Abstract
Purpose
To identify children with T-cell acute lymphoblastic leukemia (T-ALL) at high risk of induction chemotherapy failure by using DNA copy number analysis of leukemic cells collected at diagnosis.
Patients and Methods
Array comparative genomic hybridization (CGH) was performed on genomic DNA extracted from diagnostic lymphoblasts from 47 children with T-ALL treated on Children's Oncology Group Study P9404 or Dana-Farber Cancer Institute Protocol 00-01. These samples represented nine patients who did not achieve an initial complete remission, 13 who relapsed, and 25 who became long-term, event-free survivors. The findings were confirmed in an independent cohort of patients by quantitative DNA polymerase chain reaction (DNA-PCR), an assay that is well suited for clinical application.
Results
Analysis of the CGH findings in patients in whom induction chemotherapy failed compared with those in whom induction chemotherapy was successful identified the absence of biallelic TCRγ locus deletion (ABD), a characteristic of early thymocyte precursors before V(D)J recombination, as the most robust predictor of induction failure (P < .001). This feature was also associated with markedly inferior event-free (P = .002) and overall survival (P < .001) rates: 25% versus 58% and 25% versus 72%, respectively. Using a rapid and inexpensive quantitative DNA-PCR assay, we validated ABD as a predictor of a poor response to induction chemotherapy in an independent series of patients.
Conclusion
Lymphoblasts from children with T-ALL should be evaluated at diagnosis for deletion within the TCRγ locus. Patients lacking biallelic deletion, which confers a high probability of induction failure with contemporary therapy, should be assigned to alternative therapy in the context of a prospective clinical trial.
INTRODUCTION
The intensification of therapy for children with T-cell acute lymphoblastic leukemia (T-ALL) has improved clinical outcomes substantially, but first-line therapy continues to fail in approximately 25% of children and in more than 50% of adults,1–4 imparting a poor prognosis.5–7 Initial induction chemotherapy fails to induce a morphologic remission in 4% to 10% of pediatric patients2,8 (also S. Winter, unpublished data), and the vast majority of relapses occur < 2 years from diagnosis.2,9 In B-precursor ALL, the ability to accurately identify patients at high risk of treatment failure has led to remarkable improvements in prognosis for subsets of high-risk patients, such as those with the BCR-ABL1 translocation.10,11 In T-ALL, by contrast, there are still no identified subsets for which therapeutic modification based on pretreatment risk factors leads to significantly improved results. Ideally, one would like to identify, at diagnosis, those patients with a high probability of induction failure, so that they can promptly be switched to alternative first-line therapy.
This article reports the results of comparative genomic hybridization (CGH) and quantitative polymerase chain reaction (PCR) analyses of leukemic cells from children with T-ALL treated in recent Children's Oncology Group (COG) and Dana-Farber Cancer Institute (DFCI) clinical trials. We identified the absence of biallelic TCRγ deletion (ABD) as a robust predictor of induction failure in T-ALL and demonstrated its ready detection with a quantitative DNA-PCR assay that is well suited for clinical application.
PATIENTS AND METHODS
Patient Samples
To generate the primary data set, we collected diagnostic specimens (with informed consent and institutional review board approval) from pediatric patients with newly diagnosed T-ALL who were treated on the COG P9404 Study (n = 40) and DFCI Acute Lymphoblastic Leukemia Consortium Protocol 00-01 (DFCI 00-01; n = 7).12,13 Samples for patients treated in two ongoing studies—COG AALL0434 (n = 12) and DFCI 05-01 (n = 15)—were used for the first validation cohort.14–16 A second validation cohort consisted of patients with T-ALL treated on St. Jude Total Therapy Studies 13A to 15 (n = 79).3,17 Complete methods are available in the Data Supplement (online only).
RESULTS
To identify genomic abnormalities that might predict response to T-ALL therapy, we performed array CGH on genomic DNA extracted from 47 T-ALL lymphoblast specimens collected at the time of diagnosis from children treated in the COG P9404 or DFCI 00-01 studies.12,13 These trials tested similar treatments, thus minimizing the variability of prognostic marker significance, a problem often associated with changes in therapy.18 The samples analyzed represented all patients with treatment failure who were available to us: nine patients had induction failure and 13 patients relapsed after successful induction. Samples were also taken from 25 long-term, event-free survivors (control group). None of the pretreatment clinical characteristics with prognostic significance in B-precursor ALL had predictive value in the T-ALL patient samples we analyzed (Table 1), consistent with previous reports from COG and DFCI.2,19
Table 1.
