Abstract
Purpose
Despite initial treatment with surgical resection, radiotherapy, and chemotherapy, glioblastoma multiforme (GBM) virtually always recurs. Surgery is sometimes recommended to treat recurrence. In this study, we sought to devise a preoperative scale that predicts survival after surgery for recurrent glioblastoma multiforme.
Patients and Methods
The preoperative clinical and radiographic data of 34 patients who underwent re-operation of recurrent GBM tumors were analyzed using Kaplan-Meier survival analysis and Cox proportional hazards regression modeling. The factors associated with decreased postoperative survival (P < .05) were used to devise a prognostic scale which was validated with a separate cohort of 109 patients.
Results
The factors associated with poor postoperative survival were: tumor involvement of prespecified eloquent/critical brain regions (P = .021), Karnofsky performance status (KPS) ≤ 80 (P = .030), and tumor volume ≥ 50 cm3 (P = .048). An additive scale (range, 0 to 3 points) comprised of these three variables distinguishes patients with good (0 points), intermediate (1 to 2 points), and poor (3 points) postoperative survival (median survival, 10.8, 4.5, and 1.0 months, respectively; P < .001). The scale identified three statistically distinct groups within the validation cohort as well (median survival, 9.2, 6.3, and 1.9 months, respectively; P < .001).
Conclusion
We devised and validated a preoperative scale that identifies patients likely to have poor, intermediate, and good relative outcomes after surgical resection of a recurrent GBM tumor. Application of this simple scale may be useful in counseling patients regarding their treatment options and in designing clinical trials.
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common primary intrinsic brain tumor of adulthood and the most malignant glioma subtype.1 Despite significant advances in our basic understanding of tumor pathogenesis, the median overall survival of patients has increased only 3.3 months (from 11.3 months to 14.6 months) over the past 25 years.2,3 This poor prognosis is largely due to the near universal recurrence of tumors after initial treatment with maximal safe surgical resection, radiotherapy, and chemotherapy.4 At the time of tumor recurrence, additional systemic and local therapies, as well as repeat surgery, are commonly considered. While each of these therapies has potential benefits, each also carries associated risks, particularly surgery.
Previous studies have retrospectively assessed the outcomes of patients after recurrent GBM tumor resection.5–8 Among the variables significantly associated with overall survival in at least one of these studies are preoperative KPS score,5,6,8 extent of surgical resection,5 age,8 and time interval between the first and second operations.7 These studies did not, however, establish guidelines for providing preoperative advice to patients considering surgery. We therefore sought to develop an easy to use and reliable prognostic scoring system for use in counseling such patients.
Primary considerations in devising a preoperative scale were the evaluation of a broad range of potential prognostic factors and the generalizability of the scale across different health care providers and institutions. To satisfy the former, we included factors analyzed in previous surgical5–8 and nonsurgical9 studies of patients with recurrent gliomas. To address the latter, we sought to validate our scale on a cohort of patients treated at a different institution.
PATIENTS AND METHODS
Patients
The cohort initially used to devise the scale consisted of 34 consecutive patients with histologically confirmed supratentorial hemispheric GBM (WHO grade 4 astrocytoma).10 All patients received involved-field external-beam radiation therapy and either nitrosourea or temozolomide chemotherapy for treatment of their initial tumors. Tumor recurrence was defined as the appearance or enlargement since prior imaging of a contrast-enhancing mass on T1-weighted magnetic resonance imaging. At recurrence, demographic, clinical, and radiographic data were collected by the Neuro-Oncology Branch of the National Cancer Institute, National Institutes of Health (NIH), Bethesda, MD. All patients underwent maximal safe surgical resection of their recurrent tumors by a single surgeon at the NIH Clinical Center (J.K.P.). All patients were observed until the time of death.
The validation cohort consisted of patients with GBM who had undergone similar initial diagnosis and treatment at the Brigham and Women's Hospital (BWH), Boston, MA. Diagnosis of tumor recurrence was made using magnetic resonance imaging and/or contrast-enhanced computed tomography imaging and all patients underwent maximal safe surgical resection of their recurrent tumors. One hundred nine consecutive patients for whom complete demographic, clinical, and radiographic data had been collected at the time of tumor recurrence and were available for review were included. All patients were observed until the time of death. This study was approved by the institutional review boards for human studies of both institutions.
