Abstract
Human immunodeficiency virus-1 antiretroviral treatment is associated with an increased incidence of atherosclerosis. We hypothesized that antiretrovirals directly impair endothelial function after short-term exposure and that with chronic exposure, this dysfunction promotes a proliferative response, inducing neointimal hyperplasia, thus contributing to vascular lesion formation. To test this hypothesis, we treated mice with the nucleoside reverse transcriptase inhibitor azidothymidine (AZT), the protease inhibitor indinavir, or AZT + indinavir. Treatment with AZT or AZT + indinavir for 5 days impaired endothelium-dependent vessel relaxation. Though indinavir treatment alone did not alter vessel relaxation, it potentiated the impairment of endothelium-dependent relaxation induced by AZT. Coadministration of the antioxidant Mn (III) tetrakis (1-methyl-4-pyridyl) porphyrin attenuated antiretroviral-induced endothelial dysfunction, suggesting that oxidant production may have a causal role in the observed endothelial dysfunction. To test whether the antiretrovirals promote a proliferative response following endothelial dysfunction, we treated mice with antiretrovirals for 14 days and then induced a carotid endothelial injury. Two weeks later, we observed a dramatic increase in neointimal formation in all antiretroviral-treated animals, and the newly formed neointima was comprised mainly of proliferated smooth muscle cells. Although a functional endothelium surrounding the lesioned area and re-endothelialization across the area of injury is important in reducing proliferation in this model, we tested whether the neointimal hyperplasia was associated with endothelial dysfunction. Plasma levels of asymmetric dimethylarginine, a biomarker of endothelial dysfunction, increased after treatment with indinavir or AZT + indinavir. On the other hand, treatment with AZT or AZT + indinavir increased endothelial vascular cell adhesion molecule staining. We conclude that short-term treatment with antiretrovirals elicited a direct impairment in endothelial function, in part via an oxidant-dependent pathway. These antiretrovirals also exacerbated injury-induced vascular smooth muscle cell proliferation and neointimal hyperplasia, likely because of their inhibition of endothelial function.
Keywords: antiretrovirals, neointimal hyperplasia, endothelial dysfunction, oxidative stress, atherosclerosis
Human immunodeficiency virus-1 (HIV-1) antiretroviral therapy is associated with an increased incidence of atherosclerosis in HIV patients across all adult age groups. Furthermore, all stages of atherosclerotic disease have been described in HIV patients, from premature atherosclerosis, including endothelial dysfunction (de Gaetano Donati et al., 2003; Jiang et al., 2006; Stein et al., 2001) and increased intima-media thickness (Charakida et al., 2005; Lorenz et al., 2008), to advanced cardiovascular events, such as myocardial infarction and stroke (Barbaro et al., 2003; Friis-Moller et al., 2003).
At present, the relationship between antiretroviral use and the increased risk of atherosclerosis remains unclear. Traditionally, the increased early onset of atherosclerotic vascular diseases was attributed to the use of early generation protease inhibitors (PIs), especially those that caused “atherogenic” metabolic abnormalities. For example, indinavir was shown associated with insulin resistance, and ritonavir was linked to hypertriglyceridemia and increased low-density lipoprotein cholesterol levels (Lee et al., 2004). However, PI-associated alteration of lipid profiles does not account for all atherogenic effects observed for antiretroviral use. In our prior report, although the PI indinavir increased plasma lipid levels (Jiang et al., 2006), no effect on vascular reactivity was observed. Interestingly, indinavir-induced effects on vasomotor function have varied between reports. Whereas we and Chai et al. (2005a) showed that indinavir had no effect on vascular reactivity in rat aortas and porcine coronary arteries (Jiang et al., 2006), Shankar et al. (2005) and Dube et al. (2008) described impairment of endothelium-dependent vasorelaxation in humans. Nevertheless, an increasing body of evidence now indicates an atherogenic effect of nucleoside reverse transcriptase inhibitors (NRTIs). In particular, in 2008, a clinical report from the international DAD cohort study suggested that recent use of the NRTIs abacavir and didanosine appeared to increase the rate of myocardial infarction (Sabin et al., 2008). In addition, we and others reported that a commonly prescribed NRTI AZT impaired endothelium-dependent vasorelaxation (Jiang et al., 2006; Sutliff et al., 2002). In the studies conducted by Sutliff et al. (2002), ex vivo treatment of aortic rings with an antioxidant restored the AZT-induced impairment in vasorelaxation.