Clinical Characteristics of Patients with T-ALL Analyzed by Array CGH
| Characteristic | Patients With Treatment Failure (n = 22) |
Long-Term Survivors (n = 25) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .83 | ||||
| Median | 10.5 | 10 | |||
| Range | 3-17 | 2-17 | |||
| WBC at diagnosis, ×109/L | .69 | ||||
| Median | 216 | 161 | |||
| Range | 15-875 | 17-643 | |||
| Mediastinal mass | 7 of 22 | 32 | 13 of 25 | 52 | .23 |
| Sex | |||||
| Female | 6 of 22 | 27 | 6 of 25 | 24 | 1.00 |
| CNS status* | |||||
| 1 | 12 of 22 | 55 | 15 of 24 | 62 | .92 |
| 2 | 6 of 22 | 27 | 6 of 24 | 25 | |
| 3 | 4 of 22 | 18 | 3 of 24 | 13 | |
Abbreviations: T-ALL, T-cell acute lymphoblastic leukemia; CGH, comparative genomic hybridization.
No CNS data available for one long-term survivor.
ABD Robustly Predicts High-Risk T-ALL
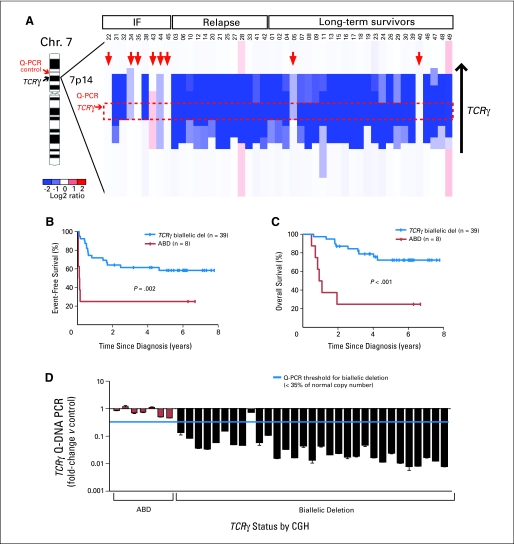
ABD, indicating that V(D)J recombination has not taken place on at least one TCRγ allele, was strongly related to a poor response to induction chemotherapy (P < .001). Six of the eight patients found to have ABD, including two that harbored monoallelic TCRγ deletions, had induction failure compared with only three of the remaining 39 patients in which both TCRγ alleles had recombined (Fig 1A). ABD was also a robust predictor of 5-year event-free survival (25% in the ABD group v 58% in patients with biallelic TCRγ deletions; P = .002; Fig 1B) and of 5-year overall survival (25% v 72%; P < .001; Fig 1C). Use of ABD as a risk factor led to classification of two of 25 long-term, event-free survivors into the high-risk group, yielding a false-positive rate of only 8%. It should be noted that our data set was enriched for patients who had treatment failure, which accounts for the lower-than-expected event-free and overall survival rates for the entire group.
Fig 1.
Relationship of absence of biallelic TCRγ locus deletion (ABD) status to clinical outcome. (A) Array comparative genomic hybridization (CGH) data obtained from diagnostic T-cell acute lymphoblastic leukemia (T-ALL) specimens from 47 children with T-ALL treated on Children's Oncology Group (COG) P9404 or Dana-Farber Cancer Institute (DFCI) 00-01 protocols are shown as a dChip plot of CGH segmented log2 copy number ratios at the TCRγ locus. Red arrows denote patients with ABD, with the threshold for biallelic TCRγ deletion defined as a CGH log2 ratio of −1.5, corresponding to 35% of normal copy number. The red box denotes the location of the intron between the most 3′ V pseudoexon (TRGV11) and the most 5′ J exon (TRGJP1), which should be involved by any deletion within the TCRγ locus as a result of V-J recombination. Location of the polymerase chain reaction (PCR) primer pairs at TCRγ and at the ANLN control, which were used for the quantitative DNA PCR (Q-PCR) assay to detect ABD, are indicated. (B, C) Kaplan-Meier event-free and overall survival rates for patients with T-ALL classified by ABD status. Tick marks indicate patients still at risk. (D) ABD status by CGH was validated by Q-PCR, with use of the TCRγ and ANLN control primer whose locations are shown in (A). Results of the CGH and DNA PCR analyses were concordant in 97% (37 of 38) samples. IF, induction failure; Chr, chromosome.
