Abstract
Purpose
Single-agent topotecan (TOPO) and combination topotecan and cyclophosphamide (TOPO/CTX) were compared in a phase II randomized trial in relapsed/refractory neuroblastoma. Because responders often underwent further therapies, novel statistical methods were required to compare the long-term outcome of the two treatments.
Patients and Methods
Children with refractory/recurrent neuroblastoma (only one prior aggressive chemotherapy regimen) were randomly assigned to daily 5-day topotecan (2 mg/m2) or combination topotecan (0.75 mg/m2) and cyclophosphamide (250 mg/m2). A randomized two-stage group sequential design enrolled 119 eligible patients. Toxicity and response were estimated. Long-term outcome of protocol therapy was assessed using novel methods—causal inference—which allowed adjustment for the confounding effect of off-study therapies.
Results
Seven more responses were observed for TOPO/CTX (complete response [CR] plus partial response [PR], 18 [32%] of 57) than TOPO (CR+PR, 11 [19%] of 59;P = .081); toxicity was similar. At 3 years, progression-free survival (PFS) and overall survival (OS) were 4% ± 2% and 15% ± 4%, respectively. PFS was significantly better for TOPO/CTX (P = .029); there was no difference in OS. Older age at diagnosis and lack of MYCN amplification predicted increased OS (P < .05). Adjusting for randomized treatment effect and subsequent autologous stem-cell transplantation, there was no difference between TOPO and TOPO/CTX in terms of the proportion alive at 2 years.
Conclusion
TOPO/CTX was superior to TOPO in terms of PFS, but there was no OS difference. After adjustment for subsequent therapies, no difference was detected in the proportion alive at 2 years. Causal inference methods for assessing long-term outcomes of phase II therapies after subsequent treatment can elucidate effects of initial therapies.
INTRODUCTION
Topotecan targets the DNA-relaxing enzyme topoisomerase I, resulting in covalent binding of the enzyme to DNA, in turn causing lethal DNA damage during replication.1 The drug was approved by the US Food and Drug Administration in 1998 for treatment of small-cell lung cancer. Topotecan combined with alkylating agents may produce synergistic tumor cell killing in multiple in vitro and in vivo models, perhaps by interfering with repair of DNA damage induced by the alkylating agent.2
Pediatric phase I studies of topotecan (administration every 21 days once daily for 5 days) demonstrated optimal clinical activity in comparison with the previous continuous-infusion regimens.3–6 This activity was confirmed in phase II studies in children with recurrent neuroblastoma, rhabdomyosarcoma, osteosarcoma, and brain tumors.7,8 A slightly higher dose of topotecan was well tolerated in a phase II investigational window study of children with rhabdomyosarcoma9; therefore, a higher dose was used in the study described here.
Pediatric phase I and II studies of the combination of topotecan and cyclophosphamide (TOPO/CTX) resulted in responses and toxicity similar to those seen with topotecan alone (TOPO).10–12 To determine which regimen, TOPO or TOPO/CTX, was superior in terms of response in recurrent or refractory neuroblastoma, randomized study Children's Oncology Group P9462 was performed. Though overall survival (OS) was not an end point, there is interest regarding long-term outcome of patients with relapsed neuroblastoma. Unfortunately, analysis of long-term survival is typically confounded by off-protocol therapy. In this study, patients were permitted to pursue autologous stem-cell transplantation (ASCT) or alternative therapy at any time without regard to response status. The duration of progression-free survival (PFS) and OS reflect not only the effect of study drugs, but also effects of subsequent treatments. To address this, a novel statistical method—causal inference analysis—was applied, in which survival probability was conditioned on response and the optimal off-protocol therapy (ASCT or no ASCT) for a given response/treatment combination.13–15
PATIENTS AND METHODS
Patients, Treatment, and Evaluation
Eligibility included neuroblastoma patients in first recurrence or progression after treatment with aggressive multidrug therapy (two or more agents, including an alkylator and a platinum-containing compound) or at second recurrence after a single regimen of aggressive chemotherapy at first recurrence. Eligibility also included age ≤ 21 years, life expectancy ≥ 4 weeks, no uncontrolled serious infection, adequate renal and hepatic function, platelet count more than 75,000/μL and absolute neutrophil count more than 750/μL except if extensive tumor in the bone marrow, Karnofsky score ≥ 50%, and either measurable disease by radiographic imaging or evaluable disease by metaiodobenzylguanidine scan or bone marrow evaluation.
