Abstract
In the testis, developing germ cells are dependent on supportive physical and paracrine interactions with Sertoli cells. The intimate nature of this relationship is demonstrated by the fact that a toxic insult compromising the stability of Sertoli cells will have deleterious effects on the associated germ cells. 2,5-Hexanedione (HD) and x-radiation (x-ray) are testicular toxicants, each with a unique cellular target. HD exposure disrupts microtubule function in Sertoli cells, and x-ray exposure causes double-strand breaks in the DNA of germ cells. Despite their differing modes of action, exposure to either toxicant has the similar ultimate effect of increased germ cell apoptosis. In this study, adult male F344 rats were exposed to 1% HD in the drinking water for 18 days with or without coexposure to 2 or 5 Gy x-ray 12 h prior to necropsy. Incidence of retained spermatid heads was increased in the HD and coexposure groups. Germ cell apoptosis was significantly increased in the x-ray and coexposure groups. There was a striking stage-dependent attenuation of apoptosis with coexposure compared with x-ray alone. Detailed histopathological analysis revealed a significant suppression of x-ray–induced germ cell apoptosis by HD pretreatment in stages I–VI of the seminiferous cycle, most noticeably at stages II/III. We hypothesize either that subacute HD pretreatment compromises the ability of the Sertoli cells to eliminate x-ray–damaged germ cells or that germ cells are more resistant to x-ray–induced damage, having adapted to a less supportive environment.
Keywords: testis; x-radiation; 2,5-hexanedione; coexposure; apoptosis
Spermatogenesis is a tightly regulated process of germ cell proliferation, maturation, and apoptosis that is dynamically regulated by intimate paracrine interactions with Sertoli cells and Leydig cells. Sertoli cells provide germ cells with both regulatory support via paracrine signaling and physical support by acting as scaffolding for the developing germ cells. Consequently, exposure to a toxicant that compromises the integrity of a Sertoli cell could have an indirect deleterious effect on its associated germ cells either by decreasing intercellular support or by signaling of apoptosis-inducing proteins. Germ cells are also susceptible to direct insults, such as irreversible toxicant-induced damage to the integrity of the cell or its genome. The net result of direct or indirect cellular insult, if severe enough, is germ cell apoptosis (Boekelheide, 2005).
The histological complexity of the seminiferous tubule presents a considerable hurdle toward deciphering the interactions of testicular toxicants with unique cell targets. This laboratory is making a concerted effort to understand the underlying principles guiding the responses to mixed chemical exposures that target the same or different cell types within the testis. A previous study established a rat coexposure model to 2,5-hexanedione (HD) and carbendazim, testicular toxicants that promote and inhibit Sertoli cell microtubule assembly, respectively. Despite their opposing mechanisms of action, a synergistic coexposure response was observed (Markelewicz et al., 2004). This study underlines the complex and unintuitive nature of the testicular response to toxicant coexposures.
In the present study, we investigate coexposure to HD and x-radiation (x-ray), two toxicants with unique cellular targets and vastly different kinetics. The environmental toxicant HD, the ultimate toxic metabolite of the common industrial solvents n-hexane and methyl n-butyl ketone, disrupts microtubule function in Sertoli cells leading to testicular injury (Boekelheide et al., 2003). Dysfunction of Sertoli cells could affect their supportive capacity, leading to germ cell loss. HD-induced testicular injury is characterized by a long latency period between initiation of exposure and the appearance of histopathological alterations; incidence of retained spermatid heads (RSH), an early marker of testicular injury, begins to increase in rats receiving 1% HD in the drinking water during the third week of exposure (Boekelheide, 1988; Bryant et al., 2008). With 5–7 weeks of continued exposure, the testicular lesions progress to include vacuolization in the Sertoli cell cytoplasm and testicular atrophy resulting from increased germ cell apoptosis (Blanchard et al., 1996; Bryant et al., 2008; Moffit et al., 2007).
X-radiation (x-ray) causes apoptosis in rapidly dividing cells as revealed by TdT-mediated dUTP biotin nick end labeling (TUNEL). The proliferating spermatogonia, located at the basal lamina of the seminiferous epithelium, are particularly susceptible to damage resulting from free radical–induced DNA breaks (Hasegawa et al., 1997; Henriksen et al., 1996). In contrast, the relatively quiescent Sertoli and Leydig cells are highly resistant to damage associated with x-ray exposure (Hasegawa et al., 1997).
