Abstract
Purpose
Men with penile squamous cell carcinoma and regional lymph node involvement have a low probability of survival with lymphadenectomy alone. A multimodal approach to treatment is desirable for such patients. We performed a phase II study of neoadjuvant chemotherapy with the objective of determining the response rate, time to progression (TTP), and overall survival (OS) among patients with bulky adenopathy.
Patients and Methods
Eligible patients had stage N2 or N3 (stage III or stage IV) penile cancer without distant metastases. Neoadjuvant treatment (four courses every 3-4 weeks) consisted of paclitaxel 175 mg/m2 administered over 3 hours on day 1; ifosfamide 1,200 mg/m2 on days 1 to 3; and cisplatin 25 mg/m2 on days 1 to 3. Clinical and pathologic responses were assessed, and patient groups were compared for TTP and OS.
Results
Thirty men received chemotherapy of whom 15 (50.0%) had an objective response and 22 (73.3%) subsequently underwent surgery. Three patients had no remaining tumor on histopathology. Nine patients (30.0%) remained alive and free of recurrence (median follow-up, 34 months; range, 14-59 months), and two patients died of other causes without recurrence. Improved TTP and OS were significantly associated with a response to chemotherapy (P < .001 and P = .001, respectively), absence of bilateral residual tumor (P = .002 and P = .017, respectively), and absence of extranodal extension (P = .001 and P = .004, respectively) or skin involvement (P = .009 and P = .012, respectively).
Conclusion
The neoadjuvant regimen of paclitaxel, ifosfamide, and cisplatin induced clinically meaningful responses in patients with bulky regional lymph node metastases from penile cancer.
INTRODUCTION
Squamous cell carcinoma of the penis is uncommon in North America and Western Europe. Approximately 1,400 new cases are diagnosed annually in the United States.1 It is more prevalent in parts of Africa, South America, and Asia, making it an important global health problem.2 Worldwide, an estimated 26,000 new cases are diagnosed annually.3
Because of its rarity, there are no published reports of completed prospective clinical trials of combined modality treatment for metastatic penile cancer as there are for squamous cell cancers of the anus, vulva, and other primary sites.4–6 Prospective and retrospective studies of inguinal and pelvic lymphadenectomy in men with penile cancer have shown that selected patients with regional lymph node metastases can be rendered free of disease surgically and enjoy long-term survival.7–9 Distant metastases of penile carcinoma are rapidly fatal, however, with an estimated median overall survival (OS) duration of 28 weeks in the largest published prospective experience with combination chemotherapy.10 Retrospective analyses of chemotherapy given as adjuvant or neoadjuvant to lymphadenectomy for regional lymph node metastases have demonstrated the feasibility of this multimodal approach but do not allow firm conclusions about its efficacy.11–13
Men with three or fewer unilateral inguinal lymph node metastases, without extranodal extension or pelvic lymph node involvement, have a low rate of disease recurrence: 10% to 20% after surgery alone.14 The recurrence rate is much higher in patients who have bilateral lymph node metastases or extranodal extension, and the rate is 80% to 90% in patients whose pelvic lymph nodes are involved.15 Surgery alone seldom results in long-term disease control for patients with both extranodal extension and pelvic lymph node metastases.16
Thus, the use of a multimodal treatment approach is desirable for men who have penile cancer with advanced inguinal and pelvic lymph node metastases. Multiple strategies are possible, including postoperative radiotherapy, chemoradiotherapy,17 adjuvant chemotherapy,11 and neoadjuvant chemotherapy.12,13,18 To explore one of these possible strategies, we performed this prospective, nonrandomized phase II clinical trial of the neoadjuvant use of paclitaxel, ifosfamide, and cisplatin chemotherapy in patients with stage TX, N2-3, M0 penile cancer to estimate the efficacy of that regimen in terms of conventional response rate, pathologic complete response (pCR) rate, OS, and time to progression (TTP). We chose this drug combination because it had activity in squamous cell carcinoma of the head and neck.19 To our knowledge, this is the first report of data from a study prospectively conducted to evaluate a combined modality treatment in metastatic penile cancer.
