Abstract
Evaluation of testicular toxicity during drug development is currently based on histopathological evaluation. A sensitive biomarker for testicular toxicology could provide an in-life and “early warning” measurement. Previous studies suggested that disruption of spermatogenesis induced leakage of germ cell proteins from seminiferous tubules (STs) into interstitial fluid (IF); such proteins have potential for use as biomarkers. To investigate this possibility further, adult male rats were treated with three testicular toxicants thought to have differing sites of action; cadmium chloride affects the blood-testis barrier (BTB), methoxyacetic acid (MAA) disrupts pachytene spermatocytes, and 1,3-dinitrobenzene (DNB) targets Sertoli cells. IF proteins were assessed by Coomassie-based dye-stained gels. Immunostaining was used to identify toxicant-induced damage (DAZL) and BTB integrity (ZO-1, occludin, N-cadherin, and β-catenin) and function (biotin). Cadmium chloride induced dose-dependent leakage of proteins from STs into IF coincident with loss of integrity and function of the BTB. Two of the “leaked” proteins were identified on Westerns as being germ cell specific, namely VASA and fatty acid–binding protein 9 (FABP9). In contrast, similar protein leakage was not evident after either MAA-induced or DNB-induced disruption of spermatogenesis and neither of these treatments affected BTB integrity or function. These results suggest that loss of BTB integrity is required for germ cell–specific proteins to leak from STs into IF, implying that use of such biomarkers has very limited potential for noninvasive monitoring of compound-induced disruption to spermatogenesis.
Keywords: blood-testis barrier, testicular toxicology, germ cell proteins, biomarker, cadmium chloride, fatty acid–binding protein 9
Assessment of testicular damage is important in toxicological evaluation during drug development as well as in clinical assessment of male fertility. Detection of testicular damage currently involves semen analysis, hormone analysis, fertility testing, measurement of organ weights, or histopathological evaluation, which is accepted to be the most sensitive end point in detection of testicular toxicity (Creasy, 2003). Development of biomarkers that could be measured in the bloodstream could aid detection of testicular damage by providing an in-life method for detection and monitoring of damage. Sensitive biomarkers could also have a role in “early warning” detection of testicular damage, which would be particularly useful in drug toxicology testing. Potential markers for testicular damage have been investigated in the past, including inhibin B (Anderson and Sharpe, 2000; Stewart and Turner, 2005), lactate dehydrogenase-C4 (Draper and Timbrell, 1998; Reader et al., 1991), creatine (Draper and Timbrell, 1998; Moore et al., 1992), and androgen-binding protein (Reader et al., 1991; Suter et al., 1998). However, these all have major limitations and have currently not been accepted for widespread use in clinical or nonclinical environments.
Previous work (Turner et al., 1996) suggested that germ cell proteins, such as phosphatidylethanolamine-binding protein and androgen-regulated protein 2, could “leak” into interstitial fluid (IF) following damage to spermatogenesis induced by scrotal heat treatment. These results suggested that if germ cell proteins can be found in IF, they could potentially enter the bloodstream, where their measurement could indicate disruption of spermatogenesis. However, leakage of germ cell–derived proteins could be a direct consequence of germ cell damage or could be due to loss of integrity of the blood-testis barrier (BTB), and these were not distinguished (Turner et al., 1996).
To further investigate whether proteins leak out of seminiferous tubules (STs) into IF following toxicant-induced testicular damage, and if so the mechanism, we have studied the effects of three testicular toxicants with different target sites of action in the adult rat testis. Cadmium chloride causes disruption of spermatogenesis at low doses by acting on the BTB (Hew et al., 1993; Siu et al., 2009). Methoxyacetic acid (MAA) exerts specific effects on pachytene spermatocytes (degeneration and depletion) (Bartlett et al., 1988; Foster et al., 1984). 1,3-Dinitrobenzene (DNB) targets the Sertoli cells, causing vacuolation, and subsequent spermatocyte degeneration (Blackburn et al., 1988; Foster et al., 1987). Doses to produce mild and moderate effects were selected so that dose-response relationships could be examined. IF samples were collected for protein analysis, and fixed testis samples were collected for analysis of the toxicant-induced damage and assessment of the integrity of the BTB. Two germ cell–specific proteins, VASA and fatty acid–binding protein 9 (FABP9), were also investigated as potential biomarker proteins.
MATERIALS AND METHODS
Animals.
Adult male Wistar rats (Harlan, UK) were maintained under standard conditions according to UK Home Office guidelines. Animals had free access to water and a soy-free diet (SDS, Dundee, UK).
Treatments.
Cadmium chloride (Sigma-Aldrich, Dorset, UK) solutions of 1, 1.75, and 3 mg/ml were prepared in 0.9% saline. Treatment (n = 8 per dose) was administered by ip injection of the required dose at a concentration of 1 ml/kg body weight. Controls (n = 8) were administered 1 ml/kg 0.9% saline by ip injection.
MAA (Fluka now Sigma-Aldrich) was adjusted to pH 7.0–7.4 with concentrated sodium hydroxide. Dosing solutions of 200 and 650 mg/kg were then made up with 0.9% saline and administered by oral gavage at a concentration of 2 ml/kg body weight (n = 6–8 per dose). Controls (n = 7/10) were administered 2 ml/kg 0.9% saline.
DNB (Sigma-Aldrich) was dissolved in dimethyl sulfoxide (DMSO; 1.5% total volume) and made up to the required volume with corn oil. Animals (n = 8 per dose) were then administered either 25 or 50 mg/kg DNB by oral gavage at a concentration of 5 ml/kg. Controls (n = 8) were dosed with 5 ml/kg corn oil + 1.5% DMSO.
Sample collection.