Germ cell development is characterized by a highly regulated sequence of proliferation and differentiation. This progression is physically mapped onto the seminiferous tubule along which are found morphologically distinct associations of germ cells at particular levels of development. These associations have been morphologically divided into 14 “stages,” indicated by roman numerals (Leblond and Clermont, 1952). The unique cellular characteristics of each stage are reflected by a distinct stage-specific gene expression pattern and varying degrees of sensitivity to particular toxicants or insults (Hikim et al., 1997; Lue et al., 1999; McClusky et al., 2007). X-ray–induced germ cell apoptosis is particularly pronounced in types A2 through B spermatogonia, reflected by an uneven distribution of germ cell damage across the various stages of the seminiferous epithelium (Hasegawa et al., 1997; Henriksen et al., 1996). The inconsistent sensitivity of germ cells across the seminiferous epithelium highlights the importance of considering the stage distribution of damage when performing mechanistic evaluations of testicular toxicity.
Despite their unique cellular targets, the net result of exposure to x-ray or HD is increased germ cell apoptosis. X-ray acts directly on the DNA of the germ cells, whereas HD acts on the supportive Sertoli cell and indirectly affects the germ cells. In this experiment, we used a coexposure paradigm consisting of a subacute 18-day exposure to HD followed by an acute exposure to x-ray to examine the consequences of simultaneous exposure to dissimilar toxicants in the testis. Histological examination and TUNEL staining revealed interesting and unexpected differences in the incidence of germ cell apoptosis between coexposure and exposure to x-ray alone.
MATERIALS AND METHODS
Animals.
Adult male Fischer 344 rats weighing 200–250 g were purchased from Charles River Laboratories (Wilmington, MA) and allowed to acclimate for 1 week prior to use. Animals were maintained in a temperature and humidity-controlled vivarium with a 12-h alternating light-dark cycle. All rats were housed two per cage in Thoren units with free access to water and Purina Rodent Chow 5001 (Farmer's Exchange, Framingham, MA). The Brown University Institutional Animal Care and Use Committee approved all experimental animal protocols in compliance with National Institutes of Health guidelines.
Chemicals.
HD was purchased from Sigma-Aldrich Corporation (St Louis, MO).
Experimental design.
Animals were randomly assigned to six different groups: control, HD alone (HD), x-ray alone (2 or 5 Gy), or coexposure of HD and x-ray (HD/2 or HD/5 Gy) (n = 8–10). HD was administered as a 1% solution in drinking water ad libitum for 18 days to the HD, HD/2 Gy, and HD/5 Gy groups. On day 17, animals from 2 Gy, 5 Gy, HD/2 Gy, and HD/5 Gy groups received x-ray exposure to the caudal half of their bodies with a single dose of 2 or 5 Gy at a dose rate of 0.31 Gy/min using a RT 250 Philips kVp x-ray machine (Philips, Hamburg, Germany) (Fig. 1). The dose rate was estimated using a Radcal radiation monitor, model 2026C (Radcal, Monrovia, CA). At 12 h after x-ray following continued HD exposure, all rats were euthanized by carbon dioxide asphyxiation and necropsied on day 18, and body and testis weights were recorded (Table 1). Left testes were fixed in 10% neutral-buffered formalin for histological examination, and the right testes were divided into two halves. One half was immersed in OCT compound (Sakura Finetek, Torrance, CA) and snap frozen in liquid nitrogen for future preparation of frozen sections. The other half was homogenized in TRI-reagent (Sigma-Aldrich), snap frozen in liquid nitrogen, and stored at −80°C for RNA isolation followed by quantitative real-time (qRT)-qPCR.
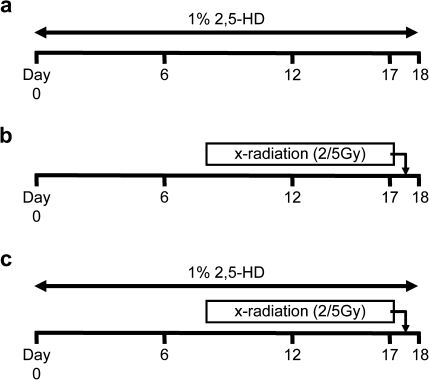
FIG. 1.