Prognostic Variables
All data with the exception of date of death were collected at the time of tumor recurrence. Demographic variables included age, sex, and relationship status (ie, single, married or divorced). Clinical and treatment variables included KPS, corticosteroid use, presence of headaches or seizures, and time from initial diagnosis to recurrence. Radiographic variables included side and lobe of tumor location. Also determined was an motor-speech-middle cerebral artery (MSM) score, a tally of tumor involvement of three prespecified eloquent/critical brain areas: the presumed motor area,11 the presumed speech area,11 and the areas directly adjacent to the M1 and/or M2 segments of the middle cerebral artery. Tumor volumes were calculated using the Medical Imaging Processing and Visualization program (http://mipav.cit.nih.gov/) or approximated using the formula for the volume of an ellipsoid (4/3 × π × radiusx × radiusy × radiusz). The sole outcome measure was survival time from the date of operation for tumor recurrence to the date of death.
Statistical Analysis
For the univariate analysis of potential prognostic factors, time-to-event distributions of the NIH patients were estimated with Kaplan-Meier plots and P values were obtained using log-rank tests. Factors with continuous variables were dichotomized using cutoff points that generated the greatest hazard ratios between the two resulting groups. Cox proportional hazards modeling was used for the multivariate analysis of potential prognostic factors. Briefly, all factors with P < .05 on univariate analysis were entered into the model and the model was refit in stepwise fashion after the sequential removal of nonsignificant factors. The process was stopped when only significant (P < .05) factors remained. Entering the significant factors sequentially and checking for and possibly removing factors that became nonsignificant confirmed the model.
The factors found to have prognostic significance on multivariate testing were used to devise a composite scale with potentially greater prognostic value than each of the individual factors. Kaplan-Meier survival analysis with log-rank testing was used to determine the prognostic significance of the scaling system. Validation of the scale was sought using a cohort of 109 patients evaluated and treated at a different institution (BWH).
Analyses were performed using MedCalc version 11.1.1.0 (MedCalc Software, Mariakerk, Belgium) and PASW Statistics 18.0 (SPSS Inc, Chicago, IL).
RESULTS
NIH Patients
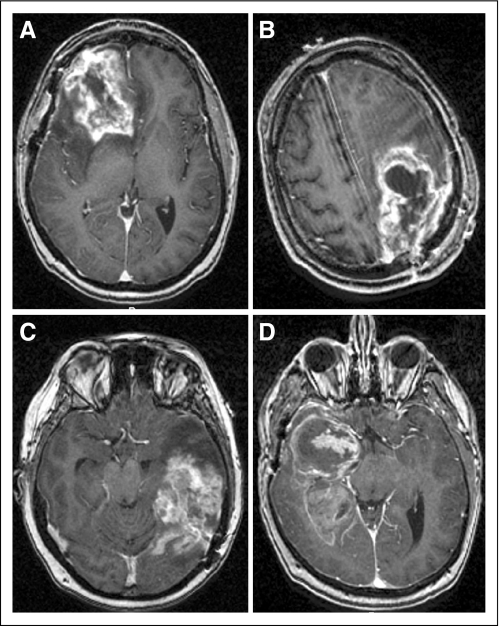
The baseline demographic, clinical, and radiographic characteristics of the patients evaluated and treated at the NIH are summarized in Table 1. The median age of the patients was 50.5 years (range, 22 to 65 years) and there were 22 males (64.7%) and 12 females (35.3%). Twenty-seven patients (79.4%) were married, four (11.8%) were single, and three (8.8%) were divorced or separated. The median KPS was 90 (range, 40 to 100), 24 patients (70.6%) were on corticosteroids, 18 (52.9%) had headaches, and 17 (50%) had seizures. The median time interval from initial diagnosis to recurrence was 11.1 month (range, −0.4 to 68.7 months). Eighteen patients (52.9%) had left-sided tumors, 19 (55.9%) had tumors in the frontal lobe, and the median tumor volume was 27.6 cm3 (range −0.8 to 98.8 cm3; Fig 1A). The median MSM score, a tally of tumor involvement of three prespecified eloquent/critical brain areas, was 1 (range, −0 to 3) and four patients (11.8%) had scores of 2 or 3 (Figs 1B and 1C, D).