Animals were killed by inhalation of CO2 followed by cervical dislocation ∼24 h following administration of cadmium chloride, MAA, or DNB. One testis was used for collection of IF as described previously (Sharpe and Cooper, 1983), whereas the contralateral testis was fixed in Bouins for 6 h, then transferred to 70% ethanol, and processed and embedded into paraffin wax using an automated processor. Five micrometer tissue sections were then cut and mounted onto glass slides. STs were isolated from a control rat, according to methods described elsewhere (Sharpe et al., 1992), homogenized, and used as a reference source of ST-derived proteins for comparison with IF samples.
Immunohistochemical detection of spermatogenic disruption.
Immunohistochemistry used an antibody to the germ cell–specific protein DAZL. Mounted tissue sections were dewaxed and rehydrated. Antigen retrieval was carried out in 0.01M citrate buffer (pH 6) by pressure-cooking slides at full pressure for 5 min and then resting for 20 min. Endogenous peroxidase activity was blocked by incubating slides in 3% hydrogen peroxide in methanol for 30 min at room temperature. Wash steps involved two 5-min incubations at room temperature in Tris-buffered saline (TBS). Nonspecific binding was blocked using normal rabbit serum (NRS) diluted 1:4 in TBS with 5% bovine serum albumin (BSA) (Sigma-Aldrich). Sections were incubated with anti-DAZL antibody (Serotec, Oxford, UK) diluted 1:500 in NRS/TBS/BSA, overnight at 4°C. Control sections were incubated as above without primary antibody. Sections were incubated with biotinylated rabbit anti-mouse (Dako, Cambridgeshire, UK) diluted 1:500 in NRS/TBS/BSA for 30 min at room temperature. Detection was carried out using streptavidin-horseradish peroxidase (Vector Laboratories, Peterborough, UK) diluted 1:500 in TBS, incubated on slides at room temperature for 30 min, followed by visualization with 3,3′-diaminobenzidine (DAB+; Dako). Sections were counterstained with Harris’ hematoxylin and mounted with Pertex. Slides were photographed using a Provis microscope fitted with a Canon DS126131 camera.
Analysis of proteins in testicular IF.
IF samples were analyzed using one dimensional (1D) gel electrophoresis. IF and ST protein concentrations were determined using the Bio-Rad DC Assay Kit (Bio-Rad, Herts, UK) and BSA as standard. Protein extracts (15 μg) in 20 μl NuPAGE Lithium Dodecyl Sulfate sample buffer, reducing agent (Invitrogen, Paisley, UK), and deionized water were separated using the NuPAGE gel electrophoresis system (Invitrogen). Samples were heat denatured at 70°C for 10 min and then loaded onto 12% acrylamide 1 mm NuPAGE Novex Bis-Tris Gels (Invitrogen). NuPAGE MOPS (3-(N-morpholino) propanesulfonic acid) buffer was used as running buffer, supplemented with NuPAGE antioxidant in the cathode chamber. SeeBlue Plus2 Pre-Stained Standard (Invitrogen) was used as a size marker. A control (standard) ST protein extract was run alongside IF samples on each gel as an internal reference against which protein bands were quantified. Gels were run at 200 V for ∼40 min, fixed in 50% methanol/7% acetic acid (15 min), washed with deionized water, and then stained with GelCode Blue Stain Reagent (Pierce now Thermo Scientific) for 1 h at room temperature before washing for 1–2 h. Stained gels were scanned using ImageScanner III (GE Healthcare, Buckinghamshire, UK) and ImageMaster 2D Platinum software (GE Healthcare). Scanned images were analyzed using ImageQuant TL (GE Healthcare). Protein bands of interest were selected and quantified based on their pixel profile. Quantified bands in IF samples were normalized to the corresponding band in the ST sample so that samples run on different gels could be compared. Results were analyzed by one-way ANOVA followed by the Tukey’s posttest and plotted using GraphPad Prism (GraphPad Software, CA).
Analysis of germ cell–specific proteins in IF.
IF proteins were separated by gel electrophoresis as described above and then transferred onto an Immobilon-FL PVDF membrane (Millipore, Hertfordshire, UK) using a Hoefer TE22 transfer chamber (GE Healthcare). Gels and membranes were sandwiched between layers of blotting paper and sponges in a blotting cassette. NuPAGE transfer buffer (Invitrogen) was supplemented with NuPAGE antioxidant (0.01%) and methanol (10%), and the transfer run for 4 h at 40 V. Membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences, Cambridge, UK) diluted with PBS (1:1) for 1 h. Primary antibody (anti-VASA 1:1000; Abcam, Cambridge, UK or anti-FABP9 1:5000; R&D Systems, MN) in the same buffer was added and incubated with the membrane overnight at 4°C. Membranes were washed four times for 5 min using PBS + 0.1% Tween-20 (Sigma) and then incubated with secondary antibody diluted 1:10,000 (for VASA, IRDye800-labeled goat anti-rabbit; Rockland and for FABP9, Alexa Fluor 680–labeled donkey anti-goat; Molecular Probes, Invitrogen) for 1 h at room temperature. Membranes were again washed in PBS + 0.1% Tween-20, with a final PBS wash. Membranes were analyzed using the Odyssey Infrared Imaging System (LI-COR Biosciences). Western blots were scanned, viewed, and protein bands quantified by measuring the intensity of the band using the Odyssey software (LI-COR Biosciences). Quantified bands were analyzed as described above.
Evaluation of the integrity of the BTB.