From November 1, 1996, to December 21, 2001, 123 patients enrolled from the Pediatric Oncology Group (POG) and the Children's Cancer Group (now the Children's Oncology Group;Fig 1). Four patients were ineligible (wrong diagnosis); 119 patients form the analytic cohort for this report. (The first 13 children entered received deferoxamine [DFO] in a therapeutic window trial;16 the window is not a subject of this analysis.) Parents or guardians provided written informed consent, and the appropriate local institutional review boards approved the study.
Fig 1.
CONSORT diagram. TOPO, topotecan alone; TOPO/CTX, topotecan plus cyclophosphamide.
Patients assigned to TOPO received intravenous topotecan 2 mg/m2/d for 5 days, and patients assigned to TOPO/CTX received intravenous topotecan 0.75 mg/m2/d and cyclophosphamide 250 mg/m2/d for 5 days. Cycles were 21 days, starting subcutaneous filgrastim 5 μg/kg/d on day 6. The protocol permitted continued treatment until disease progression or up to 1 year without progression.
Response was evaluated per Revised International Criteria for Neuroblastoma,17 based on radiologic imaging and bone marrow morphology at 6 weeks and every 12 weeks thereafter. Toxicity (grade ≥ 3) was reported per POG Common Toxicity and Complications coding system of April 1993. Initial diagnosis data, including stage,MYCN status, ploidy, and histologic classification, were available only if the patient had enrolled onto a frontline POG or Children's Cancer Group study.
Statistical Considerations
Random assignment (TOPO v TOPO/CTX) was stratified by prior ASCT and DFO window participation. Response (defined as complete response [CR] or partial response [PR] for overall best response at any time during protocol treatment) was the primary end point rather than PFS or OS because of the potential for subsequent off-protocol therapies, such as ASCT, to influence the long-term treatment difference. There was no minimum number of treatment cycles required for inclusion in the analysis of response. Per protocol, investigators could stop protocol therapy and pursue ASCT or other alternatives at any time without regard for response status.
A two-stage group sequential design tested the number of responders. The planned study would proceed as follows: of 30 patients per arm at stage 1, if seven or more responders in TOPO/CTX than in TOPO arm, stop for efficacy; if two or more responders in TOPO than in TOPO/CTX arm, stop for futility. If neither condition is met, accrue 29 more patients per arm at stage 2 (total of 59 per arm). If there are eight or more responders in TOPO/CTX than TOPO arm, then conclude that the proportion of TOPO/CTX responders is statistically significantly greater than that of TOPO. This design has a cumulative type I error of 0.098 and 80.4% power to detect a difference of 60% (TOPO/CTX) versus 40% (TOPO) response. The choice of 40% for TOPO was based on 37% response in 32 patients on POG 9340;12 however, only two courses of topotecan were given in an investigational window, so the number of responders might have been higher with further TOPO courses. A 20% improvement in response was deemed clinically significant.
Secondary analyses were performed of CR + PR + mixed response (MR), PFS, and OS. Fisher's exact test18 compared the proportion of responders between treatment arms.
Patients in the DFO investigational window were included in all analyses.16 PFS was calculated from P9462 enrollment until first occurrence of relapse, progressive disease, or death from any cause or until last contact if no event was observed. PFS was virtually synonymous with event-free survival because there was only one nonneuroblastoma event, a toxic death. Progression events were included whether they occurred while the patient was receiving study drugs or subsequent therapies. OS was calculated from enrollment until death or until last contact if alive. Survival curves were estimated according to Kaplan-Meier19 ± Greenwood SEs,20 and a one-sided log-rank test21 was used because improvement with TOPO/CTX was of interest.
Estimation of OS is complicated because some patients received ASCT after going off protocol therapy. Responders were potentially more likely to have subsequent ASCT than those who did not respond. Using a test of proportions for a difference in the proportion alive at 2 years by treatment arm, while making adjustment for the effect of off-protocol therapy ASCT, we apply causal inference methodology.13 Consider the eight possible combinations of treatment decisions: treatment arm (TOPO or TOPO/CTX) and ASCT (where ASCT or no ASCT may depend on overall best response [CR + PR, < PR, or ignore response];Table 1). In causal inference, these combinations are the dynamic treatment regimens, because the decision to administer the second treatment, ASCT, depends on response to the first treatment (TOPO or TOPO/CTX). The goal is to determine which combination is the optimal dynamic treatment regimen that, on average, will produce the highest proportion of patients alive at 2 years.14,15 This methodology makes the “sequential ignorability assumption” that a patient's treatment arm and response are the only criteria systematically governing the physician's decision of whether or not the patient should undergo ASCT. The proportion of patients alive at 2 years for each observed sequence of treatments and responses was calculated. Conditional on response and the optimal off-protocol therapy (ASCT or no ASCT) for a given response/treatment combination, the expected probability of survival at 2 years was calculated for each treatment arm, and a test of proportions was performed. In addition, the comparison was performed assuming that no patients received ASCT.