NRTIs are considered the backbone of therapy for the HIV infection and are commonly prescribed in combination with PIs or nonnucleoside reverse transcriptase inhibitors (NNRTI). AZT was the first NRTI introduced to the market and is still frequently prescribed, particularly because the advent of a single capsule, dual NRTI formulation (Combivir) including both AZT and lamivudine (Soriano et al., 2008). Though we have shown that antiretrovirals, especially NRTIs, induce vascular endothelial dysfunction in rats and that these antiretrovirals increase the production of mitochondria-derived reactive oxygen species (ROS) in cultured endothelial cells (Jiang et al., 2007), there is as yet no direct, in vivo evidence that the production of ROS has a causal role in NRTI-associated impairment of endothelium-dependent vasorelaxation.
A functional endothelium is vital to the maintenance of vascular homeostasis. Endothelial injury initiates intimal proliferation of smooth muscle cells, lipid accumulation, and monocyte infiltration, thus initiating the development of an early atherosclerotic lesion. It is as yet unknown whether antiretrovirals, especially NRTIs, can accelerate neointimal growth. In prior studies, we demonstrated that both AZT and indinavir promote vascular smooth muscle cell (VSMC) proliferation when cocultured together with endothelial cells (Hebert et al., 2004). Using a diet-induced atherosclerotic mouse model, we demonstrated that both AZT and indinavir increased aortic intima-to-media thickness (IMT) (Jiang et al., 2009), mainly via increases in the thickness of the medial layer. Another study examining IMT in HIV patients taking antiretrovirals showed that a PI-containing regimen increases IMT compared with treatment with NNRTIs (Sankatsing et al., 2009). These studies are provocative in that they suggest a vascular proliferative response may be induced by antiretroviral treatment.
Although a role for oxidant production and oxidative stress has been suggested in antiretroviral-associated endothelial dysfunction, two important questions remain: (1) Does oxidant production and oxidative stress have a causal role in antiretroviral-mediated endothelial dysfunction? If so, would cotreatment of animals with antioxidants prevent this antiretroviral-induced endothelial dysfunction? 2) Does the initial endothelial injury culminate in a vascular proliferative response after chronic treatment, thus contributing to the development of atherosclerosis? In the current study, we tested the hypothesis that short-term treatment with antiretrovirals may cause a direct injury to the vascular endothelium via increased oxidant production and that both AZT and indinavir promote vascular neointimal growth following this initial endothelial dysfunction. Together, these two events may contribute to the accelerated atherosclerosis observed for HIV-1–infected patients.
MATERIALS AND METHODS
Antiretroviral treatment of C57BL/6 mice.
Six- to eight-week-old male C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were habituated to the room for 1 week after arrival and were maintained on a normal 12-h light/dark cycle. The mice were fed standard rodent chow and were separated into eight groups of four to five mice each. The first four groups were given distilled H2O (control), AZT (100 mg/kg), indinavir sulfate (100 mg/kg), or AZT + indinavir sulfate (100 +100 mg/kg) by oral gavage for five consecutive days. Another four groups of animals received the same doses of antiretrovirals by oral gavage + 1 mg/kg Mn (III) tetrakis (1-methyl-4-pyridyl) porphyrin (MnTMPyP) (EMD Biosciences, San Diego, CA) by i.p. injection. The above doses of antiretrovirals were selected to ensure that serum concentrations were maintained above the necessary Cmin for therapeutic effects in humans, especially for this short-term treatment protocol (Note et al., 2003; Peng et al., 2009). Because of a more rapid rate of xenobiotic metabolism in mice, doses higher than that utilized for humans are required for achieving equivalent serum levels (Bogaards et al., 2000; Caldwell, 1981).