Table 1.
Demographic, Clinical, and Radiographic Characteristics of Recurrent National Institutes of Health Patients
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 50.5 | |
| Range | 22-65 | |
| Sex | ||
| Male | 22 | 64.7 |
| Female | 12 | 35.3 |
| Relationship status | ||
| Married | 27 | 79.4 |
| Single | 4 | 11.8 |
| Divorced or separated | 3 | 8.8 |
| KPS score | ||
| Median | 90 | |
| Range | 40-100 | |
| 100 | 15 | 44.1 |
| 90 | 11 | 32.4 |
| 80 | 2 | 5.9 |
| 70 | 4 | 11.8 |
| ≤ 60 | 2 | 5.9 |
| Corticosteroid therapy | ||
| Yes | 24 | 70.6 |
| No | 10 | 29.4 |
| Headaches | ||
| No | 18 | 52.9 |
| Yes | 16 | 47.1 |
| Seizures | ||
| Yes | 17 | 50 |
| No | 17 | 50 |
| Time since initial diagnosis, months | ||
| Median | 11.1 | |
| Range | 0.4 to 68.7 | |
| Side of tumor location | ||
| Left | 18 | 52.9 |
| Right | 16 | 47.1 |
| Predominant lobe of tumor location | ||
| Frontal | 19 | 55.9 |
| Temporal | 8 | 23.5 |
| Parietal | 5 | 14.7 |
| Occipital | 2 | 5.9 |
| Eloquent/critical regions involved | ||
| 0 | 15 | 44.1 |
| 1 | 15 | 44.1 |
| 2 | 2 | 5.9 |
| 3 | 2 | 5.9 |
| Tumor volume, cm3 | ||
| Median | 27.6 | |
| Range | 0.8 to 98.8 | |
Abbreviation: KPS, Karnofsky performance status.
Fig 1.
Representative T1-weighted magnetic resonance images following administration of gadolinium contrast. (A) Right frontal tumor with volume of 50 cm3; (B) left frontal-parietal tumor with involvement of motor area; (C) left temporal tumor with involvement of speech area; (D) right temporal tumor with involvement of middle cerebral artery. The MSM score is an indication of tumor involvement of the motor, speech, and middle cerebral artery region.
Prognostic Variables of Survival
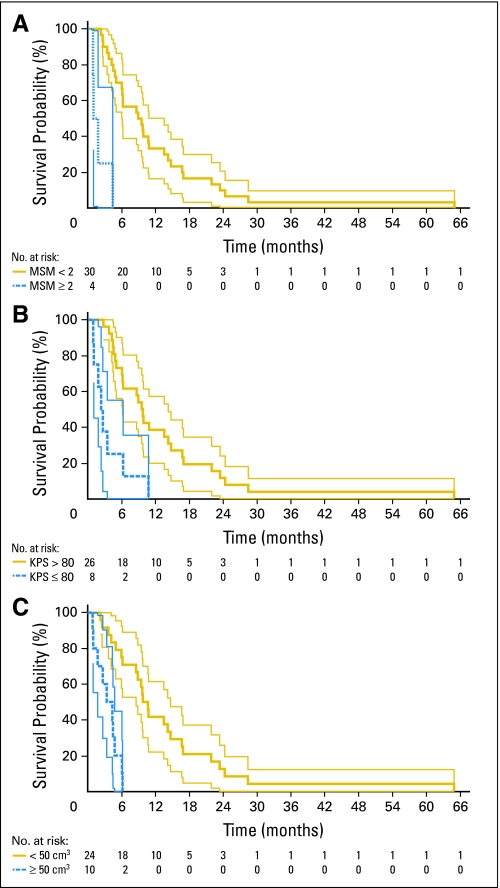
The median time interval from re-operation to death for all patients was 7.4 months (range, −0.9 to 64.9 months). The demographic, clinical, and radiographic variables collected preoperatively were analyzed using Kaplan-Meier estimation with log-rank statistics to identify those potentially prognostic of poor postoperative survival. Variables with more than two possible values (ie, age, KPS, time from initial diagnosis to recurrence, MSM score, and tumor volume) were dichotomized using the cutoff threshold that yielded the greatest hazard ratio (HR) between the two resulting groups. The three variables found to be significant (P < .05) on Kaplan-Meier analysis with log-rank testing were: MSM score ≥ 2 (P < .001; HR, 13.32; 95% CI, 3.63 to 48.88); KPS score ≤ 80 (P < .001; HR, 4.70; 95% CI, 1.96 to 11.30); and tumor volume ≥ 50 cm3 (P < .001; HR, 7.63; 95% CI, 2.78 to 20.98; Table 2, Figs 2A to 2C). The other examined variables had P > .05 and were therefore excluded from further consideration (Appendix Figs 1A to 1I, online only).
