primarily attributable to rapid cancer progression.
Analysis of Characteristics Associated With Outcome
Frequencies of baseline characteristics included in the logistic regression analysis are listed in Table 1. Variables with statistically significant association (P < .10) to the development of ≥ grade 4 toxicity using single-factor logistic regression analysis are listed in Table 3.
Table 3.
Multiple-Factor Logistic Regression Analysis Model for the Risk of Developing ≥ Grade 4 Toxicity
| Variable | OR* | P | 95% CI |
|
|---|---|---|---|---|
| L | U | |||
| Statistically significant in single-factor regression analysis | ||||
| Age, years† | 1.0 | .07 | 1.0 | 1.0 |
| Diagnosis other than osteosarcoma‡ | 4.4 | .003 | 1.7 | 11.4 |
| MTX dose, g/m2† | 0.9 | .01 | 0.8 | 1.0 |
| Infusion duration, hours† | 1.1 | .03 | 1.0 | 1.1 |
| Serum creatinine, mg/dL† | 1.3 | .057 | 1.0 | 1.6 |
| Use of diuretic‡ | 11.3 | .022 | 1.4 | 89.6 |
| Use of dialysis‡ | 2.4 | .07 | 0.9 | 5.9 |
| Appropriate leucovorin increase‡ | 0.03 | .001 | 0.004 | 0.3 |
| Grade 4§ toxicity preglucarpidase‡ | 42.4 | .000 | 5.2 | 345.7 |
| Glucarpidase rescue > 96 hours after MTX‡ | 9.7 | .000 | 3.5 | 26.6 |
| Included in multiple-factor logistic regression analysis∥ | ||||
| Age, years | 1.0 | .630 | 1.0 | 1.0 |
| Diagnosis other than osteosarcoma | 1.2 | .915 | 0.06 | 23.5 |
| MTX dose, g/m2† | 1.0 | .893 | 0.7 | 1.4 |
| Infusion duration, hours | 1.0 | .969 | 0.9 | 1.1 |
| Serum creatinine, mg/dL | 0.8 | .318 | 0.4 | 1.3 |
| Use of dialysis | 0.8 | .786 | 0.1 | 5.3 |
| Glucarpidase rescue > 96 hours after MTX | 8.2 | .033 | 1.2 | 54.5 |
Abbreviations: OR, odds ratio; L, lower; U, upper; MTX, methotrexate.
An OR > 1 indicates increased risk for the development of a ≥ grade 4 toxicity.
Continuous variables.
Binary variables.
Grade 4 toxicity defined as mucositis, neutropenia, febrile neutropenia/sepsis, skin toxicity, diarrhea, and nausea/vomiting.
The multiple-factor logistic regression analysis dropped use of diuretics, leucovorin rescue, and grade 4 toxicity before glucarpidase as a result of perfect prediction of ≥ grade 4 toxicity.
Using multiple-factor logistic regression, analysis administration of glucarpidase more than 96 hours after start of HDMTX was the only parameter that remained statistically significantly when associated with the development of ≥ grade 4 toxicity (Table 3). Inappropriate increase in leucovorin, use of diuretics, and any grade 4 toxicity before glucarpidase predicted perfectly for development of ≥ grade 4 toxicity and could not be included in this analysis.
The disposition of each patient applying the only variable associated with ≥ grade 4 toxicity in the multiple-regression analysis, as well as prior grade 4 toxicity and leucovorin rescue, is shown in Figure 3. Six of the 12 patient deaths occurred among the 14 patients with grade 4 toxicity before glucarpidase administration, and five of these occurred in patients who received inappropriate leucovorin rescue and administration of glucarpidase after more than 96 hours. In contrast, the incidence of grade 4 toxicity was lower in the 76 patients without grade 4 toxicity before glucarpidase and with appropriate leucovorin rescue. In this group (n = 64), administration of glucarpidase ≤ 96 hours after HDMTX exposure appeared to protect from the development of toxicity; only nine patients (14%) developed ≥ grade 4 toxicity compared with six (55%) of 11 patients who received glucarpidase more than 96 hours after HDMTX exposure.