A separate set of 16 C57BL/6 mice were singly housed and were dosed with distilled H2O (control), AZT (100 mg/kg), indinavir sulfate (100 mg/kg), or AZT + indinavir sulfate (100 + 100 mg/kg) in their drinking water for 1 month. The volume of water consumed was measured on a daily basis, and the drug concentrations in the water were adjusted to maintain adequate daily drug consumption. From the second week after the initiation of treatment, the mice were administered a Western diet containing 0.15% cholesterol and 21% milk fat (TD 88137, Harlan Teklad, Madison, WI) for a total of 3 weeks (Shi et al., 2004). The carotid artery injury procedure was performed at the end of the second week of treatment. The administration of the Western, i.e., high fat, diet for 3 weeks in conjunction with and beginning 1 week prior to the carotid artery injury procedure was established by other laboratories as a method for maximizing neointimal hyperplasia (Shi et al., 2004; Zhu et al., 2000). All animal protocols were performed in accordance with the guidelines of the Office of Laboratory Animal Welfare and were approved by the Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center, Shreveport, LA.
Measurement of aortic vasoreactivity.
Five days after the initiation of antiretroviral treatment, the mice were anesthetized using sodium pentobarbital (50 mg/kg, i.p.) and were sacrificed by pneumothorax. The thoracic aortas were excised, and the connective tissue was carefully removed under a dissecting microscope. The aortas were cut into 3-mm rings and were transferred to a tissue bath containing Krebs-Henseleit (KH) solution, equilibrated with 95% O2 and 5% CO2. The buffer contained 118mM NaCl, 5.8mM KCl, 27.2mM NaHCO3, 1.0mM NaH2PO4, 1.2mM MgSO4, 2.5mM CaCl2, and 11.1mM glucose. The temperature of the buffer was maintained at 37°C and the pH at 7.4. The aortic rings were attached to a Grass FT03C force-displacement transducer via a length of surgical silk, and the transducer was interfaced to a Grass model 7D recorder for the measurement of isometric force. The aortic rings were placed under an initial tension of 1 g and were equilibrated for 1 h. The KH solution was changed every 15 min. The rings were then contracted using increasing concentrations (from 10−9 to 10−6M) of phenylephrine. The contraction dose-response curve was compared between the control and MnTMPyP-treated mice. To determine antiretroviral-induced effects on vessel relaxation, the aortic rings were first contracted using 10−7M phenylephrine (determined to elicit 80% maximal contraction), and the relaxation was recorded after increasing doses of acetylcholine (Ach, 10−9 to 10−4M), for endothelium-dependent relaxation, or sodium nitroprusside (SNP, 10−9 to 10−6M), for endothelium-independent relaxation. Vessel relaxation was expressed as a percentage of the contraction induced by phenylephrine.
Determination of vascular aconitase activity.
Using the method described by Gardner (2002), aconitase activity was assessed in small sections of frozen aorta not utilized for the vessel reactivity measurements. The frozen tissues were homogenized in 50mM Tris-HCl, pH 7.4, containing 2mM sodium citrate and 0.6mM MnCl2, and were centrifuged. Aliquots of the supernatants (10 μl) were loaded into 96-well plates, and 190 μl of freshly prepared aconitase assay reaction mix, containing 0.2mM NADP+, 5mM sodium citrate, and 1 unit/ml pig heart isocitrate dehydrogenase in homogenization buffer, was immediately added. Aconitase activity was assessed by monitoring the reduction of NADP+ to NADPH at 340 nm and at 25°C over 60 min in a microplate reader. Aconitase activity was calculated using the extinction coefficient for NADPH (6.22 × 103/M·cm) and assuming the conversion of one molecule of citrate to one molecule of NADPH via isocitrate dehydrogenase. Using KC Junior software (Bio-Tek Instruments, Inc., Winooski, VT), a path length correction was utilized for standardizing the measurement to that expected for a 1-cm cuvette. One milliunit of aconitase activity was assessed as the conversion of 1 nmol of citrate to isocitrate per minute. Finally, aconitase activity was expressed relative to protein concentration, determined using the BCA assay (Pierce).
Introduction of carotid artery injury.