The absence of TCRβ deletion was also a significant predictor of induction failure (Data Supplement Fig 1A; P < .001) and of inferior event-free survival (Data Supplement Fig 1B; P = .03) and overall survival (Data Supplement Fig 1C; P = .004). Nonetheless, five of 25 long-term event-free survivors also had this feature (Data Supplement Fig 1A), resulting in a false-positive rate of 20%. The absence of biallelic TCRα/δ deletion predicted induction failure (Data Supplement Fig 2A; P = .007) and inferior event-free survival (Data Supplement Fig 2B; P = .02), but not overall survival (Data Supplement Fig 2C; P = .24), while having a false-positive rate of 24%. Despite being a statistically significant predictor of induction failure (Data Supplement Fig 3A; P = .02), the absence of biallelic CDKN2A deletion lacked a strong association with either event-free survival or overall survival (Data Supplement Figs 3B and 3C), limiting its clinical utility. Thus, among the CGH findings we identified, ABD appears to be the most reliable predictor of early treatment failure.
Development of a Quantitative DNA-PCR Assay to Detect ABD
The TCRγ locus harbors multiple V and J exons and pseudoexons that can be used during V-J recombination.20 Although the large number of potential V-J fusions complicates the development of a standard PCR assay that can reliably detect all possible productive and nonproductive recombination events on both alleles, the intron between the most 3′ V pseudoexon (TRGV11) and the most 5′ J exon (TRGJP1) should be involved by any deletion at the TCRγ locus resulting from V-J recombination. Hence, we developed a quantitative DNA-PCR (Q-PCR) assay, using primers that targeted this intron. Control Q-PCR primers targeted the ANLN locus, which encodes an actin-binding scaffolding protein21 that is located 1.9 Mbp downstream of TCRγ on chromosome 7 (Fig 1A) in a genomic region that was not affected by copy number alterations in any of the T-ALL patient samples analyzed by CGH.
The Q-PCR assay for ABD was performed on all of the T-ALL samples analyzed by CGH that had sufficient DNA available. It was successful in 88% (38 of 43) of samples analyzed, and the results were concordant with CGH in 97% (37 of 38) of the samples (Fig 1D). The lone sample that gave discordant results (patient 38) yielded CGH results consistent with biallelic TCRγ rearrangements but Q-PCR results consistent with ABD. We confirmed these findings by repeat array CGH analysis as well as repeat Q-PCR analysis, using an independent set of primers in the deleted TCRγ intron, together with a separate set of control primers in the INHBA locus, located 3.4 Mbp upstream of TCRγ (data not shown). Cytogenetically, this sample had additional material of unknown origin attached to chromosome 7 at band p15 (Data Supplement Table 1), possibly representing a translocation involving the TCRγ locus at 7p14; however, we had insufficient material to evaluate this finding further. Thus, CGH indicated an aberrant attempt at recombination of the TCRγ locus in this sample, while Q-PCR showed that the critical TCRγ intron between the most 3′ V pseudoexon (TRGV11) and the most 5′ J exon (TRGJP1) was retained.
Given that the contamination of T-ALL lymphoblast specimens by an excess of normal cells lacking TCRγ rearrangements will lead to misassignment of some patients to the high-risk ABD group by Q-PCR, we tested the degree of lymphoblast purity required for the assay to be successful. Application of Q-PCR to serial dilutions of DNA from a patient harboring biallelic TCRγ rearrangements with germline DNA demonstrated that the assay can still accurately detect the presence of biallelic TCRγ rearrangements when lymphoblast DNA is contaminated by as much as 25% germline DNA. However, we would conservatively recommend that this assay be performed only when the samples analyzed contain > 85% lymphoblasts. In our experience, this degree of purity can be readily achieved after Ficoll-Hypaque isolation of mononuclear cells from diagnostic specimens of most patients with T-ALL.