Exposure paradigm. Animals were divided into five groups: control, HD alone (a), 2 or 5 Gy x-ray alone (b), and HD/2 or 5 Gy x-ray coexposure (c). HD was administered to Fischer 344 rats as a 1% solution in drinking water ad libitum for 18 days. X-ray was given at a single dose of 2 or 5 Gy on day 17. Following continued exposure of 2,5-HD for 12 h, rats were euthanized and immediately necropsied on day 18.
TABLE 1.
Body and Testis Weights. Testis Weight is Shown as the Average of Both the Left and Right Testis Weight. Values with Different Letters are Significantly Different within Columns (p < 0.05); Mean ± SD
| Treatment group | n | Body weight (g) | Testis weight (g) |
| Control | 10 | 276.9 ± 9.0a | 1.394 ± 0.090a |
| HD | 8 | 209.1 ± 7.7b | 1.311 ± 0.108b |
| 2 Gy | 10 | 268.6 ± 9.2a | 1.378 ± 0.089a |
| 5 Gy | 10 | 269.2 ± 8.9a | 1.383 ± 0.112a |
| HD/2 Gy | 10 | 199.8 ± 7.4b | 1.215 ± 0.180b |
| HD/5 Gy | 10 | 193.1 ± 11.0b | 1.223 ± 0.115b |
Histological examination and TUNEL staining.
Two cross sections were taken from the middle of the formalin-fixed testes. One was embedded in glycol methacrylate (Technovit 7100; Heraeus Kulzer GmBH, Wehrheim, Germany) for histological examination including morphometric analyses. The other was embedded in paraffin for detection of apoptosis by TUNEL staining.
For histological examination, sections (3 μm) were stained with periodic acid-Schiff’s reagent followed by hematoxylin counterstain (PASH). A Zeiss Standard microscope (Karl Zeiss, New York, NY) and an Aperio Scan Scope (Aperio Technologies, Vista, CA) were utilized to view all histopathological sections and perform morphometric analyses, respectively. The morphometric analyses, such as diameters of seminiferous tubule, % vacuolization, % sloughing, and % RSH, were conducted randomly on 50 seminiferous tubule cross sections, each of which was required to have a major:minor axis of less than 1.5:1. Each seminiferous tubule was evaluated for its minor diameter, presence or absence of vacuolization and sloughing, and having 0, 1–3, or greater than 3 RSH.
For evaluation of apoptosis, paraffin sections (4 μm) were stained using an ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA) as directed by the manufacturer and counterstained with methyl green. All seminiferous tubules with a major:minor axis less than 1.5:1 in cross sections were included in the analysis and the number of seminiferous tubules with TUNEL-positive cells therein were counted manually using Aperio Scan Scope (Aperio Technologies). The percent of seminiferous tubules with 0, 1–3, or greater than 3 TUNEL-positive cells was also assessed.
To determine stage specificity in the TUNEL-stained sections, seminiferous tubules with greater than 3 TUNEL-positive cells were divided into four stage groups: group 1 (stages I–VI), group 2 (VII–VIII), group 3 (IX–XI), and group 4 (XII–XIV). Qualifications were made according to the position and shape of the elongated spermatid nuclei as per the standards described by Leblond and Clermont (1952)—group 1: the presence of compact elongate spermatid heads embedded deep within the seminiferous epithelium; group 2: the presence of compact elongate spermatid heads adjacent to the tubule lumen; group 3: the presence of elongated spermatids without condensation of chromatin and no compact elongate spermatid heads; and group 4: the presence of chromatin condensed elongated spermatids and large pachytene spermatocytes.
Additionally, sections from 5 Gy and HD/5 Gy groups were stained with periodic acid-Schiff reagent after TUNEL staining (TUNEL-PAS), following which the stage of each seminiferous tubule that had greater than three TUNEL-positive cells was determined.
Statistical analysis.
The student’s t-test or one-way ANOVA with Bonferronni post hoc analysis was performed using Sigma Stat software (SPSS, Chicago, IL). A p value < 0.05 was considered to be statistically significant.
RESULTS
Exposure Paradigm
Animals were randomly divided into six different groups (control, HD, 2 Gy, 5 Gy, HD/2 Gy, and HD/5 Gy) (Fig. 1), and HD was administered as a 1% solution in drinking water ad libitum for 18 days to the HD, HD/2 Gy, and HD/5 Gy groups. These doses were chosen because in preliminary studies, they gave the interesting effects described here. On day 17, animals from 2 Gy, 5 Gy, HD/2 Gy, and HD/5 Gy groups received x-ray exposure to the caudal half of their bodies with a single dose of 2 or 5 Gy. As this experiment used TUNEL staining to quantify apoptosis, x-ray doses were chosen whose effects were reliably quantifiable by histology. Rats were necropsied 12 h later on HD exposure day 18.