The procedure for the introduction of endothelial injury in the left carotid artery was performed in mice that had been administered antiretroviral treatment for 2 weeks and the Western diet for 1 week. As discussed above, the protocol for treatment of mice with the high-fat diet for 1 week prior and 2 weeks after carotid artery injury was established by other laboratories as a means for maximizing neointimal hyperplasia (Shi et al., 2004; Zhu et al., 2000). An epon-resin probe, made by forming an epon bead slightly larger than the diameter of the mouse carotid artery (0.45 mm) on a 3-0 nylon suture, was used in experiments to mechanically introduce uniform endothelial injury. The mice were anesthetized by i.p. injection of sodium pentobarbital (50 mg/kg) and were immobilized under a dissection microscope. The entire length of the left common carotid artery was exposed, and both the common and the internal carotid arteries were temporarily blocked by lifting a 3-0 nylon suture placed underneath the arteries. A 6-0 nylon suture was applied to the external carotid artery 1 cm above the bifurcation. A tranverse arteriotomy was made into the external carotid artery at the proximal side of the ligation. The epon-resin probe was inserted into the common carotid artery and was advanced toward the aortic arch and then withdrawn three times. The external carotid artery was then closed by ligation with the 6-0 suture, blood flow to the common carotid artery was reintroduced by removal of the remaining sutures, and the skin incision was closed with 5-0 sterile suture. The animals were allowed to recover in a 37°C-heated box after the surgery. An identical procedure was applied to each animal using the same experimental conditions to assure reproducible results. Studies have shown that the epon-resin probe–induced carotid artery endothelial injury model is suitable for studying neointimal hyperplasia (Zhu et al., 2000). This probe can induce consistent denudation of the endothelium, without damaging the underlying elastic laminae and with only minimal trauma to vascular smooth muscle (Zhu et al., 2000).
Tissue preparation.
To assess neointimal formation, 2 weeks after carotid artery injury, the mice were sacrificed and the chest cavity was opened. A 22-gauge butterfly angiocatheter was inserted into the left ventricle. The mice were perfused first with cold PBS under constant pressure for 20 min and then with 4% paraformaldehyde. The neck section was dissected and was fixed in 4% paraformaldehyde for >24 h. The tissues were then transferred to a formic acid EDTA buffer (Formical-2000; Decal Chemical Corp., Tallman, NY) for further decalcification. The soft tissue of the neck was dissected away, and the remaining decalcified neck tissue was embedded in paraffin using standard histological techniques. The tissues were cross-sectioned at 5 μm thickness, and for each animal, up to six parallel sections separated by ∼100μM were sampled across the lesioned area. Each were stained with Verhoeff's van Gieson staining of the elastic laminae to visualize neointimal areas.
Morphometry.
Morphometric analysis of neointimal areas was performed on the Verhoeff's van Gieson–stained cross-sections, with up to six sections analyzed for each animal. Injured carotid arteries from both the antiretroviral-treated mice were compared with injured carotids of the controls. Digitalized images from parallel sections from each animal were captured using a Nikon Labophot microscope interfaced to a Canon high-resolution digital camera. The images were analyzed using morphometric analysis software (MetaMorph 6.3, Downingtown, PA). For each section, luminal area, the area between the internal elastic lamina (IEL) and the external elastic lamina (EEL), and the area inside the IEL were measured. Vascular medial areas were calculated by subtracting the areas inside the IEL from the areas inside the EEL, and the neointimal areas were calculated by subtracting the luminal areas from the areas inside the IEL.
Measurement of plasma total cholesterol and triglyceride levels.
Plasma was collected at the termination of the experiment. Plasma total cholesterol and triglyceride levels were determined colorimetrically using total cholesterol and triglyceride reagent kits purchased from Eagle Diagnostics (De Soto, TX).
5-Bromo-2’-deoxyuridine fluorescence staining.