Table 2.
Univariate Analysis of Prognostic Factors
| Factor | Median Survival (months) |
P | |
|---|---|---|---|
| Yes | No | ||
| No. of eloquent/critical regions, ≥ 2* | 1.4 | 9.0 | < .001† |
| KPS, ≤ 80* | 2.5 | 9.6 | < .001† |
| Tumor volume, ≥ 50 cm3* | 3.9 | 10.3 | < .001† |
| Predominantly frontal lobe tumor | 6.2 | 9.7 | .227 |
| Time from initial diagnosis*, ≥ 6 months | 7.4 | 8.5 | .236 |
| Corticosteroid use | 6.1 | 9.8 | .349 |
| Relationship status, married | 8.6 | 6.0 | .459 |
| Headaches | 7.9 | 7.3 | .543 |
| Seizures | 9.7 | 6.1 | .714 |
| Sex, female | 7.9 | 7.4 | .802 |
| Age*, > 50 years | 6.1 | 8.6 | .823 |
| Tumor side, left | 7.4 | 7.5 | .825 |
Abbreviation: KPS, Karnofsky performance status.
Values were dichotomized to yield greatest hazard ratios.
Included in multivariate analysis.
Fig 2.
Kaplan-Meier plots of variables found to be significantly associated with survival using univariate testing. (A) Motor, speech, and middle cerebral artery region (MSM) score—a tally of tumor involvement of three prespecified eloquent/critical brain areas: the presumed motor area, the presumed speech area, and the areas directly adjacent to the M1 and/or M2 segments of the middle cerebral artery. Gold line represents patients with MSM lower than 2. Blue line represents patients with MSM ≥ 2. (B) Karnofsky performance status (KPS). Gold line represents patients with KPS higher than 80. Blue line represents patients with KPS ≤ 80. (C) Tumor volume. Gold line represents patients with tumor volume smaller than 50 cm3. Blue line represents patients with tumor volume ≥ 50 cm3. For all three plots, thin light lines represent 95% CIs of the enclosed thick dark lines. Below each plot, the number of patients at risk at various time points is provided.
Multivariate analysis was performed using Cox proportional hazards modeling. Both backward and forward stepwise modeling confirmed the prognostic significance of: MSM score ≥ 2 (P = .021; HR, 6.02; 95% CI, 1.32 to 27.78); KPS score ≤ 80 (P = .030; HR, 3.12; 95% CI, 1.12 to 8.62); and tumor volume ≥ 50 cm3 (P = .048; HR, 3.42; 95% CI, 1.01 to 11.62; Wald χ32 = 25.03, P < .001).