Double staining for the BTB proteins, ZO-1/occludin, or N-cadherin/β-catenin were undertaken to assess integrity of the BTB. Control sections were included in each experiment without either/both primary antibodies. For ZO-1/occludin, antigen retrieval and blocking of endogenous peroxidase activity were performed as described above. Wash steps involved two 5-min incubations at room temperature in PBS. Nonspecific binding was blocked using normal goat serum (NGS) diluted 1:4 in PBS with 5% BSA, and a Streptavidin/Biotin Blocking Kit (Vector Laboratories) was used to reduce background staining. Sections were then incubated with anti-ZO-1 antibody (Zymed now Invitrogen) diluted 1:100 in NGS/PBS/BSA, overnight at 4°C. Sections were incubated with biotinylated goat anti-rabbit antibody diluted 1:200 in NGS/PBS/BSA, followed by detection with a fluorescent-labeled streptavidin conjugate, streptavidin-488. A second NGS/PBS/BSA blocking step was performed, followed by incubation with anti-occludin antibody (Zymed) diluted 1:200 in NGS/PBS/BSA, overnight at 4°C. Occludin was detected using a goat anti-mouse peroxidase–labeled antibody, followed by incubation with the Cy3 Tyramide-Signal Amplification System (PerkinElmer, MA). Dapi nuclear counterstain was applied to slides before mounting with PermaFluor (Thermo Scientific). A similar method was used for localization of N-cadherin/β-catenin. Two antigen retrieval steps were included; citrate retrieval by pressure-cooking was initially performed, and an antigen retrieval step was carried out between incubation with the two primary antibodies, by microwaving slides in 0.01M citrate buffer (pH 6) on full power for 2.5 min, then resting for 30 min. Anti-N-cadherin was diluted 1:14,000 in NGS/PBS/BSA and detected using a goat anti-mouse peroxidase–labeled Fab fragment (Abcam), followed by tyramide Cy3. Anti-β-catenin was diluted 1:500 in NGS/PBS/BSA and detected using a goat anti-mouse peroxidase–labeled Fab fragment, followed by tyramide-fluorescein (PerkinElmer). Slides were viewed using a Zeiss LSM510 Meta confocal microscope (Carl Zeiss Ltd, Herts, UK) and Zen software (Carl Zeiss Ltd).
Evaluation of the functional integrity of the BTB.
To assess BTB function, a biotin tracer technique was used (based on the method described by Tarulli et al., 2008). EZ-link sulfo-NHS-LC-biotin (Pierce Bioscience, IL) was prepared as a 10 mg/ml solution in 0.01M CaCl2 in PBS. Two hundred microliters of this biotin tracer was injected into exposed testes of freshly killed rats. The testes were then dissected out and left at room temperature for 30 min. Testes were then fixed in Bouins and processed as described above. The biotin tracer was detected in testis sections using an immunofluorescent technique. Staining for occludin was also carried out to show the site of the BTB, in a modified double immunofluorescent protocol. Antigen retrieval and blocking steps were performed as described above. The biotin tracer was detected by incubating slides with Streptavidin-488 diluted 1:200 in PBS for 1 h. Detection of occludin was performed as described above using a goat anti-mouse peroxidase–labeled secondary antibody and tyramide Cy3.
RESULTS
Toxicant-Induced Damage
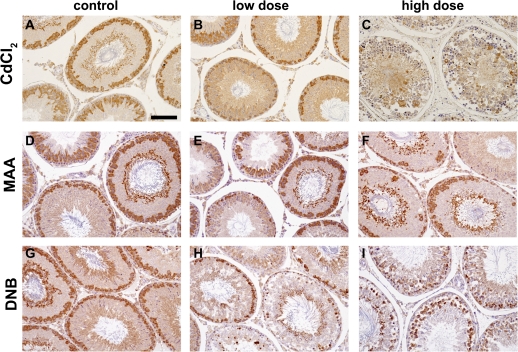
Toxicant-induced damage was assessed at 24 h by immunostaining for the germ cell protein DAZL. In control testis sections, DAZL was localized predominantly to pachytene spermatocytes and residual bodies (Figs. 1A, 1D, and 1G). Treatment with a low dose of cadmium chloride (1 mg/kg) showed no effect on the testis, and DAZL staining was comparable with control (Fig. 1B). However, a higher dose of cadmium chloride (3 mg/kg) caused severe disruption to spermatogenesis and a large reduction in DAZL staining (Fig. 1C). An intermediate dose of cadmium chloride (1.75 mg/kg) also caused some disruption to the seminiferous epithelium (results not shown). Treatment with a high dose of MAA (650 mg/kg) caused depletion of pachytene spermatocytes, clearly shown by the DAZL staining (Fig 1F), whereas treatment with 200 mg/kg MAA caused a milder depletion of pachytene spermatocytes (Fig. 1E). Treatment with DNB caused Sertoli cell vacuolation and spermatocyte depletion with changes being marginally more severe with 50 mg/kg (Fig. 1I) than with 25 mg/kg DNB (Fig. 1H).
FIG. 1.
Histological analysis of the effects of treatment with cadmium chloride, MAA, and DNB on rat testes. Rats were dosed with either a low or high dose of toxicant and testes collected 24 h later and evaluated for immunoexpression of the germ cell–specific protein DAZL (brown); counterstaining used hematoxylin (blue). Scale bar represents 100 μm. (A, D, and G). Testes from vehicle-treated rats show normal spermatogenesis and the presence of DAZL in pachytene spermatocytes and residual bodies. Testis sections from rats treated 24 h earlier with a low dose of toxicant, (B) 1 mg/kg CdCl2, (E) 200 mg/kg MAA, (H) 25 mg/kg DNB or a high dose of toxicant, (C) 3 mg/kg CdCl2, (F) 650 mg/kg MAA, and (I) 50 mg/kg DNB show dose-related toxicant-induced damage to spermatogenesis.