Overlap Between ABD and Early T-Cell Precursor ALL
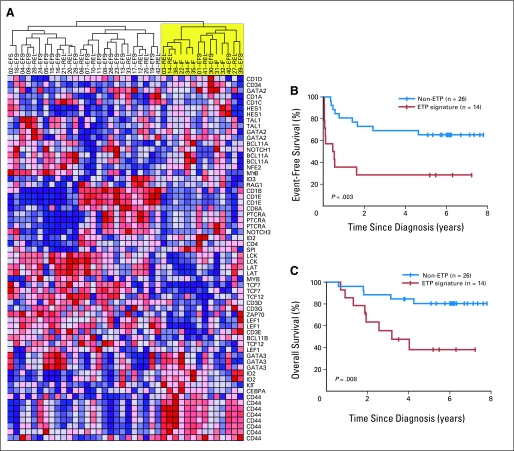
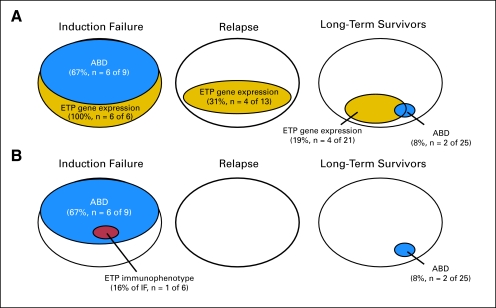
Recent work by Coustan-Smith et al22 identified an early T-cell precursor (ETP) phenotype of T-ALL, defined by either a characteristic gene expression signature or by immunophenotype, which was found to predict treatment failure in pediatric T-ALL. Given that TCRγ rearrangements occur early in normal T-cell development23,24 and that deletions of TCR loci are significantly less frequent in ETP T-ALL,22 we suspected that our ABD patients would demonstrate some biologic overlap with ETP T-ALL. Thus, using gene expression data available on 40 of the patient samples that were analyzed by CGH, we found that 14 of them had the ETP gene expression signature by hierarchical clustering (Fig 2A and Data Supplement Table 1). Indeed, five of the six ABD patient samples on which gene expression data were available possessed this signature (Data Supplement Table 1). Kaplan-Meier analyses indicated that the ETP gene expression signature and the ABD marker had similar predictive values (Figs 2B and 2C), with 5-year event-free survival rates of 28% and 25%, respectively, and 5-year overall survival rates of 38% and 25%, respectively (P = .17). However, when used as a risk factor, the ETP gene expression signature classified four of 21 long-term survivors into the high-risk group, a false-positive rate of 19%, compared with 8% for ABD.
Fig 2.
Early T-cell precursor (ETP) gene expression signature identifies a subset of patients with a poor prognosis. (A) Hierarchical clustering analysis of gene expression data based on a set of genes differentially expressed in ETP T-cell acute lymphoblastic leukemia (T-ALL)22 classifies 14 of our 40 patients as harboring the ETP gene expression signature. (B, C) Kaplan-Meier analyses of event-free survival and overall survival in T-ALL with or without the ETP gene expression signature. Tick marks indicate patients still at risk.
Immunophenotypes, available for 41 of the 47 patients in our series, were also analyzed for surface markers characteristic of ETP T-ALL.22 In marked contrast to the overlap between ABD status and the ETP gene expression signature, only one of 41 patients in our series (an ABD induction failure; Fig 3B and Data Supplement Table 1) met the immunophenotypic criteria for ETP T-ALL (CD1a, < 5%; CD8, < 5%; CD5, < 75%; and > 25% positivity for one or more of the following markers: CD117, CD34, HLA-DR, CD13, CD33, CD11b, or CD65). Each of the remaining 40 patients expressed CD5 on > 75% of blasts. Four of our patients had immunophenotypes that met all ETP criteria except for CD5 expression; these were induction failures that either demonstrated the ETP gene expression signature or had no gene expression data available (Data Supplement Table 1). Because CD5 is expressed at low levels but is generally not absent on ETP blasts (Fig 222 and D. Campana, personal communication), we suspect that the immunophenotyping data available at our participating COG and DFCI member institutions do not accurately distinguish between the low (but not absent) CD5 expression characteristic of ETP lymphoblasts and the higher CD5 expression present on the majority of T-ALL patient samples. Thus, our data underscore the need for careful interpretation of immunophenotype data, in particular CD5 expression, before the presence of the ETP immunophenotype is incorporated into clinical decision making.
Fig 3.
Overlap between absence of biallelic TCRγ locus deletion (ABD) and early T-cell precursor (ETP) T-cell acute lymphoblastic leukemia patients. (A) ABD versus ETP gene expression signature. (B) ABD versus ETP immunophenotype, which identified only one high-risk patient in this series.