Body and Testis Weights
Body weights were decreased in the HD-, HD/2 Gy-, and HD/5 Gy-treated groups compared with control, and testis weights were decreased in the HD, HD/2, and HD/5 Gy groups. There was no statistically significant difference between HD and any HD/x-ray group for either testis or body weights (Table 1).
Testicular Histopathology
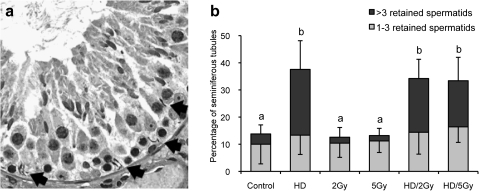
Incidence of RSH in the basal compartment of seminiferous tubules was increased in the HD and HD/x-ray groups relative to control (Fig. 2A). Morphometric analysis revealed that the percentage of seminiferous tubules with greater than 3 RSH was increased in the HD, HD/2 Gy, and HD/5 Gy groups (Fig. 2B). However, there were no statistically significant differences between HD and either co-exposure group. In addition, no dose dependence of x-ray exposure on the incidence of RSH was observed, and the proportion of seminiferous tubules with 1 to 3 RSH was unchanged across all groups. There were no significant changes in the other quantitative parameters measured, seminiferous tubule diameter, Sertoli cell vacuolization, or germ cell sloughing, in any groups compared with control (data not shown).
FIG. 2.
Spermatid head retention. (a) Histopathology of a seminiferous tubule cross section at stage X from a HD-treated rat. RSH (arrow) are present near the basement membrane of the seminiferous tubule; PASH stain. (b) Quantification of spermatid head retention in seminiferous tubule. Values are expressed as the mean percent of seminiferous tubule with 1–3 or > 3 RSH at the basal compartment among the 50 seminiferous tubules randomly selected ± SD. Different letters indicate significant difference between columns, p < 0.05.
Detection of Apoptosis
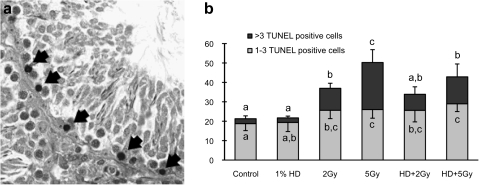
Incidence of TUNEL-positive cells was increased in both 2 and 5 Gy x-ray groups relative to control. These cells had a relatively small cytoplasm and were distributed in the basal compartment of the seminiferous tubule (Fig. 3A). In the control and HD groups, there were fewer seminiferous tubules containing TUNEL-positive cells. The cells most frequently showing positive staining were large pachytene spermatocytes or meiotic cells in stages XII, XIII, and/or XIV. The percentage of seminiferous tubules with 1–3 and greater than 3 TUNEL-positive cells was significantly increased in all x-ray groups. Dose dependence was observed in x-ray groups with or without HD exposure (Fig. 3B). The percentage of seminiferous tubules with greater than 3 TUNEL-positive cells in the 5 Gy group was 24.3 ± 6.6%, more than twice that in the 2 Gy group (11.4 ± 2.6%). These percentages were reduced to 8.3 ±3.8% and 13.9 ± 6.6% in the HD/2 and HD/5 Gy groups, respectively.
FIG. 3.
TUNEL-positive germ cells. (a) Histopathology of a seminiferous tubule cross section from a rat treated with 5 Gy radiation. TUNEL-positive nuclei (arrow) are present near the basement membrane of the seminiferous tubule. (b) Quantification of TUNEL-positive cells in seminiferous tubule. Values are expressed as mean percentage of seminiferous tubules with 1–3 or greater than 3 TUNEL-positive cells ± SD. Values with different letters are significantly different between columns (p < 0.05).