PATIENTS AND METHODS
The protocol for this trial was approved by our institutional review board, and all patients provided written informed consent to participate before they were enrolled. Because it was not a randomized trial, our study was not designed to compare the results of neoadjuvant chemotherapy with surgery alone.
Eligibility Criteria
To be eligible for enrollment, men were required to have histologically confirmed squamous cell carcinoma of the penis of any T stage and of clinical stage N2 or N3 (according to the 1987 to 2002 TNM staging system of the American Joint Committee on Cancer20), with no evidence of distant metastases. Patients with a unilateral groin mass were eligible if the largest dimension of the mass was at least 4 cm and metastatic squamous cell carcinoma was confirmed on needle-biopsy specimens or if the mass was immobile. Men with two or more discrete enlarged inguinal lymph nodes or with bilateral lymphadenopathy were also eligible, regardless of node size, if a biopsy specimen revealed metastatic squamous cell carcinoma. Patients who had enlarged pelvic lymph nodes on computed tomography imaging were eligible with or without biopsy confirmation.
Patients were also required to have a Zubrod performance status of 0 to 2. Patients who had a history of clinically significant coronary artery disease were required to have a documented estimated left ventricular ejection fraction of at least 40% before they could be enrolled.
Men were excluded from study participation if they had serum concentrations of ALT or AST that were more than twice the upper limit of the normal range, total bilirubin > 2.0 mg/dL, conjugated bilirubin > 1.5 mg/dL, a calculated glomerular filtration rate of ≤ 40 mL/min, evidence of myocardial ischemia or severe conduction abnormalities on 12-lead electrocardiography, or any uncontrolled infection, or if they had previously undergone any systemic chemotherapy for penile carcinoma or any previous radiotherapy to the inguinal or pelvic lymph nodes.
Chemotherapy
The treatment regimen consisted of four cycles of 21- to 28-days duration each, in which patients received 175 mg/m2 paclitaxel intravenously (IV) over 3 hours day 1; 1,200 mg/m2 ifosfamide IV over 2 hours days 1, 2, and 3; and 25 mg/m2 cisplatin IV over 2 hours days 1, 2, and 3. The cycle was repeated on day 22 if the patient's absolute neutrophil count was at least 1,400/μL and platelet count was at least 100,000/μL. The use of prophylactic granulocyte colony-stimulating factor was allowed but not required.
Before each dose of paclitaxel, patients were given premedication consisting of dexamethasone (8 mg IV 1 hour before or 20 mg orally at both 12 and 6 hours before the paclitaxel dose), diphenhydramine (50 mg IV), and either cimetidine (300 mg IV), ranitidine (50 mg IV), or famotidine (20 mg IV). For urothelial protection, patients were given mesna intravenously (400 mg/m2 before each dose of ifosfamide and 200 mg/m2 at both 4 and 8 hours after each dose of ifosfamide). Cisplatin was administered intravenously in 250 mL of normal saline containing 12.5 g of mannitol, which was followed by intravenous hydration with 3 L of a solution of 5% dextrose, sodium chloride, potassium chloride, magnesium sulfate, and mannitol. For subsequent cycles, a 15% dosage reduction of all three cytotoxic agents was required in the event of nonrecovery of blood counts by day 22 or if any grade 3 or higher treatment-related nonhematologic adverse event occurred.