Genetic Alterations Associated With ABD T-ALL
One of the eight specimens with ABD had a biallelic TCRα/δ rearrangement by CGH, while the others lacked any TCR rearrangement (Data Supplement Table 1). CDKN2A deletions were absent in six of the eight ABD patients, compared with eight of 31 of the patients with biallelic TCRγ deletions (Data Supplement Table 1; P = .02). NOTCH1 mutations were present in five of the eight ABD patients, with two also harboring NRAS-activating mutations (Data Supplement Table 1). No clinical features were specifically associated with ABD patients other than treatment response.
ABD T-ALL specimens were associated with specific gene expression characteristics, including overexpression of the HOXA/MEIS1 cluster, LYL1, and ERG (Data Supplement Figs 4A to 4C). Gene set enrichment analysis25 revealed similarities in gene expression profile between ABD lymphoblasts and gastric cancer cell lines in which cisplatin resistance was experimentally induced (Data Supplement Fig 4E),26 suggesting common mechanisms of drug resistance between these cell types. Furthermore, ABD T-ALL lymphoblasts showed increased expression of genes within the PI3K-PIP3-AKT and RAF-MEK-ERK signal transduction pathways (Data Supplement Figs 4F and 4G). Although these pathways are generally thought to be regulated primarily at the post-transcriptional level, our findings suggest the need for preclinical studies evaluating the therapeutic utility of small-molecule inhibitors of these pathways in ABD T-ALL.
Independent Validation of the Prognostic Value of ABD
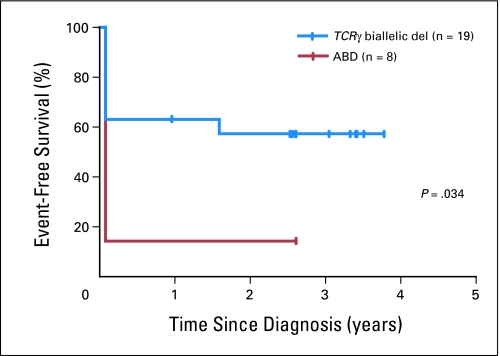
To validate ABD as a marker of high-risk disease in an independent cohort of patients, we obtained diagnostic specimens from children with T-ALL treated on clinical trials COG AALL0434 and DFCI 05-01. Because of the short follow-up times in these ongoing trials, it was not possible to assign patients to the long-term survivor versus relapse categories with any degree of certainty. Thus, we included only patients who had treatment failure (seven induction failures and one relapse), those with an inadequate response to induction chemotherapy based on minimal residual disease (MRD) > 1% at the end of induction chemotherapy (n = 7), and those in continuous complete remission ≥ 2.5 years from T-ALL diagnosis (n = 13). Q-PCR analysis for ABD, with adverse events defined as induction failure, relapse, or MRD > 1% at the end of induction chemotherapy, confirmed that ABD is a significant predictor of a poor response to induction chemotherapy (Fig 4; P = .03). Note that this group of samples was also highly enriched for patients who had treatment failure.
Fig 4.
Kaplan-Meier event-free survival analysis for an independent cohort of children with T-cell acute lymphoblastic leukemia (T-ALL) treated in the Children's Oncology Group AALL0434 and Dana-Farber Cancer Institute 05-01 clinical trials, whose absence of biallelic TCRγ locus deletion (ABD) status was evaluated by quantitative DNA polymerase chain reaction. The diagnostic specimens analyzed represented all of the high-risk patients who were available to us, including patients with treatment failure or with minimal residual disease (MRD) > 1% at the end of induction, and all patients in continuous complete remission > 2.5 years from T-ALL diagnosis. Note that MRD levels ≥ 1% at the end of induction have been associated with outcomes nearly as poor as those of patients with induction failure, with an estimated 5-year relapse-free survival rate of only 14%.33 Tick marks indicate patients still at risk.
To further examine the predictive value of ABD in an additional independent cohort of children with T-ALL, we took advantage of the T-ALL population of children treated on the St. Jude Total Therapy studies 13A to 15, in which diagnostic DNA copy number alterations were assessed by single nucleotide polymorphism array.27,28 Induction chemotherapy in the St. Jude studies is comparable to that in the COG and DFCI studies over the first 25 days of therapy, but thereafter, the St. Jude patients receive significant additional therapy before induction failure is assessed,3 so that induction failure rates are not directly comparable to those of COG and DFCI studies. Nonetheless, response to the first phase of induction chemotherapy at St. Jude is monitored by the MRD level on day 19 of therapy, prompting us to assess the prognostic value of TCRγ status at that time point. Ten of 14 patients with ABD had MRD ≥ 1% on day 19, compared with 13 of 65 patients harboring biallelic TCRγ deletions (P < .001), confirming the adverse prognostic significance of ABD in an additional independent cohort of children with T-ALL.