Table 1.
Dynamic Treatment Regimes: Consideration of All Possible Treatment/Response Sequences Leading to the Physician's Decision Regarding the Administration of ASCT
| Therapeutic Sequence | Treatment Arm | ASCT? |
|---|---|---|
| 1 | TOPO | No, regardless of response |
| 2 | TOPO | No if < PR; yes if CR or PR |
| 3 | TOPO | Yes if < PR; no if CR or PR |
| 4 | TOPO | Yes, regardless of response |
| 5 | TOPO/CTX | No, regardless of response |
| 6 | TOPO/CTX | No if < PR; yes if CR or PR |
| 7 | TOPO/CTX | Yes if < PR; no if CR or PR |
| 8 | TOPO/CTX | Yes, regardless of response |
Abbreviations: ASCT, autologous stem-cell transplantation; TOPO, topotecan alone; PR, partial response; CR, complete response; TOPO/CTX, topotecan plus cyclophosphamide.
Exploratory analyses identified factors prognostic for response (Fisher's exact test) or PFS (log-rank test), although the study was not designed or powered for this. Factors tested were those previously shown to be prognostic at diagnosis (age,MYCN status, ploidy, histology),22 plus prior ASCT and time to first relapse. P values less than .05 were considered statistically significant.
RESULTS
Patient Characteristics
Of 119 eligible patients, 71 previously underwent high-dose chemotherapy with ASCT as initial treatment, and 48 children had not. The median age at initial diagnosis was 3.6 years (range, 0.5 to 18 years) and at P9462 enrollment was 5.6 years (range, 1 to 19 years). The median time from initial diagnosis until enrollment was 18 months. Sixty-four children were randomly assigned to TOPO (two patients were ineligible) and 59 patients were randomly assigned to TOPO/CTX (two patients were ineligible). There were no differences by treatment arm in terms of age at enrollment or DFO window participation. Of patients with known data at diagnosis, there were no significant differences by age at diagnosis, age at P9462 enrollment, International Neuroblastoma Staging System stage,MYCN status, ploidy, histology, time from diagnosis to enrollment, and primary tumor site (Table 2).
Table 2.
Comparison of Patient Characteristics by Randomized Treatment Group (n = 119)
| Characteristic | TOPO (n = 62) |
TOPO/CTX (n = 57) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age at diagnosis, months | |||||
| < 18 | 6 | 10 | 8 | 15 | .5749 |
| ≥ 18 | 52 | 90 | 47 | 85 | |
| Unknown | 4 | 2 | |||
| Median | 3.7 years | 3.5 years | ND | ||
| Range | 7.1 months-17.9 years | 6.3 months-18.7 years | |||
| Age at P9462 enrollment, months | |||||
| < 18 | 2 | 3 | 0 | 0 | .4967 |
| ≥ 18 | 60 | 97 | 57 | 100 | |
| Unknown | 0 | 0 | 0 | ||
| Median | 5.6 years | 5.1 years | ND | ||
| Range | 12.4 months-18.8 years | 19.5 months-20.9 years | |||
| INSS stage at diagnosis | |||||
| 1 | 0 | 0 | 1 | 6 | .5071‡ |
| 2a/2b | 3 | 12 | 0 | 0 | |
| 3 | 5 | 20 | 3 | 18 | |
| 4 | 17 | 68 | 13 | 76 | |
| 4s | 0 | 0 | 0 | 0 | |
| Unknown† | 39 | 40 | |||
| MYCN status | |||||
| Not amplified | 16 | 70 | 12 | 63 | .7483 |
| Amplified | 7 | 30 | 7 | 37 | |
| Unknown† | 39 | 38 | |||
| Ploidy | |||||
| Hyperdiploid | 10 | 45 | 9 | 50 | 1.000 |
| Diploid | 12 | 55 | 9 | 50 | |
| Unknown† | 40 | 39 | |||
| Histology | |||||
| Favorable | 1 | 13 | 1 | 11 | 1.000 |
| Unfavorable | 7 | 87 | 8 | 89 | |
| Unknown† | 54 | 48 | |||
| Time from diagnosis to P9462 enrollment, years | |||||
| < 1 | 19 | 33 | 18 | 33 | 1.000 |
| > 1 | 39 | 67 | 37 | 67 | |
| Unknown† | 4 | 2 | |||
| DFO window | |||||
| Yes | 8 | 13 | 5 | 9 | 0.5634 |
| No | 54 | 87 | 52 | 91 | |
| Life status at last contact | |||||
| Alive | 6 | 10 | 7 | 12 | .7715 |
| Dead | 56 | 90 | 50 | 88 | |
| Primary tumor site at diagnosis | |||||
| Adrenal | 12 | 48 | 9 | 50 | |
| Abdominal | 9 | 36 | 7 | 39 | .8139§ |
| Thoracic | 2 | 8 | 1 | 6 | |
| Other | 2 | 8 | 1 | 6 | |
| Unknown† | 37 | 39 | |||
Abbreviations: TOPO, topotecan alone; TOPO/CTX, topotecan plus cyclophosphamide; ND, not done; INSS, International Neuroblastoma Staging System; DFO, deferoxamine.