Computed tomography imaging of the abdomen and pelvis with oral, rectal, and intravenous administration of contrast medium was performed after the second cycle of chemotherapy and again at the completion of chemotherapy and before surgery. Tumor responses were assessed by using Response Evaluation Criteria in Solid Tumors (RECIST) criteria, version 1.0.21 Toxic effects were tabulated according the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.22
Surgical Technique
After completing neoadjuvant chemotherapy, patients who were medically fit to undergo resection of residual disease or who had experienced a radiographic complete response (CR) underwent surgery for consolidation. Full details of the technique have been described elsewhere.13,23 Briefly, surgical incisions were planned to allow resection of grossly palpable or visible residual disease with negative surgical margins and to leave the normal surrounding tissue. Thus, a wide ellipse of normal skin was often included in the resected specimen. The resected tissue also included the underlying lymph nodes between the lateral borders of the adductor longus and sartorious muscles, with complete removal of the muscular fascia, skeletonization of the femoral vessels, and en bloc resection of the saphenous vein. Ancillary procedures occasionally used to achieve negative margins, such as resection of the femoral vessels or abdominal wall, have previously been described.13,23
Subsequently, an ipsilateral pelvic lymph node dissection was performed with the boundaries including the genitofemoral nerve laterally, the bladder medially, the ureters superiorly, and the Cloquet node within the femoral canal distally. Myocutaneous flap reconstruction by plastic surgeons was performed routinely to cover the exposed vasculature and to provide for rapid wound healing without tension.
We retrospectively assessed surgical complications by evaluating all hospital and outpatient medical records for the participants for the following specific adverse events: hemorrhage, infection, edema (lower extremities, trunk, and genitalia), soft tissue necrosis, seroma and/or abscess formation, and the need for secondary procedures to address adverse events. All adverse events were then scored by using CTCAE, version 3.0.22 Acute and chronic complications were defined as those occurring within and after 30 days of the surgical resection, respectively.
Statistical Considerations
The Bayesian method of Thall et al24 was used to monitor pCR probability (θPCR), with the primary goal of the trial being detection of a 15% or larger θPCR value. The trial was to be stopped early using the Bayesian criterion Pr[θPCR > .15 | data] < .002, assuming a beta (.15, .85) prior on θPCR. Given the planned maximum accrual of 40 patients, this criterion implied that the trial would be stopped early if there were no pCRs in the first 20 patients. If the trial continued to completion, the goal was to estimate θPCR, the probability of conventional CR or partial response, and the OS and TTP time distributions, with the latter two estimated using the Kaplan-Meier method,25 and compared between subgroups using the log-rank test.26 Because these patients tend to be older adults with multiple potential causes of mortality, TTP was defined so that death was a censoring event.
RESULTS
Patient Characteristics
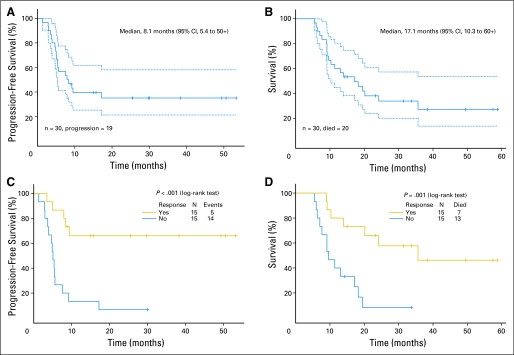
Thirty eligible patients with metastatic penile cancer were enrolled from April 2000 through September 2008. Although our original intention was to enroll 40 patients, we decided to close the study after 30 patients were enrolled, in part because the objectives of the trial had been met with that number of patients, and also because the slow accrual rate meant that several more years would have been required to reach the original target of 40 patients. The patients' characteristics are listed in Table 1 . All 30 patients received at least one course of chemotherapy, and their records could be assessed for toxicity (Table 2), conventional response, TTP, and OS (Fig 1).
Table 1.
Baseline Characteristics of Patients (N = 30)
| Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 57.5 | |
| Range | 24-78 | |
| Zubrod performance status score | ||
| 0 | 8 | 26.7 |
| 1 | 15 | 50.0 |
| 2 | 7 | 23.3 |
| Race/ethnicity | ||
| Hispanic | 8 | 26.7 |
| African American | 2 | 6.7 |
| White non-Hispanic | 20 | 66.7 |
| Primary tumor intact | 7 | 23.3 |
| Lymph node stations clinically involved | ||
| Groin only (stage III) | 9 | 30.0 |
| Deep inguinal or pelvic (stage IV) | 21 | 70.0 |
| Histopathologic type | ||
| Keratinizing squamous cell | 25 | 83.3 |
| Squamous cell, basaloid features | 4 | 13.3 |
| Mixed squamous/verrucous | 1 | 3.3 |
| Skin ulceration | 14 | 46.7 |
| Circumcised at birth | 2 | 6.7 |
| History of smoking | 22 | 73.3 |
| Current | 4 | 13.3 |
| Former | 18 | 60.0 |
Table 2.