Establishment and Validation of the NIH Recurrent GBM Scale
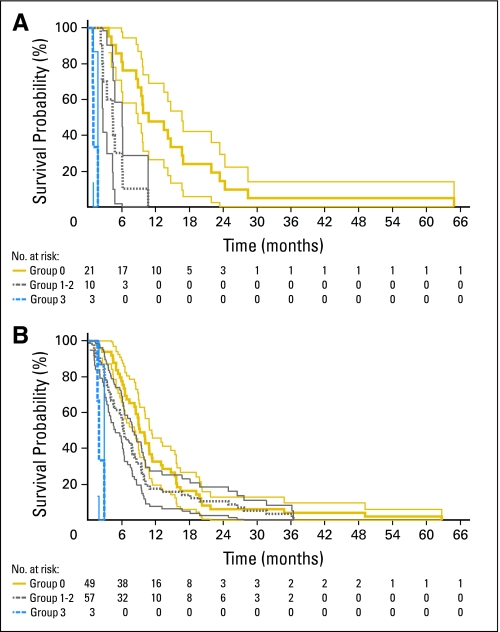
The prognostic variables found to be significant on multivariate analysis were used to devise a simple composite preoperative scale (NIH Recurrent GBM Scale). To determine a patient's score, 1 point is assigned for the presence of each of the following factors: preoperative KPS score ≤ 80, tumor volume ≥ 50 cm3, and MSM score ≥ 2. The total score is the sum of the points and ranges from 0 to 3. Of the 34 patients treated at the NIH, 21 had a score of 0 (median survival, 10.8 months; 95% CI, 8.9 to 16.7 months), seven had a score of 1 (median survival, 4.5 months; 95% CI, 2.6 to 6.1 months), three had a score of 2 (median survival, 4.4 months; 95% CI, 3.4 to 6.2 months), and three had a score of 3 (median survival, 1.0 months; 95% CI, 0.9 to 1.1 months). Patients with 1 point and those with 2 points did not significantly differ in their postoperative survivals (P = .969, log-rank test) and were therefore combined into a single prognostic group (n = 10 patients; median survival, 4.5 months; 95% CI, 2.7 to 6.1 months). Patients with a score of 3 (poor prognosis) differed significantly from those with a score of 1 to 2 (intermediate prognosis; P < .001, log-rank test) as well as from those with a score of 0 (good prognosis; P < .001, log-rank test). In addition, patients with a score of 1 to 2 differed significantly from those with a score of 0 (P < .001, log-rank test; Table 3 and Fig 3A).
Table 3.
NIH Recurrent GBM Scale
| Cohort and Score | Median Survival (months) | 95% CI | Prognostic Group |
|---|---|---|---|
| NIH | |||
| 0 | 10.8 | 8.9 to 16.7 | Good |
| 1-2 | 4.5 | 2.7 to 6.1 | Intermediate |
| 3 | 1.0 | 0.9 to 1.1 | Poor |
| BWH | |||
| 0 | 9.2 | 8.2 to 11.3 | Good |
| 1-2 | 6.3 | 4.8 to 7.9 | Intermediate |
| 3 | 1.9 | 1.7 to 2.9 | Poor |
Abbreviations: NIH, National Institutes of Health, GBM, glioblastoma multiforme; BWH, Brigham and Women's Hospital.
Fig 3.
Kaplan-Meier plots of National Institutes of Health (NIH) and Brigham and Women's Hospital (BWH) patients stratified according to the NIH Recurrent GBM Scale. (A) NIH patients. (B) BWH patients. For both plots, gold lines represent group 0 patients, gray lines represent group 1 to 2 patients, and blue lines represent group 3 patients. Thin light lines represent 95% CIs of the enclosed thick dark lines. Below each plot, the number of patients at risk at various time points is provided.
Validation of the NIH Recurrent GBM Scale was sought by applying it to patients treated at BWH. Of the 109 patients included in the study, the median survivals of those with 3 points (n = 3), 1 to 2 points (n = 57), and 0 points (n = 49) were 1.9 months (95% CI, 1.7 to 2.9 months), 6.3 months (95% CI, 4.8 to 7.9 months), and 9.2 months (95% CI, 8.2 to 11.3 months), respectively. As with the NIH patients, the BWH patients with 3 points differed significantly from those with 1 to 2 points (P < .001, log-rank test; HR, 3.00; 95% CI, 1.54 to 5.83) as well as from those with 0 points (P < .001, log-rank test; HR, 2.97; 95% CI, 1.72 to 5.12). In addition, patients with 1 to 2 points differed significantly from those with 0 points (P = .045, log-rank test; HR, 1.48; 95% CI, 1.01 to 2.20; Table 3 and Fig 3B). This difference was not as pronounced as with the NIH patients and may be due to the relatively greater number of longer term survivors (> 12 months) in the BWH 1 to 2 group. Of note, univariate analysis of the BWH patients revealed that MSM score ≥ 2 (P < .001, log-rank test) was prognostic for poor postoperative survival, but KPS score ≤ 80 (P = .262, log-rank test) and tumor volume ≥ 50 cm3 (P = .249, log-rank test) were not.