Fisher's exact test.
Data from initial diagnosis were not collected on this relapse study. Many patients had not enrolled on a Children's Oncology Group study at diagnosis; therefore, data for baseline risk factors were unknown for many patients.
Stage 4 v stage 1, 2, 3, 4s.
Mantel-Haenszel χ2 test.
Toxicity
Eighteen (32%) of 57 TOPO patients had severe or life-threatening infection, compared with 11 (19%) of 57 TOPO/CTX patients (P = .20). Twenty-eight (49%) of 57 TOPO patients had grade 3 or 4 neutropenia (absolute neutrophil count ≤ 0.9/μL), compared with 25 (44%) of 57 TOPO/CTX patients (P = .71). Thirty-five (61%) of 57 TOPO patients had grade 3 or 4 thrombocytopenia (≤ 49,000/μL) compared with 34 (60%) of 57 TOPO/CTX patients (P = 1.0). The only toxic death was a TOPO/CTX patient with hepatic failure 2 weeks after start of therapy; attribution could not be confirmed because baseline liver function tests were not performed.
Response
Per protocol, the two-stage group sequential rule was restricted to the first 59 patients per arm; however, only 57 patients were eligible in the TOPO/CTX arm. Of the 62 TOPO patients, only the first 59 patients were used for the two-stage response rule.
Six responders (CR + PR) in each arm at the first stage (n = 30 per arm) were noted, and the study continued for a total of 59 TOPO and 57 TOPO/CTX eligible patients. CR + PR was observed in 11 of 59 TOPO and 18 of 57 TOPO/CTX patients; CR + PR + MR was observed in 19 of 59 TOPO and 26 of 57 TOPO/CTX (Table 3). There was a nonstatistically significant trend toward higher response rate in TOPO/CTX: CR + PR, 32% versus 19% (P = .081), and CR + PR + MR, 47% versus 32% (P = .098). Including all eligible and evaluable patients (two additional evaluable TOPO patients accrued but not included in the two-stage evaluation), the difference was not statistically significant (P = .077; Table 4).
Table 3.
Number of Responders by Randomized Treatment Group for Two-Stage Group Sequential Design
| Stage and Response | TOPO (n = 30 at stage 1; n = 59 at stage 2) | TOPO/CTX (n = 30 at stage 1; n = 57 at stage 2) | Difference in No. of CR + PR Responders (R = R(TOPO/CTX)–R(TOPO)) | Decision Boundary | Decision |
|---|---|---|---|---|---|
| Stage 1, n = 60 | 0 | R ≥ 7: stop (success) | Continue to stage 2 | ||
| CR + PR | 6 | 6 | –2 < R < 7: continue | ||
| CR + PR + MR | 10 | 13 | R ≤ –2: stop (futile) | ||
| Stage 2, n = 116 | 7 | ≥ 8: success | Insufficient evidence of benefit | ||
| CR + PR | 11 | 18 | < 8: insufficient | ||
| CR + PR + MR | 19 | 26 | evidence of benefit |
Abbreviations: TOPO, topotecan alone; TOPO/CTX, topotecan plus cyclophosphamide; CR, complete response; PR, partial response; R, responders.
Table 4.