Number of Grades 3 and 4 Adverse Events Occurring During Chemotherapy
| Adverse Event | Chemotherapy Course |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Allergic reaction, grade 4 | 1 | 0 | 0 | 0 |
| Neutropenia, grade 4 | 0 | 0 | 0 | 1 |
| Anemia, grade 3 | 0 | 0 | 0 | 1 |
| Central venous catheter–related thrombosis, grade 3 | 0 | 1 | 0 | 0 |
| Deep vein thrombosis, grade 3 | 0 | 1 | 0 | 0 |
| Febrile neutropenia, grade 3 | 0 | 0 | 0 | 1 |
| Hyperglycemia, grade 3 | 1 | 0 | 0 | 0 |
| Infection, grade 3 | 3 | 2 | 0 | 0 |
| Motor neuropathy, grade 3 | 0 | 0 | 0 | 1 |
| Myocardial ischemia, grade 3 | 0 | 1 | 0 | 1 |
| Thrombocytopenia, grade 3 | 0 | 0 | 0 | 1 |
NOTE. All 30 patients enrolled completed at least one course of chemotherapy and thus had records that could be evaluated for adverse effects.
Fig 1.
Kaplan-Meier plots (with 95% CIs as dotted lines) of (A) time to progression of disease, (B) overall survival; patients are grouped by response for (C) time to progression and (D) overall survival.
Neoadjuvant Chemotherapy
Twenty-three patients (76.7%) completed the planned four courses of chemotherapy. The other seven patients discontinued chemotherapy after one to three courses; the reasons were rapid tumor progression (three patients), hypersensitivity to paclitaxel (one patient), cardiac event (one patient), and patient's decision not to receive further treatment (two patients).
Conventional Response Rate
Three CRs and 12 PRs were achieved, for an overall empirical response probability of 0.50 (95% CI, 0.31 to 0.69). Nine of the 30 patients (30.0%) had stable disease, and the disease progressed in the remaining six (20.0%).
Surgery and Complications
Bilateral inguinal lymph node dissections and unilateral or bilateral pelvic lymph node dissections were performed in 22 of the 23 patients who had completed the full four courses of neoadjuvant chemotherapy. Four patients who did not complete the full protocol-specified chemotherapy regimen also underwent surgery: one patient after having completed two courses and experiencing tumor progression and three patients after having undergone other preoperative chemotherapy treatments.
The results of histopathologic analyses of resected tissue are listed in Table 3, and postsurgical complications are listed in Table 4. The latter rates were either lower than or comparable to our contemporary lymphadenectomy experience with or without chemotherapy.23 Data from the patients who underwent surgery outside the context of the clinical trial (n = 4) were analyzed separately with respect to postsurgical complications, which included one fatality (Appendix Table A1, online only). No deaths were related to the treatment protocol.
Table 3.
Histopathologic Data on Residual Tumor From the Patients Who Completed All Four Courses of Neoadjuvant Chemotherapy and Then Underwent Lymphadenectomy (n = 22)
| Presence of Histopathologic Finding | No. of Patients | % | Univariate Analyses |
|||
|---|---|---|---|---|---|---|
| Median TTP (months) | Log-Rank P | Median OS (months) | Log-Rank P | |||
| Bilateral residual metastatic tumor | ||||||
| Yes | 8 | 36.4 | 5 | 10 | ||
| No | 14* | 63.6 | > 50 | .002 | 36 | .017 |
| Extranodal extension | ||||||
| Yes | 9 | 40.9 | 5 | 10 | ||
| No | 13 | 59.1 | > 50 | .001 | > 50 | .004 |
| Skin or subcutaneous involvement | ||||||
| Yes | 10 | 45.5 | 6 | 9 | ||
| No | 12 | 54.5 | > 50 | .009 | 36 | .012 |
Abbreviations: TTP, time to progression; OS, overall survival.