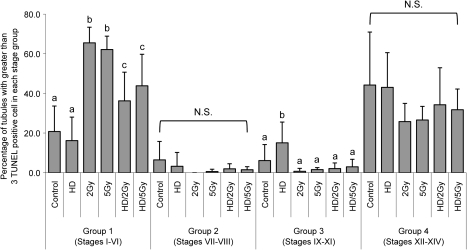
To determine stage specificity in the TUNEL-stained sections, seminiferous tubules with greater than 3 TUNEL-positive cells were divided into four stage groups (groups 1–4) according to the position and shape of the elongated spermatid nuclei (Fig. 4). The majority of seminiferous tubules with greater than 3 TUNEL-positive cells was observed in groups 1 and 4 (Fig. 5). For each treatment group, group 1 (stages I–VI) contained the highest proportion of tubules with greater than 3 TUNEL-positive cells in x-ray and HD/x-ray groups (2 Gy: 65.5%, 5 Gy: 62.1%, HD/2 Gy: 36.2%, and HD/5 Gy: 49.1%), whereas group 4 contained the highest in control (44.2%) and HD (43.1%) groups. In contrast, group 2 had the lowest percentage of tubules with > 3 TUNEL-positive cells (control: 6.5%, HD: 3.2%, 2 Gy: 0.0%, 5 Gy: 0.6%, HD/2 Gy: 1.9%, and HD/5 Gy: 1.5%).
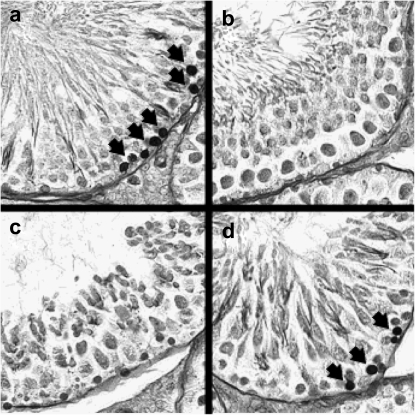
FIG. 4.
Stage specificity of TUNEL-PAS staining in 5 Gy groups. Histopathology of TUNEL-PAS staining, TUNEL-positive nuclei (arrow) are present near the basement membrane in (a) group 1 (stages I–VI) and (d) group 4 (stages XII–XIV). Minimal TUNEL-positive nuclei were found in (b) group 2 (stages VII–VIII) and (c) group 3 (stages IX–XI).
FIG. 5.
Quantification of TUNEL-positive germ cells by stage group. Values are expressed as the mean percent of seminiferous tubules showing > 3 TUNEL-positive germ cells ± SD in each group within all seminiferous tubules showing > 3 TUNEL-positive germ cells. Significant differences are indicated by different letters, p < 0.05, NS; not significant in any groups.
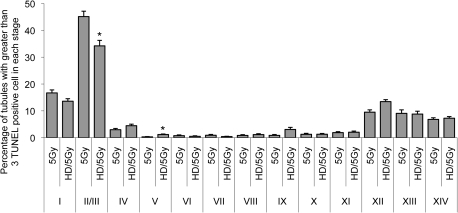
To further investigate the stage specificity of the decreased apoptotic effect in the coexposed testis, sections from 5 Gy and HD/5 Gy groups were stained with TUNEL-PAS for more detailed histology. This allowed for the evaluation of seminiferous tubules with > 3 TUNEL-positive cells by stage rather than stage group (Fig. 6). The percentage of seminiferous tubules with 0, 1–3, or greater than 3 TUNEL-positive cells was comparable between TUNEL staining and TUNEL-PAS staining (data not shown). For both 5 Gy and HD/5 Gy groups, stage II/III exhibited the greatest percentage of > 3 TUNEL-positive cells (45.1 ± 3.0% and 34.2 ± 11.1%, respectively). In stage II/III, this value was significantly decreased in the coexposure HD/5 Gy group compared with 5 Gy alone (Fig. 6).
FIG. 6.
Quantification of stage specificity by TUNEL-PAS staining in 5 Gy and HD/5 Gy groups. Values are expressed as the mean percent of seminiferous tubules with > 3 TUNEL-positive germ cells divided by the total number of all seminiferous tubule with greater than 3 TUNEL-positive germ cells ± SD. Asterisks (*) indicate significant difference between HD/5 Gy and 5 Gy of the same stage (p < 0.05).
DISCUSSION
The present study investigated the response to combined exposure of unique testicular toxicants with dissimilar cellular targets in the testis. The significance of the coexposure model has long been an area of interest for this laboratory. A previous study that examined histopathological markers of exposure to two well-described testicular toxicants, HD and carbendazim, with the same cellular (Sertoli cell) and subcellular (microtubule) targets revealed a synergistic interaction. The coexposure model has begun to provide mechanistic insight into the complexity of real-life exposures to more than one testicular toxicant at a time (Markelewicz et al., 2004). However, the cellular complexity of the testis and the importance of paracrine signaling make interpretation of toxicant coexposure challenging.