Overall Survival (n = 119) and Response (n = 118) by Patient Characteristics and Treatment Group of Eligible Patients on Study P9462
| Characteristic | No. | % | 3-Year OS Rate | SE | Log-RankP | Responders (CR +PR + MR) | ResponseP* |
|---|---|---|---|---|---|---|---|
| Age at initial diagnosis, years | |||||||
| 0-1.5 | 14 | 12.3 | 7.1 | 6.9 | 35.7 | ||
| ≥ 1.5 | 100 | 87.7 | 17.4 | 4.1 | .0007 | 80.0 | 1.00 |
| Unknown | 5 | ||||||
| Prior ASCT | |||||||
| No | 48 | 40.3 | 23.4 | 6.2 | 34.0 | ||
| Yes | 71 | 59.7 | 9.2 | 3.7 | .068 | 40.9 | .56 |
| Unknown | 0 | 0 | |||||
| Time to relapse, months | |||||||
| < 6 | 15 | 13.1 | 33.3 | 12.2 | 33.3 (10.2%) | ||
| ≥ 6 | 99 | 86.8 | 13.4 | 3.6 | .074 | 40.4 (4.3%) | .78 |
| Unknown | 5 | ||||||
| Histology | |||||||
| Favorable | 2 | 11.8 | 50 | 35.4 | 50.0 | ||
| Unfavorable | 15 | 88.2 | 13.3 | 8.8 | .42 | 33.3 | 1.0 |
| Unknown | 102 | ||||||
| Ploidy | |||||||
| Diploid | 20 | 51.3 | 13.3 | 8.1 | 50.0 | ||
| Hyperdiploid | 19 | 48.7 | 15.8 | 8.4 | .97 | 26.3 | .19 |
| Unknown | 80 | ||||||
| MYCN status | |||||||
| Amplified | 14 | 34.2 | 7.1 | 6.9 | 35.7 | ||
| Not amplified | 27 | 65.8 | 20.8 | 8.0 | .0002 | 37.0 | 1.0 |
| Unknown | 78 | ||||||
| Treatment group | |||||||
| TOPO | 62 | 52.1 | 13.8 | 4.5 | 42.2 | ||
| TOPO/CTX | 57 | 47.9 | 17.3 | 5.6 | .72 | 57.8 | .077 |
Abbreviations: OS, overall survival; CR, complete response; PR, partial response; MR, minimal response; ASCT, autologous stem-cell transplantation; TOPO, topotecan; TOPO/CTX, topotecan plus cyclophosphamide.
Fisher's exact test.
Survival
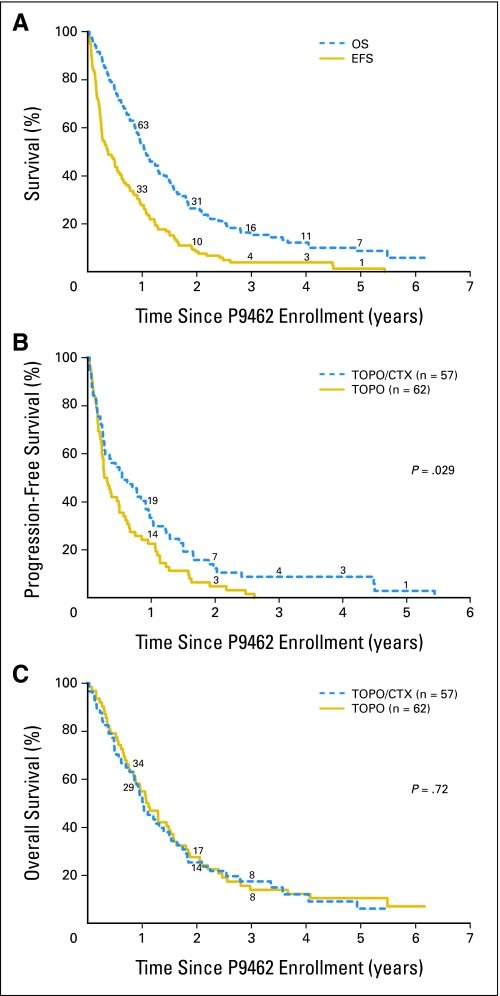
Overall, the median follow-up in patients without an event was 3.9 years, and 3-year PFS and OS were 4% ± 2% and 15% ± 4%, respectively (n = 119,Fig 2A). Overall, median PFS was 4.5 months, and median OS was 1.1 year. There were 117 events, and 106 patients subsequently died. PFS was statistically significantly better for children who received TOPO/CTX versus TOPO (P = .029;Fig 2B). All 62 TOPO patients experienced disease progression before 3 years, whereas progression occurred after 4 years for three patients on TOPO/CTX. OS was similar for both groups (P = .72; Fig 2C).
Fig 2.
(A) Event-free survival (EFS) and overall survival (OS) for 119 patients with relapsed or refractory neuroblastoma on study P9462. (B) Progression-free survival for 62 topotecan alone (TOPO) versus 57 topotecan plus cyclophosphamide (TOPO/CTX) patients on study P9462 (P = .029). (C) OS for 62 TOPO versus 57 TOPO/CTX patients on study P9462 (P = .72). The numbers of patients at risk are shown along the curves.