Includes three patients with a pathologic complete response.
Table 4.
Postsurgical Complications Among Patients Who Completed All Four Courses of Neoadjuvant Chemotherapy and Then Underwent Lymphadenectomy (n = 22)
| Timing and Grade of Complication* | Type of Postsurgical Complication |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noninfectious Wound Separation or Skin Breakdown |
Hemorrhage or Bleeding |
Skin Infection |
Lower Extremity Edema |
Edema in Trunk and/or Genitalia |
Soft Tissue Necrosis |
Seroma |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Early (within 30 days of surgery) | ||||||||||||||
| Grade 4 | 0 | 0 | 1 | 4.5† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 3 | 2 | 9.1 | 2 | 9.1 | 1 | 4.5 | 0 | 0 | 1 | 4.5 | 2 | 9.1 | 0 | 0 |
| Grade 1 or 2 | 3 | 13.6 | 0 | 0 | 0 | 0 | 6 | 27.3 | 2 | 9.1 | 2 | 9.1 | 1 | 4.5 |
| Delayed (more than 30 days after surgery) | ||||||||||||||
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 3 | 0 | 0 | 0 | 0 | 1 | 4.5 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 13.6 |
| Grade 1 or 2 | 0 | 0 | 0 | 0 | 2 | 9.1 | 5 | 22.7 | 2 | 9.1 | 0 | 0 | 0 | 0 |
All complications were graded by using the Common Terminology Criteria for Adverse Events, version 3.
This grade 4 hemorrhage was noted on postoperative day 2 and required operative intervention but was no further issue.
Postoperative Radiotherapy
Eleven of the 22 patients who underwent surgery are known to have experienced tumor progression. Radiotherapy or chemoradiotherapy was administered to five of these patients for treatment of tumor recurrence. No patients were given adjuvant radiotherapy.
pCRs
Because two of the first 20 patients enrolled experienced a pCR, the trial was not stopped early. One additional patient experienced a pCR, for a total of three pCRs, or 10.0% of the enrolled patients and 13.6% of the 22 patients who completed the full neoadjuvant chemotherapy regimen and underwent consolidation surgery.
Survival
Twenty of the 30 patients have died, with an estimated median TTP of 8.1 months (95% CI, 5.4 to 50+) and OS of 17.1 months (95% CI, 10.3 to 60+; Figs 1A and 1B). The median follow-up time for the 10 surviving patients was 34 months (range, 14 to 59 months). Seventeen of the 20 deaths were attributed to progressive metastatic penile cancer, one was caused by postoperative bleeding (in this particular case, the surgery was performed outside of the specified study protocol and after disease progression), and the other deaths were from unrelated or unknown causes in patients with no clinical evidence of tumor (10 months and 36 months, respectively).
We performed univariate analyses of TTP and OS for patient subgroups according to pretreatment variables, response to chemotherapy, and the anatomic extent of residual disease found at surgery. TTP and OS were not statistically associated with any of the pretreatment variables we selected, although we did observe the expected trends toward shorter survival times in patients with an unfavorable performance status, an immobile groin mass, a skin ulceration, and/or leukocytosis at baseline (Table 5). An unexpected observation was that patients with enlarged pelvic lymph nodes on baseline imaging had a longer median OS than did patients with no evidence of pelvic lymphadenopathy, although the difference was not significant (19 months v 11 months; P = .35).
Table 5.