Two hours before sacrifice, a single dose of 25 mg/kg 5-bromo-2’-deoxyuridine (BrdU) was injected i.p. A BrdU immunofluorescence labeling and detection kit purchased from Roche Applied Science (Penzburg, Germany) was utilized, and the tissue cross-sections were stained according to kit instructions. Specifically, paraffin-embedded sections were thoroughly dewaxed and were rehydrated by the standard xylene, differential concentrations of ethanol, and water procedure. After washing three times with washing buffer, the sections were incubated with an anti-BrdU primary antibody (1:10) for 30 min at 37°C. The slides were again washed three times with washing buffer, and the sections were incubated with anti-mouse IgG-fluorescein working solution for another 30 min at 37°C. The slides were washed once more, and fluorescence mounting medium and cover slips were applied. The fluorescence was detected at a wavelength of 488 nm, and the images were acquired using a Zeiss LSM 510 confocal microscope at a magnification of ×40.
Immunostaining for VCAM-1 and α-actin.
Representative paraffin-embedded cross sections were immunostained for vascular cell adhesion molecule-1 (VCAM-1) and α-actin using goat polyclonal antibody (1:200) obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and mouse monoclonal antibody (1:200) purchased from Sigma, respectively. This was followed by biotinylated horse anti-goat IgG-streptavidin-HRP (1:2000; Santa Cruz), for VCAM-1 staining, and horse anti-mouse IgG-streptavidin-HRP (1:2000; Santa Cruz), for α-actin. Bound antibodies were visualized by the chromogenic substrate 3,3-diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin. Images were taken using a Motic B1 Series light microscope interfaced to a high-resolution digital camera. Endothelial staining was analyzed by Image J software (National Institutes of Health) with the Landini color deconvolution plug-in for hemotoxylin and eosin-DAB described by Ruifrok and Johnston (2001).
Measurement of plasma levels of asymmetric dimethylarginine.
Plasma levels of asymmetric dimethylarginine (ADMA) were determined using a reversed phase liquid chromatography with AccQ-Fluor fluorescence detection, as described before (Heresztyn et al., 2004). L-homoarginine was spiked with 20-μl mouse plasma samples as an internal standard. The samples were mixed with 25-μl ice-cold methanol, were precipitated in ice for 10 min, and were centrifuged at 10,000 rpm at 4°C for 5 min (Zhang and Kaye, 2004). The extraction was repeated after the supernatant was collected, and two supernatants were pooled and dried under a stream of nitrogen. The samples were then reconstituted in 90-μl AccQ-Fluor reagent buffer and 10-μl fluorescent derivatizing reagent (AccQ-Fluor) before filtering through sterile 0.22μM microfiltration tubes (Ultrafree MC, Millipore). Separation was achieved using the mobile phases 100mM sodium acetate, pH'ed to 5 with citric acid, for an approximate concentration of 15mM (A) and acetonitrile:methanol:water at a ratio of 2:1:1 (B). An Agilent Zorbax C18-SB reverse-phase column (3 × 150 mm, 3.5 μm particle size) was maintained at 34°C with a flow rate of 0.5 ml/min. The gradient elution was as follows: 0–5 min isocratic at 10%B, 5–25 min to 20%B on a gradient curve of +3, 25–35 min linear to 40%B, 35–49 min linear to 100%B, followed by a 7-min 100%B organic flush, and a 10-min 10%B column re-equilibration. Fluorescence was monitored at an excitation wavelength of 250 nm and an emission wavelength of 395 nm. Mixed standards containing arginine metabolites ADMA, symmetric dimethylarginine, and NG-monomethyl-L-arginine, along with internal standard L-homoarginine injected intermixed with the samples, were used for quantitation. The linear range studied was 0.05 to 20μM. Analyte peaks were identified by retention time and by coinjection with standards.
Statistical analysis.
Data were expressed as mean ± SE. Statistical analysis was performed using one-way ANOVA with Tukey’s or least significant difference Fisher’s post hoc tests. Vascular reactivity data were analyzed using two-way ANOVA with repeated measures. For measurements of aconitase activities, stratified across both antiretroviral and antioxidant treatments, ANOVA was followed by Dunnett's multiple comparison test. A value of p < 0.05 was considered statistically significant.