Consistent with previous findings, rats subjected to a subacute exposure of 1% HD in drinking water for 18 days exhibited decreased body and testis weights as well as an increased incidence of seminiferous tubules with greater than 3 RSH (Bryant et al., 2008; Moffit et al., 2007). Apoptosis was not increased by HD exposure alone after 18 days, as demonstrated in this study, while extended treatment with HD has been reported to induce an increase in apoptosis via the Sertoli cell-mediated pathway (Blanchard et al., 1996). Simultaneous exposure to x-ray did not have an additional effect on RSH or other histopathological end points such as seminiferous tubule diameter, Sertoli cell vacuolization, or germ cell sloughing. The incidence of apoptosis as measured by TUNEL staining was significantly increased in a dose-dependent manner by x-ray exposure alone. Intriguingly, an 18-day priming period with HD resulted in an attenuation in the incidence of x-ray–induced germ cell apoptosis (Fig. 3).
It is well known that x-ray exposure induces apoptosis in germ cells undergoing DNA replication (Hasegawa et al., 1997; Henriksen et al., 1996). In this study, x-ray exposure resulted in an increased incidence of seminiferous tubules with greater than 3 TUNEL-positive cells. Apoptosis was primarily distributed in group 1 (stages I–VI) and peaked at stage II/III in both the 5 Gy and HD/5 Gy groups, suggesting that types A4 and intermediate spermatogonia, which are prevalent in this stage range, are most susceptible to x-ray–induced damage. These results are consistent with previous reports that A2 through B spermatogonia are most sensitive to x-ray exposure in rodents (Hasegawa et al., 1997; Henriksen et al., 1996). Interestingly, the coexposure model did not change the cell specificity of the x-ray killing effect seen here. This is a reasonable finding, as x-ray affects DNA replication of proliferating germ cells, but not the relatively quiescent Sertoli cells in the adult testis (Hasegawa et al., 1997). The greatest percentage of TUNEL-positive cells observed in the HD group was found in stage group 4 (stages XII–XIV) at a distribution and rate comparable with that of the control group, indicating that at these doses, 18-day HD treatment does not affect the induction or distribution of apoptosis in any stage.
The stage-specific nature of seminiferous tubule sensitivity to toxic insult has been suggested previously. Wright et al. (1983) demonstrated the presence of stage-specific proteins in the rat and more recently Johnston et al. (2008), using gene array technology, revealed that tubule stages are associated with unique transcriptomes. These data, combined with the histological distinctions between stages, support the hypothesis that the seminiferous tubule would exhibit a stage-related sensitivity to particular toxicants, a prospect that has been supported by numerous observations (Hikim et al., 1997; Lue et al., 1999; McClusky et al., 2007).
The magnitude of x-ray–induced apoptosis in the HD/5 Gy group was attenuated by an 18-day priming exposure of HD (Fig. 4). However, the relative distribution of apoptosis across stages remained unchanged with coexposure, indicating that pretreatment with HD affected x-ray–induced apoptosis in a quantitative but not qualitative manner (Fig. 5). Interestingly, when examined as an average across all stages, the attenuative effect on apoptosis was stronger in the HD/5 Gy group than in the HD/2 Gy group.
Apoptosis of male germ cells can be induced in response to various internal or external cellular signals via the intrinsic or extrinsic pathways (Boekelheide et al., 2000; Lee et al., 1997). These pathways are differentially regulated depending on whether the toxicant targets are Sertoli or germ cells. Lee et al. (1999) found that a toxicant-induced insult to the Sertoli cell will cause the secretion of proteins by the Sertoli cell that subsequently initiate the apoptosis-signaling cascade in germ cells. A direct insult to the germ cell, however, will bypass the Sertoli cell-signaling pathway and directly trigger apoptosis in the germ cell.