DISCUSSION
Given the near inevitability of GBM tumor recurrence, most patients eventually consider further treatment options. Among the alternatives are supportive care, additional systemic or local therapies, and/or additional surgery. In addition to an immediate decrease in tumor burden, a potential benefit of surgery is the improvement of tumor related neurologic symptoms and deficits. A potential risk is the exacerbation or new onset of the same, as well as a temporary or permanent exclusion from other therapies. We therefore devised and validated a simple scale—the NIH Recurrent GBM Scale—that utilizes easily obtainable preoperative data to provide objective information regarding likely postsurgical outcomes. To determine a particular patient's score, 1 point is assigned for the presence of each of the following: MSM score ≥ 2, KPS score ≤ 80, and tumor volume ≥ 50 cm3. The total score is the sum of the points and can range from 0 to 3. The advantage of using a composite score rather than individual factors to obtain prognostic information is clearly demonstrated by the lack of significance KPS score and tumor volume each by themselves had on survival in the validation cohort.
The brain areas directly adjacent to the M1 and/or M2 segments of the middle cerebral artery have not traditionally been considered eloquent brain regions. From a surgical point of view, however, injury to these vessels can result in damage to the eloquent brain regions they supply. They were therefore considered critical regions and included in determining the MSM score. We also examined the proximity of tumors to the anterior choroidal, posterior cerebral, and anterior cerebral arteries, but the small numbers of tumors involving these arteries did not allow us to draw any statistically significant conclusions.
Application of the NIH Recurrent GBM Scale facilitates the preoperative estimation of a patient's postoperative survival. Patients with a score of 3 points, regardless of treating institution, were found to have poor survival after surgery and appear to have derived little, if any, survival benefit. Because their numbers were small, however, surgical recommendations to future patients in this group should be tailored to the individual circumstances of each particular patient. As an example, patients in this prognostic group who wish to enroll in clinical trials requiring a life expectancy of ≥ 2 months will likely be advised to do so without a re-operation if they otherwise qualify. The patients in our study with intermediate prognosis scores (1 to 2 points) had survival times consistent with those of the intermediate prognosis patients in various New Approaches to Brain Tumor Therapy CNS Consortium conducted trials (recursive partitioning analysis classes 5 and 6).9 Likewise, the good prognosis patients (0 points) in our study had survival times in keeping with those of the good prognosis patients in the aforementioned New Approaches to Brain Tumor Therapy trials (recursive partitioning analysis class 4).9 Future patients with scores of 0 to 2 points will be counseled to undergo surgery if indicated, and encouraged to subsequently enroll in clinical trials of experimental therapies if eligible.
The NIH Recurrent GBM Scale, because of its prognostic power and ease of use, may also be helpful for stratifying patients during the enrollment or analysis of clinical trials. While patients with a score of 3 (median survival, 1.0 months) are unlikely to qualify for the majority of trials, those with scores of 1 to 2 (median survival, 4.5 months) and 0 (median survival, 10.8 months) should be enrolled or analyzed as separate groups given their significant differences in survival (HR for score of 1 to 2 v score of 0, 5.85; 95% CI, 2.33 to 14.73).
One limitation of our study is that it does not determine the survival benefit of re-operation per se, as all patients underwent surgery. A randomized clinical trial designed to determine a survival benefit would be difficult to conduct given the obvious symptomatic benefits that surgery can provide in many instances. Another limitation is that data on therapies administered after re-operation were not included in our analyses. Doing so, however, would have been contrary to the intent of our study, the establishment of a scale comprised solely of preoperative factors. In addition, given the overall lack of efficacy of phase II tested therapies for recurrent gliomas,9,12 their contribution to postoperative survival is likely to have been negligible. A possible exception to this is bevacizumab, which has resulted in median overall survival times of 31 weeks and 9.2 months.13,14 The two patients in the NIH cohort who received bevacizumab had relatively good survivals (ie, 16.7 and 23.4 months), but this would have been predicted given their scores of 0 points. It would be of interest to stratify patients in future phase III trials of bevacizumab using our scale.