Evaluation of Proteins in IF Following Toxicant Treatment
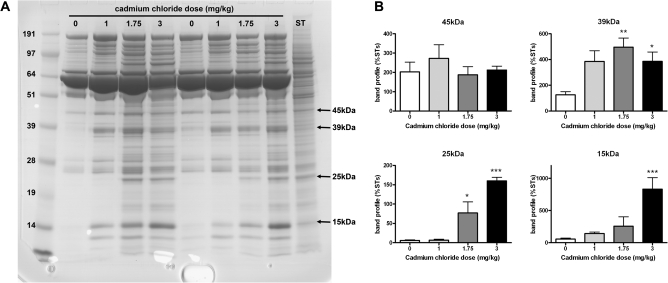
Protein bands in IF samples were selected for quantification in the different treatment groups. Analysis of proteins in IF from rats treated with cadmium chloride showed a dose-related increase in the number of proteins present in IF following treatment (representative gel shown in Fig. 2A), and as protein bands of similar molecular weight were present in the ST extract, it was deduced that leakage of proteins from STs into IF may have occurred following treatment with high doses of cadmium chloride. Comparison of the protein profile between IF and blood plasma was not routinely undertaken in the present studies, but in general, the profile on 1D gels for both media is similar (Turner et al., 1996; Elkin, Piner, and Sharpe, unpublished data), consistent with the view that IF is simply an exudate of blood plasma (Setchell et al., 1994). Quantification of four protein bands from all IF samples collected from cadmium chloride–treated animals showed that some proteins (25 and 15 kDa; Fig. 2B) increased in concentration in IF in a dose-related manner. Other proteins, such as the 45-kDa band, did not appear to change in concentration, and the 39 kDa protein appeared to increase in concentration following all doses of cadmium chloride treatment (Fig. 2B).
FIG. 2.
Effects of cadmium chloride (1, 1.75, or 3 mg/kg) treatment on leakage of ST proteins into IF collected 24 h later. A protein extract of isolated ST from a control rat were run on each gel for normalization. A dose-related increase in the number of proteins in IF samples was observed. (A) Representative 1D gel with arrows indicating protein bands that were quantified. (B) Quantification (mean + SEM) of four proteins (45, 39, 25, and 15 kDa) in IF from the different treatment groups. (n = 8 samples per group except for 1 mg/kg CdCl2 where n = 5). Samples (two per dose) were run on four different gels, and quantification of proteins was normalized to the ST sample run on each of the gels. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control IF.
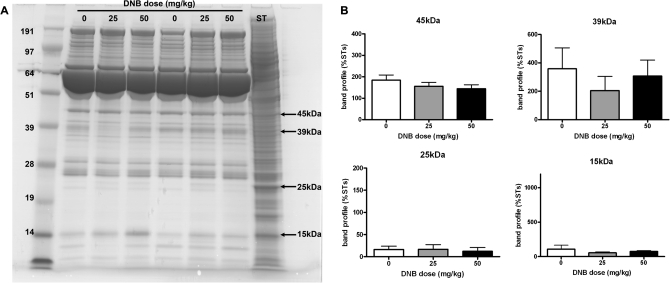
In contrast, no differences in the proteins present in IF samples from low- and high-dose DNB-treated animals were observed when compared with controls (Fig. 3A). Quantification of four protein bands (45, 39, 25, and 15 kDa) supported this conclusion (Fig. 3B) and suggested that ST proteins were not leaking into IF following DNB treatment. Similar results were observed with IF samples collected from rats treated with MAA, suggesting that this treatment also did not result in ST proteins leaking into IF (results not shown).
FIG. 3.
Effect of DNB (25 or 50 mg/kg) treatment on leakage of ST proteins into IF collected 24 h later. A protein extract of isolated ST from a control rat were run on each gel for normalization. (A) Representative 1D gel with arrows indicating protein bands that were quantified. No differences in the proteins present in IF were noted following DNB treatment. (B) Quantification (mean + SEM) of four proteins (45, 39, 25, and 15 kDa) in IF from the different treatment groups (n = 8 samples per group). Samples (two per dose) were run on four different gels, and quantification of proteins was normalized to the ST sample run on each of the gels.
BTB Integrity
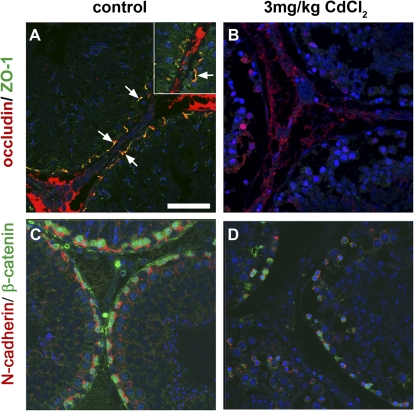
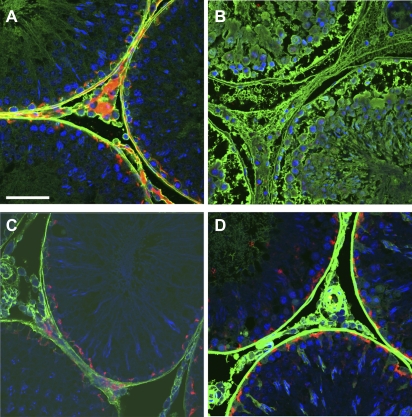
The results above suggested that germ cell damage alone does not cause leakage of ST proteins into IF. To investigate whether an effect on the BTB was responsible for the protein leakage, integrity of the BTB was evaluated in the different treatment groups using two techniques. First, co-staining for BTB proteins, and second, analysis of BTB function using biotin tracer evaluation. Occludin is a tight junction protein, which has been detected at the site of the BTB (reviewed in Mruk and Cheng, 2004). Immunofluorescence co-staining for the tight junction adaptor protein ZO-1 and occludin was undertaken to assess the state of the tight junctions at the BTB. In control testes, occludin and ZO-1 clearly colocalized at the site of the BTB, parallel to the basement membrane of tubules (Fig. 4A). Following high-dose (3 mg/kg) cadmium chloride treatment, occludin and ZO-1 appeared to be absent from the STs (Fig. 4B). To assess the state of adherens junctions in the BTB we used immunofluorescent co-staining for the adherens junction protein N-cadherin and the adaptor protein β-catenin. N-cadherin was shown to localize to the site of the BTB in control testis sections with β-catenin localized to the spermatocytes adjacent to the BTB (Fig. 4C). Following high-dose (3 mg/kg) cadmium chloride treatment, the organization of both N-cadherin and β-catenin was disrupted from the site of the BTB with an apparent reduction in expression of both proteins (Fig. 4D). These results suggested that treatment with 3 mg/kg cadmium chloride has disrupted the tight junctions and adherens junctions at the BTB. Some disruption to the BTB proteins was observed following treatment with 1.75 mg/kg CdCl2, but BTB protein expression in testis sections from rats treated with 1 mg/kg CdCl2 was comparable with controls (data not shown). Similar investigations were undertaken with tissue collected from the MAA- and DNB-treated animals, but no differences in BTB protein expression were observed in comparison with controls, suggesting that neither treatment had affected the BTB (data not shown).