Pretreatment Characteristics and Survival of Patients (N = 30)
| Presence of Pretreatment Characteristic | No. of Patients | % | Univariate Analysis |
|||
|---|---|---|---|---|---|---|
| Median TTP (months) | Log-Rank P | Median OS (months) | Log-Rank P | |||
| Zubrod performance status | ||||||
| 0-1 | 23 | 76.7 | 9 | 19 | ||
| 2 | 7 | 23.3 | 3 | .10 | 9 | .16 |
| Immobile groin mass | ||||||
| Yes | 7 | 23.3 | 4 | 10 | ||
| No | 23 | 76.7 | 8 | .33 | 19 | .48 |
| Skin ulceration | ||||||
| Yes | 14 | 46.7 | 7 | 12 | ||
| No | 16 | 53.3 | 13 | .36 | 20 | .43 |
| Leukocytosis | ||||||
| Yes | 9 | 30.0 | 10 | 10 | ||
| No | 21 | 70.0 | 8 | .62 | 18 | .81 |
| Pelvic lymphadenopathy | ||||||
| Yes (stage IV) | 21 | 70.0 | 9 | 19 | ||
| No (stage III) | 9 | 30.0 | 5 | .52 | 11 | .35 |
Abbreviations: TTP, time to progression; OS, overall survival.
We found a statistically significant improvement in TTP and OS among patients who experienced an objective response to chemotherapy compared with those among patients who did not (Figs 1C and 1D). Univariate analyses revealed statistically significantly worse median TTP and OS among patients who had bilateral residual tumor at resection compared with those among patients who did not (Table 3; P = .002 and P = .017, respectively). Even with a Bonferroni correction for multiple testing, the effect of bilateral residual tumor at resection remained significant at level 0.05. Finally, patients who had extracapsular extension into extranodal tissue or involvement of the resected skin or subcutaneous tissue also had statistically significantly shorter TTP and OS than did the patients without such involvement (Table 3).
DISCUSSION
In this single-institution, nonrandomized, phase II clinical trial of neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy, we found that 36.7% of the enrolled patients with penile cancer and bulky regional lymph node metastases remained free of recurrence at theirlast clinical assessment (Fig 1A). This is the first prospective study that we know of with sufficient size to reliably estimate the outcomes of multimodality therapy for metastatic penile carcinoma. The results of several retrospective studies of neoadjuvant chemotherapy have been published but were inconclusive.11–13 Because it was not a randomized trial, our study was not designed to compare the results of neoadjuvant chemotherapy with surgery alone.
The results of many patient series documenting the expected TTP and OS for men with stage TX, N2-3, M0 penile cancer have been published.9,16,27,28 The goal of long-term, disease-free survival is rarely achieved with surgery alone for patients with pelvic lymph node metastases and extranodal extension. We observed residual viable tumor in lymph nodes with extranodal extension in a subset of our patients who underwent postchemotherapy surgery, and that characteristic was associated with shorter survival. Most of the patients in this study had radiographic evidence of pelvic lymph node metastases and/or extranodal extension at baseline, and we estimated that their expected rate of long-term survival with surgery alone would be at most 10% to 15%.9,15
pCRs occurred in 13.6% of our patients who completed the chemotherapy regimen and underwent consolidation surgery. We used an estimated pCR rate of 15% of enrolled patients as a measure of clinical benefit for the purpose of early stopping, although this end point was not preselected as the primary objective of the trial. In the univariate analysis, a pCR was not a statistically significant predictor of TTP (> 50 months v 9 months; P = .11) but was a marginally significant predictor of OS (> 60 months v 18 months; P = .07), and the absence of bilateral residual tumor was significantly beneficial for TTP (Table 3).
The largest prospective clinical trial of chemotherapy in metastatic penile cancer conducted so far was a multicenter effort by the Southwest Oncology Group (SWOG)10 in which patients were treated with bleomycin, methotrexate, and cisplatin. In that trial, an overall response rate of 32.5% and a median OS time of 28 weeks were found. However, although that response rate exceeded the investigators' predetermined target response rate of 30%, the treatment benefit was offset by a 13.9% rate of treatment-related mortality. Conversely, the regimen we used was well tolerated, and none of the deaths that occurred were due to chemotherapy-related toxicity. On the basis of this safety profile and an overall response rate of 50%, we therefore recommend the use of paclitaxel, ifosfamide, and cisplatin for neoadjuvant treatment, as in this study, and for the palliative treatment of patients with visceral or distant metastases. There is also a role for salvage radiotherapy and for chemoradiotherapy as an alternative to surgery, although published data for chemoradiotherapy as definitive therapy for metastatic penile cancer are lacking.