The NIH Recurrent GBM Scale was devised and validated to generate objective information with which to advise patients with recurrent GBM tumors. In the broader health care context, it is an initial step in using comparative-effectiveness data to inform medical practices in the treatment of GBM recurrence.15 Patients with a score of 3 points are less likely to derive a survival benefit from surgery and the attendant risks should therefore be evaluated on a case-by-case basis. In comparison, patients with scores of 1 to 2 or 0 points have significantly longer expected postoperative survival periods, and re-operation, if indicated, should be pursued. Since surgery is not curative, enrollment in clinical trials of additional systemic or local therapies should also be encouraged to increase the chances of rigorously identifying potentially effective treatments.
Supplementary Material
Footnotes
Supported by the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke, the National Cancer Institute, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Presented in part at the Annual Meeting of the Congress of Neurological Surgeons, October 24-29, 2009, New Orleans, LA.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: John K. Park
Financial support: John K. Park, Howard A. Fine, Peter McL. Black
Administrative support: John K. Park, Howard A. Fine, Peter McL. Black
Provision of study materials or patients: John K. Park, Teri Nguyen Kreisl, Fabio M. Iwamoto, Joohee Sul, Howard A. Fine, Peter McL. Black
Collection and assembly of data: John K. Park, Tiffany Hodges, Leopold Arko, Michael Shen, Donna Dello Iacono, Adrian McNabb, Nancy Olsen Bailey, Teri Nguyen Kreisl, Fabio M. Iwamoto, Joohee Sul
Data analysis and interpretation: John K. Park, Tiffany Hodges, Leopold Arko, Michael Shen, Donna Dello Iacono, Adrian McNabb, Nancy Olsen Bailey, Sungyoung Auh, Grace E. Park, Peter McL. Black
Manuscript writing: John K. Park, Tiffany Hodges, Leopold Arko, Sungyoung Auh, Grace E. Park, Howard A. Fine, Peter McL. Black
Final approval of manuscript: John K. Park, Tiffany Hodges, Leopold Arko, Michael Shen, Donna Dello Iacono, Adrian McNabb, Nancy Olsen Bailey, Teri Nguyen Kreisl, Fabio M. Iwamoto, Joohee Sul, Sungyoung Auh, Grace E. Park, Howard A. Fine, Peter McL. Black
REFERENCES
- 1.Central Brain Tumor Registry of the United States. Central Brain Tumor Registry of the United States Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2010. [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 5.Ammirati M, Galicich JH, Arbit E, et al. Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery. 1987;21:607–614. doi: 10.1227/00006123-198711000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Barker FG, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42:709–720. doi: 10.1097/00006123-199804000-00013. discussion 720-723, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Dirks P, Bernstein M, Muller PJ, et al. The value of reoperation for recurrent glioblastoma. Can J Surg. 1993;36:271–275. [PubMed] [Google Scholar]
- 8.Harsh GR, Levin VA, Gutin PH, et al. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615–621. doi: 10.1227/00006123-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Carson KA, Grossman SA, Fisher JD, et al. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang EF, Smith JS, Chang SM, et al. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109:817–824. doi: 10.3171/JNS/2008/109/11/0817. [DOI] [PubMed] [Google Scholar]
- 12.Arko L, Katsyv I, Park GE, et al. Experimental approaches for the treatment of malignant gliomas. Pharmacol Ther. 2010 doi: 10.1016/j.pharmthera.2010.04.015. (in press) doi: 10.1016/j.pharmthera.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 14.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mushlin AI, Ghomrawi H. Health care reform and the need for comparative-effectiveness research. N Engl J Med. 2010;362:e6. doi: 10.1056/NEJMp0912651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.