Fig 3.
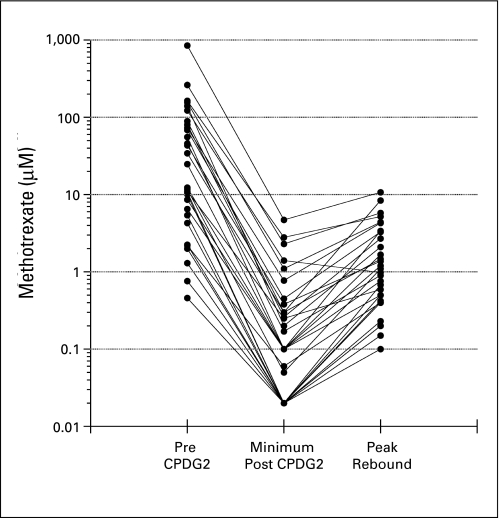
Maximum decrease and rebound increase in plasma methotrexate (MTX) concentration after administration of glucarpidase (CPDG2) in 30 patients. The median peak rebound increase plasma MTX concentration was 3.3% (range, 0.8% to 13.7%) for 20 patients with plasma MTX concentration ≥ 10 μmol/L before glucarpidase and 20.1% (range, 3.7% to 30.8%) for 10 patients with plasma MTX concentration less than 10 μmol/L before glucarpidase.
DISCUSSION
The development of renal dysfunction during HDMTX therapy is a medical emergency, because it can markedly enhance MTX toxicity. Glucarpidase provides an alternative route of elimination for MTX in this circumstance. In line with previous studies,5 after the initial rapid and dramatic (> 98%) reduction in plasma MTX concentration after glucarpidase, MTX was eliminated slowly, with a median elimination half-life of more than 72 hours, compared with 5.5 hours for its inactive metabolite, DAMPA, which is partially eliminated by metabolism.25 Second and third scheduled doses of glucarpidase did not result in an additional substantial decrease in plasma MTX concentration. The persistence of low but potentially toxic concentration of MTX postglucarpidase emphasizes the importance of continued administration of leucovorin until plasma MTX concentration is less than 0.05 to 0.1 μmol/L.
DAMPA interferes with commercial immunoassay-based assays of MTX, and after glucarpidase administration, these methods overestimate the true plasma MTX concentration.25,26 This overestimation, however, provides a margin of safety, given that the additional leucovorin will continue to provide rescue until the true MTX concentration is in the nontoxic range. A small rebound increase in plasma MTX concentration after glucarpidase was observed in 30 of 75 evaluable patients, but with exception of one patient with a plasma MTX concentration of 849 μmol/L preglucarpidase, MTX concentration remained less than 10 μmol/L at peak rebound.
As anticipated, we documented that glucarpidase, which has a half-life of ten hours in humans,27 also hydrolyzes 5-mTHF. Leucovorin concentration greatly exceeded 5-mTHF concentration, but this likely represents primarily the inactive d-isomer, which is eliminated more slowly than the l-isomer and accumulates.28 The hydrolysis of 5-mTHF by glucarpidase can be offset by continued leucovorin administration postglucarpidase treatment.