In conclusion, neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy was effective in terms of the conventional response rate, TTP, and OS, and it produced pCRs in 10% of patients. Surgery was shown to be feasible and without increased complications. The postoperative extent of residual disease was statistically significantly associated with the rates of recurrence and death, suggesting that those findings could be used to identify the patients at highest risk of recurrence. We recommend the use of this neoadjuvant regimen as a new standard of care for multimodal treatment of men with regional metastatic penile cancer.
Supplementary Material
Acknowledgment
We thank Karen Phillips, ELS, for editorial assistance.
Appendix
Table A1.
Postsurgical Complications Among the Patients Who Received Off-Protocol Treatment or Less Than Four Courses of Neoadjuvant Chemotherapy and Then Underwent Lymphadenectomy (n = 4)
| Timing and Grade of Complication* | No. of Patients With Type of Postsurgical Complication | ||||||
|---|---|---|---|---|---|---|---|
| Noninfectious Wound Separation or Skin Breakdown | Hemorrhage or Bleeding | Skin Infection | Lower Extremity Edema | Edema in Trunk and/or Genitalia | Soft Tissue Necrosis | Seroma | |
| Early (within 30 days of surgery) | |||||||
| Grade 4 or 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Grade 1 or 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Delayed (more than 30 days after surgery) | |||||||
| Grade 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Grade 3 or 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 1 or 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
All complications were graded by using the Common Terminology Criteria for Adverse Events, version 3.
Footnotes
Supported by National Cancer Institute Cancer Center Core Grant No. CA016672.
Presented in part at the Annual Meeting of the American Urological Association, May 20-26, 2006, Atlanta, GA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00512096.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lance C. Pagliaro, Danai Daliani, Peter F. Thall, Curtis A. Pettaway
Administrative support: Lance C. Pagliaro, Dallas L. Williams
Provision of study materials or patients: Lance C. Pagliaro, Danai Daliani, Curtis A. Pettaway
Collection and assembly of data: Lance C. Pagliaro, Dallas L. Williams, Michael B. Williams, William Osai, Michael Kincaid, Curtis A. Pettaway
Data analysis and interpretation: Lance C. Pagliaro, Michael B. Williams, Sijin Wen, Peter F. Thall, Curtis A. Pettaway
Manuscript writing: Lance C. Pagliaro, Dallas L. Williams, Danai Daliani, Michael B. Williams, William Osai, Michael Kincaid, Sijin Wen, Peter F. Thall, Curtis A. Pettaway
Final approval of manuscript: Lance C. Pagliaro, Dallas L. Williams, Danai Daliani, Michael B. Williams, William Osai, Michael Kincaid, Sijin Wen, Peter F. Thall, Curtis A. Pettaway
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Misra S, Chaturvedi A, Misra NC. Penile carcinoma: A challenge for the developing world. Lancet Oncol. 2004;5:240–247. doi: 10.1016/S1470-2045(04)01427-5. [DOI] [PubMed] [Google Scholar]
- 3.Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: Epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141–150. doi: 10.1007/s00345-008-0302-z. [DOI] [PubMed] [Google Scholar]
- 4.Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin—UKCCCR Anal Cancer Trial Working Party, UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 5.Montana GS, Thomas GM, Moore DH, et al. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: A Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 2000;48:1007–1013. doi: 10.1016/s0360-3016(00)00762-8. [DOI] [PubMed] [Google Scholar]
- 6.Winquist E, Oliver T, Gilbert R. Postoperative chemoradiotherapy for advanced squamous cell carcinoma of the head and neck: A systematic review with meta-analysis. Head Neck. 2007;29:38–46. doi: 10.1002/hed.20465. [DOI] [PubMed] [Google Scholar]