FIG. 4.
Localization of BTB proteins occludin (red) and ZO-1 (green) (arrows, panels A and B and insert, panel A) and N-cadherin (red) and β-catenin (green) (panels C and D), in testes from vehicle-treated control rats (A and C) and rats treated 24 h earlier with 3 mg/kg cadmium chloride (B and D). Note the depletion of all BTB proteins from the site of the BTB following treatment with 3 mg/kg cadmium chloride. Scale bar represents 50 μm. Blue = nuclear counterstain (Dapi). Note also that the red “staining” present in the interstitium represents nonspecific binding of the tyramide Cy3 as it was also present in slides for which the occludin antibody was omitted (not shown).
Further analysis of the BTB was carried out using a biotin tracer, injected into freshly dissected testes, to assess the function of the BTB. The tracer cannot penetrate cell membranes (manufacturers literature) and so should be localized to the interstitium and in the STs up to the site of the BTB if the BTB is functional. Figure 5A shows the biotin tracer localized to the interstitium and surrounding the germ cells up to the site of the BTB (indicated by occludin) in control testes. Following treatment with 3 mg/kg CdCl2, the biotin tracer penetrated fully into the STs (Fig. 5B), indicating loss of BTB function and supporting the results obtained with the BTB protein localization studies (Fig. 4). Treatment with 1.75 mg/kg CdCl2 did not obviously alter biotin tracer penetration in most testis cross-sections studied (not shown), suggesting that this technique is perhaps less sensitive than is direct evaluation of BTB protein expression in situ, as outlined above. Treatment with high-dose MAA (650 mg/kg) or DNB (50 mg/kg) did not affect BTB function, with the biotin tracer localized to the interstitium and up to the site of the BTB as in controls (Figs. 5C and 5 D compared with Fig. 5A).
FIG. 5.
Localization of biotin tracer (green) in testes from a vehicle-treated control rat (A) and rats treated with either 3 mg/kg CdCl2 (B), 650 mg/kg MAA (C), or 50 mg/kg DNB (D). Occludin protein is shown in red to indicate the site of the BTB. The biotin tracer is localized to the interstitium and up to the site of the BTB in control testes, and testes from animals treated with 650 mg/kg MAA or 50 mg/kg DNB (A, C, and D) but has penetrated the STs following treatment with 3 mg/kg CdCl2 (B). Scale bar represents 50 μm. Blue= nuclear counterstain (Dapi).
Evaluation of Germ Cell–Specific Proteins in IF Following Toxicant Treatment
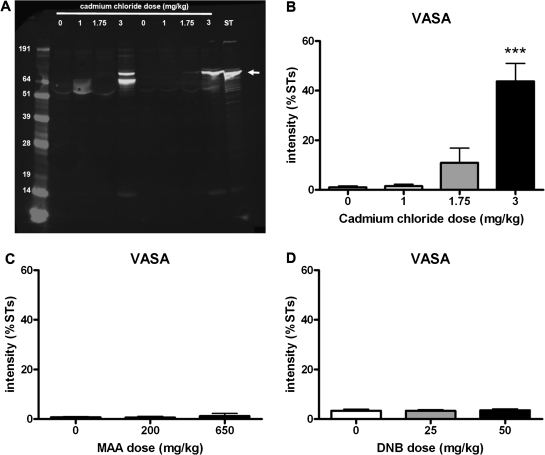
The germ cell–specific proteins, VASA and FABP9, were selected for investigation as potential biomarker candidate proteins. VASA and FABP9 were measured in IF samples from animals treated with the toxicants, using Western blotting. VASA was detected as a clear band ∼76 kDa in size in samples from animals treated with 3 mg/kg cadmium chloride and in the ST extract (representative blot shown in Fig. 6A). A smaller molecular weight band was observed in some lanes and was thought to be nonspecific detection of albumin. Quantification of VASA across all samples run on four blots showed a significantly higher concentration of VASA in IF following treatment with 3 mg/kg cadmium chloride (Fig. 6B). A slight increase in VASA in IF following 1.75 mg/kg cadmium chloride was also observed, with VASA in IF from 1 mg/kg cadmium chloride–treated animals comparable with control (Fig. 6B). VASA in IF from animals treated with either dose of MAA or DNB was comparable with control (Figs. 6C and 6D) with little or no protein detected.
FIG. 6.
Effects of toxicant treatment on leakage of VASA protein into IF collected 24 h later. (A) Representative Western blot with IF samples collected 24 h following administration of 1, 1.75, or 3 mg/kg cadmium chloride or vehicle control compared with ST sample, probed with VASA antibody. VASA was detected at ∼76 kDa. (B) Quantification of VASA protein band (∼76 kDa) (mean + SEM) in IF from the different treatment groups. Samples (two per dose) were run on four different blots and quantification of VASA protein band was normalized to the ST sample run on each of the blots (n = 8, except 0 mg/kg where n = 7 and 1 mg/kg where n = 5). (C) Quantification of VASA protein band (∼76 kDa) in IF collected from rats treated with 200 or 650 mg/kg MAA (mean + SEM). Samples (two per dose) were run on two different blots, and quantification of VASA protein band was normalized to the ST sample run on each of the blots (n = 4). (D) Quantification of VASA protein band (∼76 kDa) in IF collected from rats treated with 25 or 50 mg/kg DNB (mean + SEM). Samples (two per dose) were run on two different blots, and quantification of VASA protein band was normalized to the ST sample run on each of the blots (n = 4). ***p < 0.001 compared with control IF.