Effect of Subsequent Therapies on Survival: Causal Inference Analysis
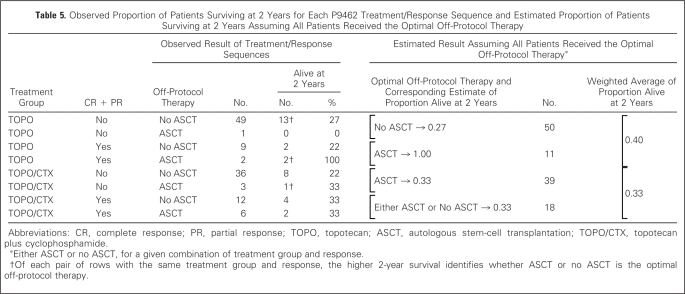
Nine TOPO/CTX and three TOPO patients received off-protocol ASCT. If ASCT results in increased survival, and if patients exposed to TOPO/CTX are more likely to undergo ASCT, then our ability to test for the hypothesized survival benefit of TOPO/CTX is confounded by ASCT. Using causal inference analysis, we can assess this possibility, taking into account response and physician decision making based on response. Because a decision for ASCT (or not) was made after assessment of response to TOPO or TOPO/CTX, eight patient treatment paths are possible (Table 1). Causal inference analysis accounts for the observed outcome of each pathway and chooses one optimal (in terms of higher proportion alive at 2 years) path for each arm (TOPO or TOPO/CTX) to use in analysis. For example, for the subgroup who received TOPO and did not achieve CR or PR, a higher proportion who did not undergo ASCT (27%) were alive at 2 years compared with ASCT (0%;Table 5). Therefore, “No ASCT” is the optimal off-protocol therapy for this subgroup. Similarly, for the TOPO/CTX patients who achieved CR or PR, 33% were alive at 2 years whether or not ASCT was done; therefore, there is not an optimal off-protocol therapy for this subgroup, and either can be used in the analysis.
Table 5.
Observed Proportion of Patients Surviving at 2 Years for Each P9462 Treatment/Response Sequence and Estimated Proportion of Patients Surviving at 2 Years Assuming All Patients Received the Optimal Off-Protocol Therapy
Abbreviations: CR, complete response; PR, partial response; TOPO, topotecan; ASCT, autologous stem-cell transplantation; TOPO/CTX, topotecan plus cyclophosphamide.
Either ASCT or no ASCT, for a given combination of treatment group and response.
Of each pair of rows with the same treatment group and response, the higher 2-year survival identifies whether ASCT or no ASCT is the optimal off-protocol therapy.
We now create a hypothetical situation in which all patients receive the optimal off-protocol therapy for their given subgroup. On the basis of that assumption, the estimated proportion surviving at 2 years is not statistically significantly higher for TOPO (0.40) than for TOPO/CTX (0.33;P = .215,Table 5). Assuming no patient underwent subsequent ASCT, the estimated proportion alive at 2 years was 0.26 for both TOPO and TOPO/CTX (P = .475).
Prognostic Factors
Older age at diagnosis (P = .0007) and single-copyMYCN (P = .0002) were statistically significantly predictive of increased OS (Table 4). Other factors were not predictive of OS, although patients with no prior ASCT, or with a shorter time from initial diagnosis to entry on P9462, showed a trend toward longer OS (P < .1). No factors were predictive of response (CR + PR + MR; Table 4).
DISCUSSION
This is the largest phase II randomized trial ever conducted in children with refractory or recurrent neuroblastoma. Patients were randomly assigned to TOPO or TOPO/CTX. Although there was a suggestion of improved response with the combination therapy, the difference was not statistically significant. Unfortunately, lacking two TOPO/CTX patients for the required 59 per arm, the two-stage rule is noninformative. It is possible, though not likely, that one or both of the missing TOPO/CTX patients could have been responders, thus triggering the rule to conclude superiority of TOPO/CTX. Hematopoietic toxicity was similar in both regimens without unexpected significant toxicities. PFS was statistically significantly better for children who received TOPO/CTX (P = .029), but OS did not differ between treatment arms. Predictors of improved OS included single-copy MYCN and older age at diagnosis. A trend toward improved survival was observed in patients who had not received a prior ASCT or were within 6 months of diagnosis.