- 7.de Kernion JB, Tynberg P, Persky L, et al. Proceedings: Carcinoma of the penis. Cancer. 1973;32:1256–1262. doi: 10.1002/1097-0142(197311)32:5<1256::aid-cncr2820320534>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Horenblas S, van Tinteren H, Delemarre JF, et al. Squamous cell carcinoma of the penis: III. Treatment of regional lymph nodes. J Urol. 1993;149:492–497. doi: 10.1016/s0022-5347(17)36126-8. [DOI] [PubMed] [Google Scholar]
- 9.Ravi R. Correlation between the extent of nodal involvement and survival following groin dissection for carcinoma of the penis. Br J Urol. 1993;72:817–819. doi: 10.1111/j.1464-410x.1993.tb16273.x. [DOI] [PubMed] [Google Scholar]
- 10.Haas GP, Blumenstein BA, Gagliano RG, et al. Cisplatin, methotrexate and bleomycin for the treatment of carcinoma of the penis: A Southwest Oncology Group study. J Urol. 1999;161:1823–1825. [PubMed] [Google Scholar]
- 11.Pizzocaro G, Piva L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 1988;27:823–824. doi: 10.3109/02841868809094366. [DOI] [PubMed] [Google Scholar]
- 12.Leijte JA, Kerst JM, Bais E, et al. Neoadjuvant chemotherapy in advanced penile carcinoma. Eur Urol. 2007;52:488–494. doi: 10.1016/j.eururo.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Bermejo C, Busby JE, Spiess PE, et al. Neoadjuvant chemotherapy followed by aggressive surgical consolidation for metastatic penile squamous cell carcinoma. J Urol. 2007;177:1335–1338. doi: 10.1016/j.juro.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Novara G, Galfano A, De Marco V, et al. Prognostic factors in squamous cell carcinoma of the penis. Nat Clin Pract Urol. 2007;4:140–146. doi: 10.1038/ncpuro0751. [DOI] [PubMed] [Google Scholar]
- 15.Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93:133–138. doi: 10.1002/jso.20414. [DOI] [PubMed] [Google Scholar]
- 16.Lont AP, Kroon BK, Gallee MP, et al. Pelvic lymph node dissection for penile carcinoma: Extent of inguinal lymph node involvement as an indicator for pelvic lymph node involvement and survival. J Urol. 2007;177:947–952. doi: 10.1016/j.juro.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 17.Crook J, Ma C, Grimard L. Radiation therapy in the management of the primary penile tumor: An update. World J Urol. 2009;27:189–196. doi: 10.1007/s00345-008-0309-5. [DOI] [PubMed] [Google Scholar]
- 18.Theodore C, Skoneczna I, Bodrogi I, et al. A phase II multicentre study of irinotecan (CPT 11) in combination with cisplatin (CDDP) in metastatic or locally advanced penile carcinoma (EORTC Protocol 30992) Ann Oncol. 2008;19:1304–1307. doi: 10.1093/annonc/mdn149. [DOI] [PubMed] [Google Scholar]
- 19.Shin DM, Glisson BS, Khuri FR, et al. Phase II trial of paclitaxel, ifosfamide, and cisplatin in patients with recurrent head and neck squamous cell carcinoma. J Clin Oncol. 1998;16:1325–1330. doi: 10.1200/JCO.1998.16.4.1325. [DOI] [PubMed] [Google Scholar]
- 20.Greene F, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. ed 6. New York, NY: Springer; 2002. [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf.
- 23.Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: The M.D. Anderson Cancer Center Experience. J Urol. 2002;167:1638–1642. [PubMed] [Google Scholar]
- 24.Thall PF, Sung HG. Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med. 1998;17:1563–1580. doi: 10.1002/(sici)1097-0258(19980730)17:14<1563::aid-sim873>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 27.Srinivas V, Morse MJ, Herr HW, et al. Penile cancer: Relation of extent of nodal metastasis to survival. J Urol. 1987;137:880–882. doi: 10.1016/s0022-5347(17)44281-9. [DOI] [PubMed] [Google Scholar]
- 28.Lopes A, Hidalgo GS, Kowalski LP, et al. Prognostic factors in carcinoma of the penis: Multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J Urol. 1996;156:1637–1642. doi: 10.1016/s0022-5347(01)65471-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.