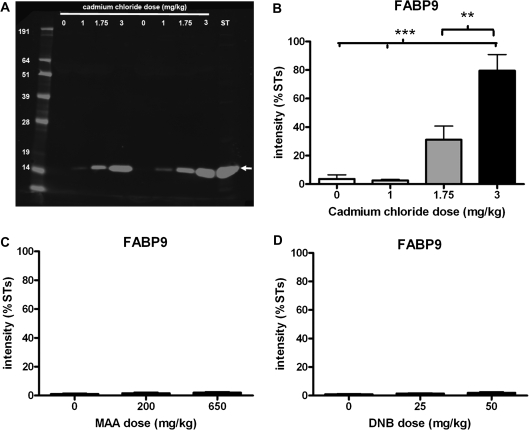
Similarly, FABP9 was detected by Western blotting with a clear band ∼15 kDa in size observed (representative blot shown in Fig. 7A). A clear dose-related increase in the concentration of FABP9 in IF from animals treated with cadmium chloride was observed on the blots and supported by quantification of the protein bands (Fig. 7B). FABP9 in IF from animals treated with either MAA or DNB were comparable with control (Figs. 7C and 7D) with little or no protein detected.
FIG. 7.
Effects of toxicant treatment on leakage of FABP9 protein into IF collected 24 h later. (A) Representative Western blot with IF samples collected 24 h following administration of 1, 1.75, or 3 mg/kg cadmium chloride or vehicle control compared with ST sample, probed with FABP9 antibody. FABP9 was detected at ∼15 kDa. (B) Quantification of FABP9 protein band (∼15 kDa) (mean + SEM) in IF from the different treatment groups. Samples (two per dose) were run on four different blots and quantification of FABP9 protein band was normalized to the ST sample run on each of the blots (n = 8, except 0 mg/kg where n = 7 and 1 mg/kg where n = 5). (C) Quantification of FABP9 protein band (∼15 kDa) in IF collected from rats treated with 200 or 650 mg/kg MAA (mean + SEM). Samples (two per dose) were run on three different blots, and quantification of VASA protein band was normalized to the ST sample run on each of the blots (n = 6 except 0 mg/kg where n = 7). (D) Quantification of FABP9 protein band (∼15 kDa) in IF collected from rats treated with 25 or 50 mg/kg DNB (mean + SEM). Samples (two per dose) were run on three different blots and quantification of FABP9 protein band was normalized to the ST sample run on each of the blots (n = 6). **p < 0.01, ***p < 0.001 compared with control IF.
Results with the specific proteins, VASA and FABP9, support earlier results, suggesting that proteins leak from STs into IF following cadmium chloride treatment but not following MAA or DNB treatment.
DISCUSSION
The objective of this study was to investigate whether germ cell–derived proteins leak from STs into IF following toxicant-induced damage to spermatogenesis and whether leaked proteins could be used to detect testicular damage regardless of mechanism of toxicant action. Our results suggest that proteins only leak from STs following loss of integrity of the BTB and that toxicant-induced disruption of spermatogenesis does not lead to protein leakage if the BTB remains intact.
The primary detection method employed in the present studies was to compare the protein profile in IF collected from individual control and toxicant-treated adult rats. IF is an exudate from blood and exhibits a similar protein profile to blood plasma (Turner et al., 1996). Initial identification of the appearance of putative ST-derived proteins in IF was achieved by comparing the IF protein profile with that present in a homogenate of isolated adult STs; the latter will contain proteins deriving from peritubular myoid, Sertoli, and germ cells. Some of these proteins, especially those deriving from nongerm cells, may normally leak or be secreted into IF, whereas proteins from meiotic and postmeiotic germ cells are presumed not to normally leak into IF because they are potentially immunogenic. Appearance of a protein band in IF in toxicant-treated animals that was not present in control rat IF (or in lower amounts) was taken as potential evidence for its leakage from STs if a corresponding band was present in the ST extract. The most likely source of these “new” protein bands is germ cells as proteins from these cells will be most abundant in ST extracts, but it is also possible that they could emanate from a source other than the ST, e.g., from the vasculature or from Leydig/other interstitial cells. These options were considered less likely because both our earlier (Turner et al., 1996) and later (Elkin, Piner, and Sharpe, unpublished data) studies have shown no supporting evidence for these possibilities. In contrast, as we have shown presently, two particular protein bands in IF from cadmium chloride–exposed animals can be identified as germ cell–specific proteins (see below).
Analysis of proteins present in IF following cadmium chloride treatment showed a dose-related increase in the number of proteins detected in IF in comparison with controls. Quantification of some protein bands from the 1D gels suggested increases in the amount of some proteins in IF (39, 25, and 15 kDa) following cadmium chloride treatment. Other protein bands, such as the 45-kDa band, did not appear to leak from STs into IF following cadmium chloride treatment with a fairly constant amount of protein present in IF from all dose groups. Although these protein bands are of an unknown nature and could potentially represent a range of similar sized proteins (as 1D gels were used), their potential origin from STs was evidenced by the presence of a comparable band in the ST sample run on each gel. The 45 kDa protein was present in IF at a constant amount, irrespective of cadmium chloride treatment, which could suggest that it is Sertoli cell derived and is secreted constitutively into IF. In contrast, protein bands that were not normally present in IF but which appeared after cadmium chloride treatment and corresponded with a similar sized protein band in the ST extract could potentially be germ cell derived. To establish whether or not this was the case, IF samples from the different treatment groups were subjected to Western blotting for two germ cell–specific proteins, VASA and FABP9.