A strength of this study is the excellent long-term follow-up, resulting in informative survival estimates. The overall median OS on this study was 1.1 year, compared with 8.4 months in a recent single-institution report.23 Appropriate historical comparison studies of PFS or OS in patients with relapsed neuroblastoma are difficult to identify. The results of this study will be very useful as a baseline comparator to other agents in relapsed/refractory patients.
OS analysis was complicated by permitted use of ASCT or alternative therapy at any time without regard to response status. The duration of PFS and OS reflect not only the effect of study drugs, but also effects of subsequent treatments. To address this issue, a novel method—causal inference analysis—was applied. This method permits adjustment for off-protocol therapy (eg, either ASCT or no ASCT). We found no evidence of a TOPO/CTX advantage for the proportion of patients surviving at 2 years, taking into account the effects of optimal subsequent therapy. There was also no TOPO/CTX advantage assuming no patients undergo subsequent ASCT. However, these causal inference nonsignificant results do not imply that the observed significantly higher PFS for TOPO/CTX (compared with TOPO) is independent of subsequent therapy; the nonsignificant result simply means we have insufficient evidence to conclude a difference affecting survival in the initial therapy (TOPO/CTXv TOPO) after adjusting for optimal off-protocol therapy, or assuming none of the patients got ASCT. Our sequential ignorability assumption (assuming that the ASCT treatment decision was based solely on the patient's response to the P9462 treatment arm) seems not unreasonable; however, if in fact other factors more strongly influenced that decision, then our causal inference results are questionable. As true for any analytic approach, small sample size limits our ability to infer to a larger population.
In summary, the weight of the evidence lends modest support to use of the TOPO/CTX combination. These results led to incorporation of TOPO/CTX into the COG induction chemotherapy regimen for patients with newly diagnosed high-risk neuroblastoma.24 TOPO/CTX proved superior to TOPO in PFS, with a trend toward superior response rate, but no improvement in OS. There was no detectable difference in the proportion of patients surviving at 2 years after adjustment for optimal off-protocol therapy through application of causal inference methods. Causal inference methods are a useful approach to assessing the long-term effect of an initial therapy with statistical adjustment for subsequent therapy. This methodology will be particularly important for long-term survival analyses in trials of biologically based therapies with shorter term end points.
Footnotes
See accompanying editorial on page 3800
Supported by Grant No. U10 CA98413 from the National Institutes of Health to the Children's Oncology Group, Grant No. U10 CA29139 to the Pediatric Oncology Group, and Grant No. U10 CA 13539 to the Children's Cancer Group.
Presented in part at the 40th Annual Meeting of the American Society of Clinical Oncology, June 5-8, 2004, New Orleans, LA (abstr 8512).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Wendy B. London, Christopher N. Frantz, Katherine K. Matthay, Robert P. Castleberry, Lisa Diller
Administrative support: Wendy B. London, Christopher N. Frantz, Lisa Diller
Provision of study materials or patients: Christopher N. Frantz, Laura A. Campbell, Susan L. Cohn, Katherine K. Matthay, Lisa Diller
Collection and assembly of data: Wendy B. London, Christopher N. Frantz, Laura A. Campbell, Robert C. Seeger, Lisa Diller
Data analysis and interpretation: Wendy B. London, Christopher N. Frantz, Babette A. Brumback, Katherine K. Matthay, Lisa Diller
Manuscript writing: Wendy B. London, Christopher N. Frantz, Laura A. Campbell, Babette A. Brumback, Susan L. Cohn, Katherine K. Matthay, Robert P. Castleberry, Lisa Diller
Final approval of manuscript: Wendy B. London, Christopher N. Frantz, Laura A. Campbell, Robert C. Seeger, Babette A. Brumback, Susan L. Cohn, Katherine K. Matthay, Robert P. Castleberry, Lisa Diller
REFERENCES
- 1.Takimoto CH, Arbuck SG. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. Topoisomerase I targeting agents: The camptothecins, in Chabner BA, Longo DL (eds): Cancer Chemotherapy and Biotherapy; pp. 579–604. [Google Scholar]
- 2.Oguro M. In Proceedings of The Third Conference on DNA Topoisomerases in Therapy. New York: 1990. Oct 15-18, A topoisomerase I inhibitor, CPT-11: Its enigmatic antitumor activity in combination with other agents in vitro; p. 35. [Google Scholar]