There were 12 deaths of the 100 patients who received glucarpidase, six of which were directly attributed to irreversible MTX toxicity. Our multiple-regression analysis model identified the presence of grade 4 toxicity before administration of glucarpidase, inadequate leucovorin rescue within the first 3 days after HDMTX exposure, diuretic use, and administration of glucarpidase more than 96 hours after start of HDMTX exposure as statistically significantly associated with the development of ≥ grade 4 toxicity. The pharmacology of MTX supports these findings. Once cells are exposed for prolonged time periods to high concentration of methotrexate in the absence of an increased concentration of leucovorin, toxicity is expected to be irreversible. These findings emphasize the need for early recognition and treatment of HDMTX-induced renal dysfunction, which should include timely increase in leucovorin rescue. Although glucarpidase and thymidine did not seem to be beneficial to patients presenting with severe toxicity before glucarpidase administration, our analysis suggests that glucarpidase administration within 96 hours after the start of MTX may decrease the development of grade 4 and 5 toxicity. There was only one MTX-related death of 81 patients who had not developed grade 4 toxicity before administration of glucarpidase, and in that group of patients, early rescue with glucarpidase resulted in a lower incidence of grade 4 toxicity. Our analysis does not support additional benefit from the coadministration of thymidine. All six patients who died as a result of MTX toxicity had received thymidine. In addition, we observed a similar degree of MTX-related myelosuppression in patients with osteosarcoma, who received MTX as single chemotherapy and were treated with or without thymidine.
Limitations of this study primarily relate to those of a retrospective analysis of a heterogeneous patient population, with data collection appropriate to our special exception program across multiple participating sites. Thus collection of certain data points was incomplete; for example, information regarding concomitant treatment with cytotoxic chemotherapy was not available for a substantial number of patients, which limits our ability to attribute toxicity solely to MTX.
There is concern that DAMPA, which is less soluble than MTX, could exacerbate HDMTX-induced nephrotoxicity. Despite significant increases in serum creatinine and the need for dialysis in some patients, patients recovered renal function within a median of 3 weeks from the initial insult, consistent with our observation of the initial 21 patients treated with glucarpidase.5 DAMPA was eliminated more rapidly than the residual MTX after glucarpidase administration, presumably through conjugation with glucuronide and biliary excretion.25
Despite the fact that glucarpidase is a recombinant bacterial enzyme, we did not document antibody formation to glucarpidase. Repeated use of glucarpidase as a MTX rescue agent in settings other than HDMTX-induced nephrotoxicity may thus be feasible.
In summary, our analysis of 100 patients who were treated consecutively demonstrates that early intervention with pharmacokinetically guided leucovorin and glucarpidase is highly effective in treating patients who develop HDMTX-induced renal dysfunction. However, mortality in patients for whom effective intervention is delayed was high and occurred despite treatment, including treatment with thymidine. A higher mortality rate in elderly patients with cancer with MTX-induced renal dysfunction and in patients for whom the start of glucarpidase and leucovorin rescue was delayed has been observed by other investigators.3,4
In patients receiving HDMTX, early recognition of renal dysfunction, manifested primarily by increasing serum creatinine, should warrant urgent intervention with a combination of increased and sustained leucovorin dosage before and after systemic administration of glucarpidase.22
Acknowledgment
We thank Seth Steinberg, PhD, for providing advice on the statistical analysis.
Footnotes
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, Bethesda, MD.
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, May 13-17, 2005, Orlando, FL.