VASA is a member of the DEAD-box family of genes, which encodes an ATP-dependent RNA helicase and is expressed specifically in germ cells and is involved in the regulation of cell proliferation and differentiation (Tanaka et al., 2000). FABP9 (PERF15/TLBP) is a small transport protein and a member of the FABP family of conserved intracellular lipid–binding proteins (Furuhashi and Hotamisligil, 2008; Kido and Namiki, 2000). FABP9 is present in the rat perforatorium at the inner acrosomal and outer face of the nuclear envelope of the sperm head and can be identified in pachytene spermatocytes and round and elongate spermatids (Korley et al., 1997; Oko and Morales, 1994). It has been suggested to have roles in mediating structural rearrangement and stability of the acrosome (Oko and Morales, 1994), controlling sperm quality (Kido et al., 2005) and germ cell apoptosis in the developing testis (Kido and Namiki, 2000). Western blot analysis of both proteins showed a dose-related increase in their amounts in IF following cadmium chloride treatment. Both VASA and FABP9 are localized to postmeiotic germ cells, so the results support the idea that the proteins are leaking into IF from behind the BTB, following testicular damage. Indeed, the band for FABP9 probably corresponds to the 15-kDa protein band detected with Coomassie blue staining in our studies of overall protein leakage into IF. The dose-related increase in VASA and FABP9 levels in IF following cadmium chloride treatment supports the use of either protein as a biomarker for cadmium chloride–induced disruption to STs.
As cadmium chloride treatment caused considerable disruption of spermatogenesis and germ cell damage/loss, it was further investigated if other treatments (MAA and DNB) that also caused disruption of spermatogenesis/germ cells via other mechanisms also resulted in leakage of proteins into IF. However, in both MAA- and DNB-treated animals, no evidence of ST protein leakage into IF in general, or of VASA or FABP9 in particular, was detected despite widespread disruption of spermatogenesis. This suggested that ST protein leakage was specific to cadmium chloride treatment. This compound is established to alter vascular permeability in the testis (Setchell and Waites, 1970; Siu et al., 2009), so theoretically this could be responsible for the altered protein profile observed in IF from these animals. However, as most plasma proteins normally pass readily from plasma into testicular IF (Setchell et al., 1994; Turner et al., 1996), it is not obvious why an increase in microvessel permeability would lead to the appearance of proteins in IF, especially when they correspond in size to proteins present in ST. More recent studies have shown that, at low doses, cadmium chloride causes disruption of the BTB (reviewed in Siu et al., 2009), and at face value, this appeared to offer a more logical explanation for our present findings. If this was the case, then our expectation was that neither MAA nor DNB would affect the BTB, as in contrast to cadmium chloride, neither of these toxicants induced the appearance of putative ST-derived proteins in IF.
The BTB is a complex structure of interacting proteins with an important function in the maintenance of the specific microenvironment for spermatogenesis. Tight and adherens junctions between adjacent Sertoli cells constitute the BTB, with junction proteins connecting to the Sertoli cell cytoskeleton via adaptor proteins. Immunofluorescent evaluation of BTB component proteins (occludin, ZO-1, N-cadherin, and β-catenin) and functional analysis of the BTB (biotin tracer injection) were therefore applied to the present treatment groups to identify if BTB disruption explained leakage of ST proteins into IF.
The present studies show unequivocally that only cadmium chloride, at doses of 1.75–3.0 mg/kg, disrupted the BTB as evidenced by loss of BTB components (occludin, ZO-1, N-cadherin, and β-catenin), whereas MAA and DNB had no discernible effect. Consistent with these findings, evidence for loss of BTB functional integrity based on exclusion of biotin, showed a broadly similar relationship to treatment, although it was reckoned to be a less sensitive indicator of BTB damage than was direct visualization of BTB protein components in situ. Moreover, only treatment groups/animals that showed loss of BTB integrity showed evidence of ST and germ cell protein leakage into IF. It is therefore concluded that such leakage is probably dependent upon loss of BTB integrity and is not related per se to disruption of spermatogenesis/germ cell damage. We cannot completely exclude the possibility that cadmium chloride–induced alteration of vascular permeability (Setchell and Waites, 1970; Prozialeck et al., 2008) plays a part in these events, but this would not provide a logical explanation for why proteins derived specifically from germ cells that are sequestered behind the BTB would leak into IF.
Therefore, our results, obtained using three differently acting testicular toxicants, show that loss of integrity of the BTB is probably mandatory for leakage of (germ cell–specific) proteins from STs into IF, rather than just damage to the germ cells or Sertoli cells. The results, however, do not identify whether loss of one component of the BTB is required for loss of integrity of the barrier or whether several components must be depleted. It is unlikely that disruption of one component would lead to a loss of integrity of the BTB based on the mechanism of BTB rearrangement when the preleptotene/leptotene spermatocytes must traverse the BTB at stages VII–IX of the rat spermatogenic cycle (reviewed in Mruk and Cheng, 2004). It has been suggested that the adherens junctions compensate for disengagement of tight junction components and vice versa, and this allows the spermatocytes to cross the BTB without a loss of its overall integrity (Yan and Cheng, 2005). If this is the case, then it is likely that such a mechanism would also compensate for the toxicant-induced selective depletion of any one component of the BTB. In turn, this suggests that changes in levels of a biomarker would probably only be able to indicate extensive damage to the BTB and not disruption of one component.
Our identification of germ cell–specific proteins that leak from STs into IF following cadmium chloride treatment raises the possibility of developing appropriately sensitive assay systems to detect changes in concentration of the target proteins in the bloodstream. Although this might be feasible, our present conclusion is that this would be of little practical use in toxicological studies as it would only be able to detect spermatogenic damage when this was associated with major loss of integrity of the BTB; it would not detect damage to spermatogenesis where the BTB remained largely intact, which is probably the more common scenario. Therefore, prospects for biomonitoring of testicular damage per se using germ cell–specific protein biomarkers appear, unfortunately, to be of limited utility.