ABD in Relapsed T-ALL
The presence of ABD at diagnosis predicted the failure of induction chemotherapy but not relapse, indicating that ABD identifies T-ALL blasts with high-level resistance to chemotherapy. To test the hypothesis that relapse might occur in some non-ABD T-ALL patients because of outgrowth of a minor ABD subclone, we took advantage of matched newly diagnosed and relapse patient samples that were collected by the Mullighan and Ferrando laboratories.28,29 Single nucleotide polymorphism array or Q-PCR analysis of copy number at the TCRγ locus revealed that of 26 patients with biallelic TCRγ deletions at diagnosis, five had relapse clones characterized by ABD (Data Supplement Fig 5). It is difficult to imagine how a cell might reacquire genomic DNA that was biallelically deleted; hence, these data strongly suggest that the drug-resistant subclone responsible for relapse underwent T cell developmental arrest at an ABD stage, earlier than the majority of blasts at diagnosis.
DISCUSSION
We have identified ABD as a robust predictor of early treatment failure in children with T-ALL treated on contemporary protocols for this disease and have developed a quantitative DNA-PCR assay for reliable evaluation of ABD status in clinical settings. To the best of our knowledge, all contemporary front-line induction regimens for pediatric T-ALL rely heavily on most or all of the drugs used in our studies; thus, ABD is likely to confer a poor response to induction therapy on all modern protocols.
Normally, developing thymocytes proceed through a series of distinct (so-called double-negative) stages of differentiation before they begin to express CD4 or CD8. Murine double-negative thymocytes are subclassified into four stages—DN1-DN4—on the basis of their expression of CD44 and CD25, while three double-negative stages can be identified in human thymocytes, with the human prothymocyte stage (CD34+CD38+CD1a−) largely corresponding to the murine DN1 and DN2 stages.30 In normal human T-cell development, TCRγ rearrangements can be detected in rare prothymocytes by using PCR-based assays, but they are not common until the prethymocyte (CD34+CD38+CD1a+) stage,23,24 indicating that most normal human prothymocytes have not rearranged the TCRγ gene. Thus, T-ALL lymphoblasts identified by both ABD and the ETP gene expression signature likely represent patients showing developmental arrest at the prothymocyte stage of T-cell development. By contrast, half the T-ALL lymphoblast specimens with the ETP gene expression signature in our series had biallelic TCRγ rearrangements, indicating that this marker also identifies T-ALL blasts at more mature stages of T-cell development. Indeed, a subset of the data used to generate the ETP gene expression signature was derived from a population of mouse thymocytes predominantly at the DN3 stage,22,31 when TCRγ rearrangements are common.32 We conclude that patients with T-ALL with developmental arrest at the prethymocyte stage account for a significant fraction of those identified with the ETP gene expression signature. Thus, by using both the TCRγ DNA-PCR assay and the ETP gene expression signature, one can readily divide early thymic ALL patients into subsets arising before and immediately after the onset of TCRγ gene rearrangement.
Despite the close correlation between ABD and the ETP gene expression signature, only one of the 17 patients with treatment failure for whom we had complete immunophenotypes displayed the ETP profile of surface markers described by Coustan-Smith et al.22 This discrepancy can be attributed to the detection of CD5 on > 75% of cells in all but one of our patients. Typically, CD5 expression is 10-fold lower on early T-cell precursor ALL than on mature T cells but is not absent altogether (see Fig 2 of Coustan-Smith et al22 and D. Campana, personal communication). We suspect that the use of a threshold based on the percentage of positive blasts may have failed to accurately distinguish CD5-low ETP blasts from CD5-high patients with T-ALL in our series. Nonetheless, differences in the analysis and interpretation of ETP immunophenotypes could affect clinical decision making and will need to be resolved in future clinical trials.
Given the high rate of induction failure in patients with T-ALL with ABD and their dismal outcome regardless of salvage efforts, there is an urgent need to test alternative induction chemotherapy for these patients. We propose that ABD status be evaluated by quantitative DNA-PCR immediately at diagnosis in patients presenting with T-ALL and that candidate chemotherapy regimens be tested for ABD patients in the context of a prospective international collaborative trial.