The views expressed do not necessarily represent views of the National Institutes of Health or the US government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Peter C. Adamson, BTG-Protherics (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Brigitte C. Widemann, Frank M. Balis, Aiman Shalabi, Peter C. Adamson
Administrative support: Michelle Eby, Percy Ivy
Provision of study materials or patients: Frank M. Balis, Matthew Boron, Aiman Shalabi, Michelle Eby
Collection and assembly of data: Brigitte C. Widemann, Matthew Boron, Nalini Jayaprakash, Aiman Shalabi, Michelle O'Brien, Michelle Eby, Diane E. Cole, Robert F. Murphy
Data analysis and interpretation: Brigitte C. Widemann, Frank M. Balis, AeRang Kim, Nalini Jayaprakash, Michelle O'Brien, Diane E. Cole, Robert F. Murphy, Elizabeth Fox, Percy Ivy, Peter C. Adamson
Manuscript writing: Brigitte C. Widemann, Frank M. Balis, AeRang Kim, Michelle Eby, Peter C. Adamson
Final approval of manuscript: Brigitte C. Widemann, Frank M. Balis, AeRang Kim, Matthew Boron, Nalini Jayaprakash, Aiman Shalabi, Michelle O'Brien, Michelle Eby, Diane E. Cole, Robert F. Murphy, Elizabeth Fox, Percy Ivy, Peter C. Adamson
REFERENCES
- 1.Bleyer WA. Methotrexate: Clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977;4:87–101. doi: 10.1016/s0305-7372(77)80007-8. [DOI] [PubMed] [Google Scholar]
- 2.Djerassi I. High-dose methotrexate (NSC-740) and citrovorum factor (NSC-3590) rescue: Background and rationale. Cancer Chemother Rep. 1975;6:3–6. [Google Scholar]
- 3.Buchen S, Ngampolo D, Melton RG, et al. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer. 2005;92:480–487. doi: 10.1038/sj.bjc.6602337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz S, Borner K, Müller K, et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. Oncologist. 2007;12:1299–1308. doi: 10.1634/theoncologist.12-11-1299. [DOI] [PubMed] [Google Scholar]
- 5.Widemann BC, Balis FM, Murphy RF, et al. Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol. 1997;15:2125–2134. doi: 10.1200/JCO.1997.15.5.2125. [DOI] [PubMed] [Google Scholar]
- 6.Howell SB, Herbst K, Boss GR, et al. Thymidine requirements for the rescue of patients treated with high-dose methotrexate. Cancer Res. 1980;40:1824–1829. [PubMed] [Google Scholar]
- 7.Widemann BC, Hetherington ML, Murphy RF, et al. Carboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicity. Cancer. 1995;76:521–526. doi: 10.1002/1097-0142(19950801)76:3<521::aid-cncr2820760325>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Estève MA, Devictor-Pierre B, Galy G, et al. Severe acute toxicity associated with high-dose methotrexate (MTX) therapy: Use of therapeutic drug monitoring and test-dose to guide carboxypeptidase G2 rescue and MTX continuation. Eur J Clin Pharmacol. 2007;63:39–42. doi: 10.1007/s00228-006-0212-1. [DOI] [PubMed] [Google Scholar]
- 9.Grill J, Amigo-Ferreiro ME, Schoepfer C, et al. Carboxypeptidase-G2 rescue of methotrexate intoxication [in French] Bull Cancer. 1998;85:1066. [PubMed] [Google Scholar]
- 10.Harned TM, Mascarenhas L. Severe methotrexate toxicity precipitated by intravenous radiographic contrast. J Pediatr Hematol Oncol. 2007;29:496–499. doi: 10.1097/MPH.0b013e3180683c04. [DOI] [PubMed] [Google Scholar]
- 11.Hum M, Kamen BA. Successful carboxypeptidase G2 rescue in delayed MTX-elimination due to renal failure. Pediatr Hematol Oncol. 1995;12:521–524. doi: 10.3109/08880019509030765. [DOI] [PubMed] [Google Scholar]
- 12.Krackhardt A, Schwartz S, Korfel A, et al. Carboxypeptidase G2 rescue in a 79 year-old patient with cranial lymphoma after high-dose methotrexate induced acute renal failure. Leuk Lymphoma. 1999;35:631–635. doi: 10.1080/10428199909169631. [DOI] [PubMed] [Google Scholar]