FUNDING
UK Biotechnology and Biological Sciences Research Council Industrial Collaborative Award in Science and Engineering studentship(Ref: 13077) to N.D.E.
Acknowledgments
We thank Mark Fisken for animal husbandry and treatments and Mike Millar, Shelia MacPherson, and Arantza Esnal for histological support.
References
- Anderson RA, Sharpe RM. Regulation of inhibin production in the human male and its clinical applications. Int. J. Androl. 2000;23:136–144. doi: 10.1046/j.1365-2605.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Bartlett JM, Kerr JB, Sharpe RM. The selective removal of pachytene spermatocytes using methoxy acetic acid as an approach to the study in vivo of paracrine interactions in the testis. J. Androl. 1988;9:31–40. doi: 10.1002/j.1939-4640.1988.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Blackburn DM, Gray AJ, Lloyd SC, Sheard CM, Foster PMD. A comparison of the effects of the 3 isomers of dinitrobenzene on the testis in the rat. Toxicol. Appl. Pharmacol. 1988;92:54–64. doi: 10.1016/0041-008x(88)90227-x. [DOI] [PubMed] [Google Scholar]
- Creasy DM. Evaluation of testicular toxicology: a synopsis and discussion of the recommendations proposed by the Society of Toxicologic Pathology. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2003;68:408–415. doi: 10.1002/bdrb.10041. [DOI] [PubMed] [Google Scholar]
- Draper RP, Timbrell JA. Comparison of urinary creatine with other biomarkers for detection of cadmium-induced testicular damage. Biomarkers. 1998;3:335–346. doi: 10.1080/135475098231147. [DOI] [PubMed] [Google Scholar]
- Foster PMD, Creasy DM, Foster JR, Gray TJB. Testicular toxicity produced by ethylene-glycol monomethyl and monoethyl ethers in the rat. Environ. Health Perspect. 1984;57:207–217. doi: 10.1289/ehp.8457207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster PMD, Lloyd SC, Prout MS. Toxicity and metabolism of 1,3-dinitrobenzene in rat testicular cell-cultures. Toxicol. In Vitro. 1987;1:31–37. doi: 10.1016/0887-2333(87)90035-x. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in-vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol. Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- Kido T, Arata S, Suzuki R, Hosono T, Nakanishi Y, Miyazaki J, Saito I, Kuroki T, Shioda S. The testicular fatty acid binding protein PERF15 regulates the fate of germ cells in PERF15 transgenic mice. Dev. Growth Differ. 2005;47:15–24. doi: 10.1111/j.1440-169x.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- Kido T, Namiki H. Expression of testicular fatty acid-binding protein PERF 15 during germ cell apoptosis. Dev. Growth Differ. 2000;42:359–366. doi: 10.1046/j.1440-169x.2000.00520.x. [DOI] [PubMed] [Google Scholar]
- Korley R, Pouresmaeili F, Oko R. Analysis of the protein composition of the mouse sperm perinuclear theca and characterization of its major protein constituent. Biol. Reprod. 1997;57:1426–1432. doi: 10.1095/biolreprod57.6.1426. [DOI] [PubMed] [Google Scholar]
- Moore NP, Creasy DM, Gray TJB, Timbrell JA. Urinary creatine orofiles after administration of cell-specific testicular toxicants to the rat. Arch. Toxicol. 1992;66:435–442. doi: 10.1007/BF02035135. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Oko R, Morales CR. A novel testicular protein, with sequence similarities to a family of lipid binding proteins, is a major component of the rat sperm perinuclear theca. Dev. Biol. 1994;166:235–245. doi: 10.1006/dbio.1994.1310. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The vascular system as a target of metal toxicity. Toxicol. Sci. 2008;102:207–218. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader SCJ, Shingles C, Stonard MD. Acute testicular toxicity of 1,3-dinitrobenzene and ethylene-glycol monomethyl ether in the rat—evaluation of biochemical effect markers and hormonal responses. Fundam. Appl. Toxicol. 1991;16:61–70. doi: 10.1016/0272-0590(91)90135-q. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Maddocks S, Brooks DE. Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2nd ed. New York: Raven Press; 1994. pp. 1063–1173. [Google Scholar]
- Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J. Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Cooper I. Testicular interstitial fluid as a monitor for changes in the intratesticular environment in the rat. J. Reprod. Fertil. 1983;69:125–135. doi: 10.1530/jrf.0.0690125. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Maddocks S, Millar M, Kerr JB, Saunders PT, McKinnell C. Testosterone and spermatogenesis. Identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J. Androl. 1992;13:172–184. [PubMed] [Google Scholar]
- Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Turner KJ. Inhibin B as a potential biomarker of testicular toxicity. Cancer Biomark. 2005;1:75–91. doi: 10.3233/cbm-2005-1109. [DOI] [PubMed] [Google Scholar]
- Suter L, Clemann N, Koch E, Bobadilla M, Bechter R. New and traditional approaches for the assessment of testicular toxicity. Reprod. Toxicol. 1998;12:39–47. doi: 10.1016/s0890-6238(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, Yokoyama M, Noce T. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev. 2000;14:841–853. [PMC free article] [PubMed] [Google Scholar]
- Tarulli GA, Meachem SJ, Schlatt S, Stanton PG. Regulation of testicular tight junctions by gonadotrophins in the adult Djungarian hamster in vivo. Reproduction. 2008;135:867–877. doi: 10.1530/REP-07-0572. [DOI] [PubMed] [Google Scholar]
- Turner KJ, McKinnell C, McLaren TT, Qureshi SJ, Saunders PT, Foster PM, Sharpe RM. Detection of germ cell-derived proteins in testicular interstitial fluid: potential for monitoring spermatogenesis in vivo. J. Androl. 1996;17:127–136. [PubMed] [Google Scholar]
- Yan HH, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]