Supplementary Material
Acknowledgment
We thank the children with T-cell acute lymphoblastic leukemia (T-ALL) and their families for permission to study T-ALL clinical specimens. Array comparative genomic hybridization analyses were performed and supported in part by the Belfer Institute for Applied Cancer Science. We also thank Dario Campana for critical review and helpful discussion, Giuseppe Basso for some of the matched diagnosis-relapse samples analyzed in this study, and John Gilbert for editorial review.
Footnotes
Supported by National Institutes of Health Grants No. NCI 5P01CA68484 (S.E.S., L.B.S., A.T.L.), NCI 1K08CA133103 (A.G.), NCI R01CA120196, and R01CA129382 (A.A.F.), the William Lawrence Foundation (A.G., A.T.L.), the Wine Advocate Fund for Philanthropy of The Community Foundation of the National Capital Region (S.E.S.), Children's Oncology Group 9900 cell biology study, and Grants No. CA98543, CA114766, and CA98413 from the National Institutes of Health. A.G. is a scholar of the American Society of Hematology-Harold Amos Medical Faculty Development Program, V.T. is a Lady Tata Memorial Trust Research Fellow, A.A.F. is a Leukemia and Lymphoma Society Scholar, and S.P.H. is the Ergen Family Chair in Pediatric Cancer at The Children's Hospital, Aurora, CO.
Presented in part at the 51st Annual Meeting of the American Society of Hematology, December 5-8, 2009, New Orleans, LA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00165178.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Alejandro Gutierrez, Stephen E. Sallan, A. Thomas Look
Administrative support: Alejandro Gutierrez
Provision of study materials or patients: Valeria Tosello, Jeffery Kutok, Mignon L. Loh, Adolfo A. Ferrando, Stuart S. Winter, Charles G. Mullighan, Lewis B. Silverman, Stephen P. Hunger
Collection and assembly of data: Alejandro Gutierrez, Jianhua Zhang, Ruta Grebliunaite, Alexei Protopopov, Valeria Tosello, Richard S. Larson, Michael J. Borowitz, Adolfo A. Ferrando, Stuart S. Winter, Charles G. Mullighan, Lynda Chin, A. Thomas Look
Data analysis and interpretation: Alejandro Gutierrez, Suzanne E. Dahlberg, Donna S. Neuberg, Jianhua Zhang, Takaomi Sanda, Valeria Tosello, Michael J. Borowitz, Adolfo A. Ferrando, Lewis B. Silverman, Lynda Chin, Stephen E. Sallan, A. Thomas Look
Manuscript writing: Alejandro Gutierrez, Mignon L. Loh, Stuart S. Winter, Charles G. Mullighan, Lewis B. Silverman, A. Thomas Look
Final approval of manuscript: Alejandro Gutierrez, Suzanne E. Dahlberg, Donna S. Neuberg, Jianhua Zhang, Ruta Grebliunaite, Takaomi Sanda, Alexei Protopopov, Valeria Tosello, Jeffery Kutok, Richard S. Larson, Michael J. Borowitz, Mignon L. Loh, Adolfo A. Ferrando, Stuart S. Winter, Charles G. Mullighan, Lewis B. Silverman, Lynda Chin, Stephen P. Hunger, Stephen E. Sallan, A. Thomas Look
REFERENCES
- 1.Annino L, Vegna ML, Camera A, et al. Treatment of adult acute lymphoblastic leukemia (ALL): Long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–871. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg JM, Silverman LB, Levy DE, et al. Childhood T-cell acute lymphoblastic leukemia: The Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21:3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks DI, Paietta EM, Moorman AV, et al. T-cell acute lymphoblastic leukemia in adults: Clinical features, immunophenotype, cytogenetics, and outcome from the large randomised prospective trial (UKALL XII/ECOG 2993) Blood. 2009;114:5136–5145. doi: 10.1182/blood-2009-08-231217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einsiedel HG, von Stackelberg A, Hartmann R, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: Results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Münster Group 87. J Clin Oncol. 2005;23:7942–7950. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 6.Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: A Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28:648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oudot C, Auclerc MF, Levy V, et al. Prognostic factors for leukemic induction failure in children with acute lymphoblastic leukemia and outcome after salvage therapy: The FRALLE 93 study. J Clin Oncol. 2008;26:1496–1503. doi: 10.1200/JCO.2007.12.2820. [DOI] [PubMed] [Google Scholar]
- 9.Ballerini P, Landman-Parker J, Cayuela JM, et al. Impact of genotype on survival of children with T-cell acute lymphoblastic leukemia treated according to the French protocol FRALLE-93: The effect of TLX3/HOX11L2 gene expression on outcome. Haematologica. 2008;93:1658–1665. doi: 10.3324/haematol.13291. [DOI] [PubMed] [Google Scholar]
- 10.Aricò M, Valsecchi MG, Camitta B, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342:998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- 11.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: A Children's Oncology Group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ClinicalTrials.gov. Treatment of Acute Lymphoblastic Leukemia in Children, DFCI 00-001. http://clinicaltrials.gov/ct2/show/NCT00165178?term=NCT00165178&rank=1.