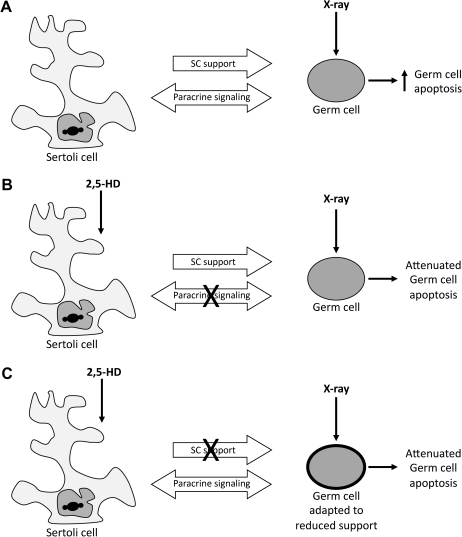
These phenomena combined with the increased complexity of the coexposure model demonstrated here have led us to posit two hypotheses explaining the attenuation of x-ray–induced germ cell apoptosis by HD pretreatment. Exposure to x-ray alone results in an increase in germ cell apoptosis (Fig. 7A). Although this toxicant-cell interaction is direct, the germ cell remains under the influence of general support and paracrine signaling by the Sertoli cell. Conditioning by HD prior to x-ray exposure may have one of the following effects: HD may compromise the signaling ability of Sertoli cells, including that which is responsible for normal induction of apoptosis (Fig. 7B); on the other hand, HD might hinder the general supportive ability of the Sertoli cell, encouraging the vulnerable germ cell to adapt characteristics that lend increased resistance to potential insults (Fig. 7C). Both pathways allow for the same ultimate effect of attenuated apoptosis: the first by decreasing normal levels of apoptotic signaling by the Sertoli cell and the second by conferring increased toxicant resistance on germ cells by decreasing the Sertoli cell's supportive ability.
FIG. 7.
Proposed mechanisms of HD-mediated attenuation of germ cell apoptosis. Sertoli cells normally support and nurture the developing germ cells. Following a direct toxic insult to germ cells by x-ray exposure, the susceptible germ cells undergo apoptosis (A). Sertoli cell dysfunction caused by 2,5-HD pretreatment may impair production of apoptotic signals by Sertoli cells, resulting in reduced elimination of x-ray–damaged germ cells (B). Alternatively, disruption of Sertoli cell support by 2,5-HD pretreatment may result in an adaptive response of the germ cells rendering them less sensitive to x-ray effects. Germ cell resistance is depicted by a thickened cell outline (C).
To address these hypotheses and elucidate the pathway by which this coexposure model exerts its effect, we will have to move beyond simple histology. We have identified those tubule stages and cells most affected by exposure; now an analysis of the gene expression pathways is required to further elucidate the mechanistic pathways. This laboratory has conducted a simultaneous study using a gene array platform to examine a wide variety of gene expression changes allowing for an unbiased examination of potential alterations in genetic pathways and physiological functions affected by coexposure. In the companion studies, insights were drawn from both the gene array and histological analyses to identify candidate stages, cells and genes in the spermatogonial population that were most affected by exposure (Campion et al., 2010a). To obtain a clear picture of gene changes by cell type, we utilized the laser capture microdissection technique which allowed for the capture of cell-specific RNA by histology (Campion et al., 2010b). Candidate gene expression changes were analyzed by qRT-PCR, and conclusions were drawn based on the synthesis of histological and genetic data.
In summary, these experiments revealed an interesting and unexpected antagonistic effect on apoptosis following coexposure of two testicular toxicants with differing modes of action. Although a valuable observation in and of itself, investigation into the mechanisms of this attenuative effect will yield greater insight into the nature of real-world mixtures of exposures.
FUNDING
National Institute of Environmental Health Sciences (NIEHS) (5 P42 ES013660); NIH.
Acknowledgments
Conflict of interest: The authors have no conflict of interest.