- 13.Krause AS, Weihrauch MR, Bode U, et al. Carboxypeptidase-G2 rescue in cancer patients with delayed methotrexate elimination after high-dose methotrexate therapy. Leuk Lymphoma. 2002;43:2139–2143. doi: 10.1080/1042819021000032953. [DOI] [PubMed] [Google Scholar]
- 14.Mantadakis E, Rogers ZR, Smith AK, et al. Delayed methotrexate clearance in a patient with sickle cell anemia and osteosarcoma. J Pediatr Hematol Oncol. 1999;21:165–169. doi: 10.1097/00043426-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Mohty M, Peyriere H, Guinet C, et al. Carboxypeptidase G2 rescue in delayed methotrexate elimination in renal failure. Leuk Lymphoma. 2000;37:441–443. doi: 10.3109/10428190009089446. [DOI] [PubMed] [Google Scholar]
- 16.Nowicki TS, Bjornard K, Kudlowitz D, et al. Early recognition of renal toxicity of high-dose methotrexate therapy: A case report. J Pediatr Hematol Oncol. 2008;30:950–952. doi: 10.1097/MPH.0b013e318182e73e. [DOI] [PubMed] [Google Scholar]
- 17.Peyriere H, Cociglio M, Margueritte G, et al. Optimal management of methotrexate intoxication in a child with osteosarcoma. Ann Pharmacother. 2004;38:422–427. doi: 10.1345/aph.1D237. [DOI] [PubMed] [Google Scholar]
- 18.Poblozki A, Dempke W, Schmoll H. Carboxypeptidase-G2-Rescue bei einerPatientin mit Methotrexat-induziertem Nierenversagen [in German] Med Klin. 2000;95:457–460. doi: 10.1007/s000630050008. [DOI] [PubMed] [Google Scholar]
- 19.Snyder RL. Resumption of high-dose methotrexate after methotrexate-induced nephrotoxicity and carboxypeptidase G2 use. Am J Health Syst Pharm. 2007;64:1163–1169. doi: 10.2146/ajhp060187. [DOI] [PubMed] [Google Scholar]
- 20.Zoubek A, Zaunschirm HA, Lion T, et al. Successful carboxypeptidase G2 rescue in delayed methotrexate elimination due to renal failure. Pediatr Hematol Oncol. 1995;12:471–477. doi: 10.3109/08880019509009477. [DOI] [PubMed] [Google Scholar]
- 21.Frei E, 3rd, Blum RH, Pitman SW, et al. High dose methotrexate with leucovorin rescue. Rationale and spectrum of antitumor activity. Am J Med. 1980;68:370–376. doi: 10.1016/0002-9343(80)90105-9. [DOI] [PubMed] [Google Scholar]
- 22.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 23.Adamson PC, Balis FM, McCully CL, et al. Methotrexate pharmacokinetics following administration of recombinant carboxypeptidase-G2 in Rhesus monkeys. J Clin Oncol. 1992;10:1359–1364. doi: 10.1200/JCO.1992.10.8.1359. [DOI] [PubMed] [Google Scholar]
- 24.Widemann BC, Balis FM, Shalabi A, et al. Treatment of accidental intrathecal methotrexate overdose with intrathecal carboxypeptidase G2. J Natl Cancer Inst. 2004;96:1557–1559. doi: 10.1093/jnci/djh270. [DOI] [PubMed] [Google Scholar]
- 25.Widemann BC, Sung E, Anderson L, et al. Pharmacokinetics and metabolism of the methotrexate metabolite 2, 4-diamino-N10-methylpteroic acid. J Pharmacol Exp Ther. 2000;294:894–901. [PubMed] [Google Scholar]
- 26.Widemann BC, Balis FM, Adamson PC. Dihydrofolate reductase enzyme inhibition assay for plasma methotrexate determination using a 96-well microplate reader. Clin Chem. 1999;45:223–228. [PubMed] [Google Scholar]
- 27.Phillips M, Smith W, Balan G, et al. Pharmacokinetics of glucarpidase in subjects with normal and impaired renal function. J Clin Pharmacol. 2008;48:279–284. doi: 10.1177/0091270007311571. [DOI] [PubMed] [Google Scholar]
- 28.Hempel G, Lingg R, Boos J. Interactions of carboxypeptidase G2 with 6S-leucovorin and 6R-leucovorin in vitro: Implications for the application in case of methotrexate intoxications. Cancer Chemother Pharmacol. 2005;55:347–353. doi: 10.1007/s00280-004-0910-2. [DOI] [PubMed] [Google Scholar]