- 13.Winter SS, Jiang Z, Khawaja HM, et al. Identification of genomic classifiers that distinguish induction failure in T-lineage acute lymphoblastic leukemia: A report from the Children's Oncology Group. Blood. 2007;110:1429–1438. doi: 10.1182/blood-2006-12-059790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov. Combination Chemotherapy in Treating Young Patients With Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia, COG AALL0434. http://clinicaltrials.gov/ct2/show/NCT00408005?term=winter+stuart+children%27s+oncology+group&rank=1.
- 15.ClinicalTrials.gov. Pegasparaginase or Asparaginase and Combination Chemotherapy in Treating Young Patients With Newly Diagnosed Acute Lymphoblastic Leukemia, DFCI 05001. http://clinicaltrials.gov/ct2/show/NCT00400946?term=NCT00400946&rank=1.
- 16.Silverman LB, Supko JG, Stevenson KE, et al. Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood. 2020;115:1351–1353. doi: 10.1182/blood-2009-09-245951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vrooman LM, Silverman LB. Childhood acute lymphoblastic leukemia: Update on prognostic factors. Curr Opin Pediatr. 2009;21:1–8. doi: 10.1097/MOP.0b013e32831f1f24. [DOI] [PubMed] [Google Scholar]
- 19.Pullen J, Shuster JJ, Link M, et al. Significance of commonly used prognostic factors differs for children with T cell acute lymphocytic leukemia (ALL), as compared to those with B-precursor ALL: A Pediatric Oncology Group (POG) study. Leukemia. 1999;13:1696–1707. doi: 10.1038/sj.leu.2401555. [DOI] [PubMed] [Google Scholar]
- 20.Lefranc MP, Giudicelli V, Ginestoux C, et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2009;37:D1006–D1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piekny AJ, Glotzer M. Anillin is a scaffold protein that links RhoA, actin, and myosin during cytokinesis. Curr Biol. 2008;18:30–36. doi: 10.1016/j.cub.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 22.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: A subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blom B, Verschuren MC, Heemskerk MH, et al. TCR gene rearrangements and expression of the pre-T cell receptor complex during human T-cell differentiation. Blood. 1999;93:3033–3043. [PubMed] [Google Scholar]
- 24.Dik WA, Pike-Overzet K, Weerkamp F, et al. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med. 2005;201:1715–1723. doi: 10.1084/jem.20042524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang HC, Kim IJ, Park JH, et al. Identification of genes with differential expression in acquired drug-resistant gastric cancer cells using high-density oligonucleotide microarrays. Clin Cancer Res. 2004;10:272–284. doi: 10.1158/1078-0432.ccr-1025-3. [DOI] [PubMed] [Google Scholar]
- 27.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 28.Mullighan CG, Phillips LA, Su X, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322:1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tosello V, Mansour MR, Barnes K, et al. WT1 mutations in T-ALL. Blood. 2009;114:1038–1045. doi: 10.1182/blood-2008-12-192039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staal FJ, Weerkamp F, Langerak AW, et al. Transcriptional control of t lymphocyte differentiation. Stem Cells. 2001;19:165–179. doi: 10.1634/stemcells.19-3-165. [DOI] [PubMed] [Google Scholar]
- 31.Tydell CC, David-Fung ES, Moore JE, et al. Molecular dissection of prethymic progenitor entry into the T lymphocyte developmental pathway. J Immunol. 2007;179:421–438. doi: 10.4049/jimmunol.179.1.421. [DOI] [PubMed] [Google Scholar]
- 32.Capone M, Hockett RD, Jr, Zlotnik A. Kinetics of T cell receptor beta, gamma, and delta rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44(+) CD25(+) Pro-T thymocytes. Proc Natl Acad Sci U S A. 1998;95:12522–12527. doi: 10.1073/pnas.95.21.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willemse MJ, Seriu T, Hettinger K, et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood. 2002;99:4386–4393. doi: 10.1182/blood.v99.12.4386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.