References
- Blanchard KT, Allard EK, Boekelheide K. Fate of germ cells in 2,5-hexanedione-induced testicular injury. I. Apoptosis is the mechanism of germ cell death. Toxicol. Appl. Pharmacol. 1996;137:141–148. doi: 10.1006/taap.1996.0066. [DOI] [PubMed] [Google Scholar]
- Boekelheide K. Rat testis during 2,5-hexanedione intoxication and recovery. I. Dose response and the reversibility of germ cell loss. Toxicol. Appl. Pharmacol. 1988;92:18–27. doi: 10.1016/0041-008x(88)90223-2. [DOI] [PubMed] [Google Scholar]
- Boekelheide K. Mechanisms of toxic damage to spermatogenesis. J. Natl Cancer Inst. Monogr. 2005;34:6–8. doi: 10.1093/jncimonographs/lgi006. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Fleming SL, Allio T, Embree-Ku ME, Hall SJ, Johnson KJ, Kwon EJ, Patel SR, Rasoulpour RJ, Schoenfeld HA, et al. 2,5-Hexanedione-induced testicular injury. Annu. Rev. Pharmacol. Toxicol. 2003;43:125–147. doi: 10.1146/annurev.pharmtox.43.100901.135930. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA. Role of Sertoli cells in injury-associated testicular germ cell apoptosis. Proc. Soc. Exp. Biol. Med. 2000;225:105–115. doi: 10.1046/j.1525-1373.2000.22513.x. [DOI] [PubMed] [Google Scholar]
- Bryant BH, Yamasaki H, Sandrof MA, Boekelheide K. Spermatid head retention as a marker of 2,5-hexanedione-induced testicular toxicity in the rat. Toxicol. Pathol. 2008;36:552–559. doi: 10.1177/0192623308317426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion SN, Houseman EA, Sandrof MA, Hensley J, Sui Y, Gaido KW, Wu Z, Boekelheide K. Suppression of radiation-induced testicular germ cell apoptosis by 2,5-hexanedione pretreatment. II. Gene array analysis reveals adaptive changes in cell cycle and cell death pathways. Toxicol. Sci. 2010 doi: 10.1093/toxsci/kfq204. Advance Access published on July 8, 2010; doi: 10.1093/toxsci/kfq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion SN, Sandrof MA, Yamasaki H, Boekelheide K. Suppression of radiation-induced testicular germ cell apoptosis by 2,5-hexanedione pretreatment. III. Candidate gene analysis identifies a role for fas in the attenuation of x-ray–induced apoptosis. Toxicol. Sci. 2010 doi: 10.1093/toxsci/kfq205. Advance Access published on July 8, 2010; doi: 10.1093/toxsci/kfq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M, Wilson G, Russell LD, Meistrich ML. Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiat. Res. 1997;147:457–467. [PubMed] [Google Scholar]
- Henriksen K, Kulmala J, Toppari J, Mehrotra K, Parvinen M. Stage-specific apoptosis in the rat seminiferous epithelium: quantification of irradiation effects. J. Androl. 1996;17:394–402. [PubMed] [Google Scholar]
- Hikim AP, Lue Y, Swerdloff RS. Separation of germ cell apoptosis from toxin-induced cell death by necrosis using in situ end-labeling histochemistry after glutaraldehyde fixation. Tissue Cell. 1997;29:487–493. doi: 10.1016/s0040-8166(97)80034-1. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Wright WW, Dicandeloro P, Wilson E, Kopf GS, Jelinsky SA. Stage-specific gene expression is a fundamental characteristic of rat spermatogenic cells and Sertoli cells. Proc. Natl Acad. Sci. USA. 2008;105:8315–8320. doi: 10.1073/pnas.0709854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K. The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology. 1999;140:852–858. doi: 10.1210/endo.140.2.6479. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- Markelewicz RJ, Jr., Hall SJ, Boekelheide K. 2,5-Hexanedione and carbendazim coexposure synergistically disrupts rat spermatogenesis despite opposing molecular effects on microtubules. Toxicol. Sci. 2004;80:92–100. doi: 10.1093/toxsci/kfh140. [DOI] [PubMed] [Google Scholar]
- McClusky LM, de Jager C, Bornman MS. Stage-related increase in the proportion of apoptotic germ cells and altered frequencies of stages in the spermatogenic cycle following gestational, lactational, and direct exposure of male rats to p-nonylphenol. Toxicol. Sci. 2007;95:249–256. doi: 10.1093/toxsci/kfl141. [DOI] [PubMed] [Google Scholar]
- Moffit JS, Bryant BH, Hall SJ, Boekelheide K. Dose-dependent effects of sertoli cell toxicants 2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol. Pathol. 2007;35:719–727. doi: 10.1080/01926230701481931. [DOI] [PubMed] [Google Scholar]
- Wright WW, Parvinen M, Musto NA, Gunsalus GL, Phillips DM, Mather JP, Bardin CW. Identification of stage-specific proteins synthesized by rat seminiferous tubules. Biol. Reprod. 1983;29:257–270. doi: 10.1095/biolreprod29.1.257. [DOI] [PubMed] [Google Scholar]