- 3.Blaney SM, Balis FM, Cole DE, et al. Pediatric phase I trial and pharmacokinetic study of topotecan administered as a 24-hour continuous infusion. Cancer Res. 1993;53:1032–1036. [PubMed] [Google Scholar]
- 4.Tubergen DG, Stewart CF, Pratt CB, et al. Phase I trial and pharmacokinetic (PK) and pharmacodynamics (PD) study of topotecan using a five-day course in children with refractory solid tumors: A Pediatric Oncology Group study. J Pediatr Hematol Oncol. 1996;18:352–361. doi: 10.1097/00043426-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Cole D, Blaney S, Balis F, et al. A phase I and pharmacokinetic study of topotecan in pediatric patients. Proc Am Soc Clin Oncol. 1992;11:116. abstr XXXX. [Google Scholar]
- 6.Pratt CB, Stewart C, Santana VM, et al. Phase I study of topotecan for pediatric patients with malignant solid tumors. J Clin Oncol. 1994;12:539–543. doi: 10.1200/JCO.1994.12.3.539. [DOI] [PubMed] [Google Scholar]
- 7.Nitschke R, Parkhurst J, Sullivan J, et al. Topotecan in pediatric patients with recurrent and progressive solid tumors: A Pediatric Oncology Group phase II study. J Pediatr Hematol Oncol. 1998;20:315–318. doi: 10.1097/00043426-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Blaney SM, Needle MN, Gillespie A, et al. Phase II trial of topotecan administered as 72-hour continuous infusion in children with refractory solid tumors: A collaborative Pediatric Branch, National Cancer Institute, and Children's Cancer Group Study. Clin Cancer Res. 1998;4:357–360. [PubMed] [Google Scholar]
- 9.Pappo AS, Lyden E, Breneman J, et al. Up-front window trial of topotecan in previously untreated children and adolescents with metastatic rhabdomyosarcoma: An Intergroup Rhabdomyosarcoma study. J Clin Oncol. 2001;19:213–219. doi: 10.1200/JCO.2001.19.1.213. [DOI] [PubMed] [Google Scholar]
- 10.Saylors RL, 3rd, Stewart CF, Zamboni WC, et al. Phase I study of topotecan in combination with cyclophosphamide in pediatric patients with malignant solid tumors: A Pediatric Oncology Group Study. J Clin Oncol. 1998;16:945–952. doi: 10.1200/JCO.1998.16.3.945. [DOI] [PubMed] [Google Scholar]
- 11.Saylors RL, 3rd, Stine KC, Sullivan J, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: A Pediatric Oncology Group phase II study. J Clin Oncol. 2001;19:3463–3469. doi: 10.1200/JCO.2001.19.15.3463. [DOI] [PubMed] [Google Scholar]
- 12.Kretschmar CS, Kletzel M, Murray K, et al. Response to paclitaxel, topotecan, and topotecan-cyclophosphamide in children with untreated disseminated neuroblastoma treated in an upfront phase II investigational window: A Pediatric Oncology Group study. J Clin Oncol. 2004;22:4119–4126. doi: 10.1200/JCO.2004.08.174. [DOI] [PubMed] [Google Scholar]
- 13.Brumback BA, London WB. Causal inference in cancer clinical trials. In: Tu X, Tang W, Kowalski J, editors. Modern Clinical Trial Analysis. New York, NY: Springer; 2008. (in press) [Google Scholar]
- 14.Murphy SA. Optimal dynamic treatment regimes (with discussion) J R Stat Soc B. 2003;65:331–366. [Google Scholar]
- 15.Robins JM. Optimal structural nested models for optimal sequential decisions. In: Lin DY, Heagerty P, editors. Proceedings of the Second Seattle Symposium on Biostatistics. New York, NY: Springer; 2004. pp. 189–326. [Google Scholar]
- 16.Frantz CN, Bernstein M, Castleberry R, et al. Dose escalation study of desferrioxamine (DFO) in children with refractory neuroblastoma. Proc Am Soc Clin Oncol. 1994;13:1420. [Google Scholar]
- 17.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 18.Agresti A. Categorical Data Analysis (ed 2) Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete O-observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Greenwood M. London, United Kingdom: His Majesty's Stationery Office; 1926. The natural duration of cancer, in Reports On Public Health and Medical Subjects 33; pp. 1–26. [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data (ed 2) Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 22.Cohn SL, Pearson ADJ, London WB, et al. The International Neuroblastoma Risk Group (INRG) Classification System. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau L, Tai D, Weitzman S, et al. Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol Oncol. 2004;26:227–232. doi: 10.1097/00043426-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Park JR, Stewart CF, London WB, et al. A topotecan-containing induction regimen for treatment of high risk neuroblastoma. J Clin Oncol. 2006;24(suppl):505s. doi: 10.1200/JCO.2010.34.3293. abstr 9013. [DOI] [PMC free article] [PubMed] [Google Scholar]