RESULTS
MnTMPyP Attenuated AZT-Induced and AZT + Indinavir–Induced Endothelial Dysfunction
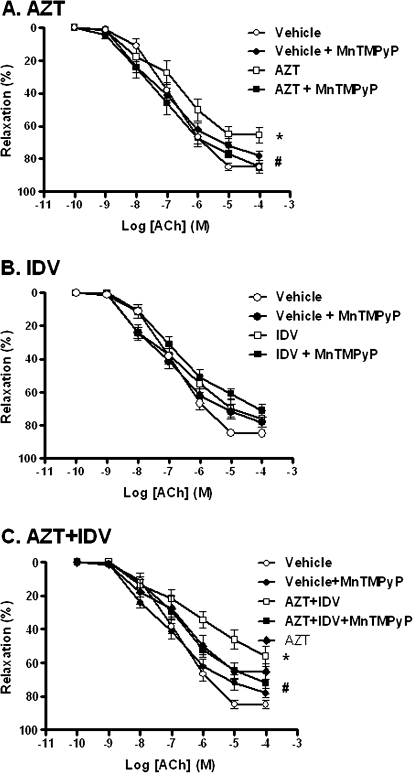
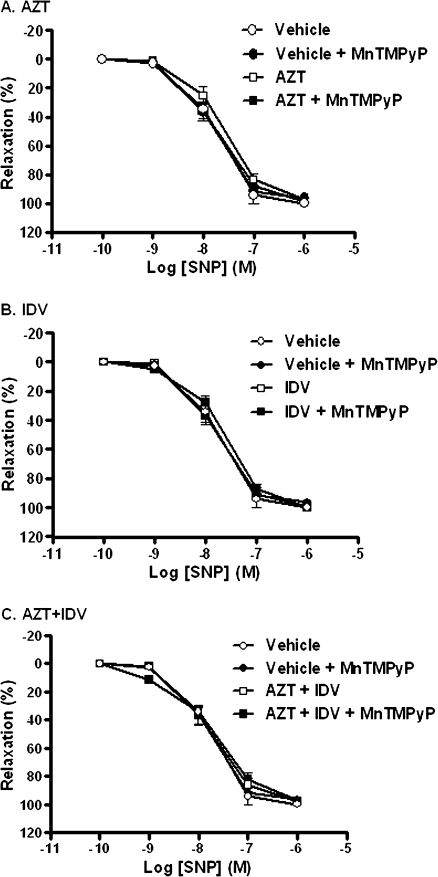
Preliminary studies indicated that antiretroviral treatment did not alter phenylephrine-induced contraction (data not shown). However, treatment of C57BL/6 mice with AZT (100 mg/kg) or AZT + indinavir (100 mg/kg) for 5 days significantly attenuated Ach-mediated vasorelaxation (Fig. 1), without affecting that induced by the NO donor SNP (Fig. 2). Two-way ANOVA with repeated measures applied to the resulting curves for Ach-induced relaxation revealed significant differences for these two treatment groups compared with controls. In addition, maximal relaxation was reduced from 85.0 ± 2.3% for controls to 65.5 ± 4.7% for AZT and 56.3 ± 6.1% for AZT + indinavir. This indicates that treatment with AZT or AZT + indinavir specifically impaired endothelium-dependent, but not endothelium-independent relaxation. Indinavir treatment (100 mg/kg) alone for 5 days did not alter endothelium-dependent vasorelaxation. However, coadministration with indinavir potentiated AZT-induced endothelial dysfunction compared with AZT treatment alone, as determined by two-way ANOVA with repeated measures. In this case, maximal relaxation was reduced from 65.5 ± 4.7% for AZT to 56.3 ± 6.1% for AZT + indinavir. Treatment with MnTMPyP alone affected neither mouse aortic contraction in response to phenylephrine nor endothelium-dependent (Fig. 1) or endothelium-independent vasorelaxation (Fig. 2). Most importantly, coadministration of MnTMPyP with AZT or AZT + indinavir attenuated the observed antiretroviral-induced impairment in endothelium-dependent vasorelaxation (Figs. 1A and 1C).
FIG. 1.
Effects of antiretroviral treatment on endothelium-dependent vasorelaxation. Aortic ring relaxation was determined as the percent relaxation to increasing concentrations of Ach, after precontraction with 10−7M phenylephrine. (A) As revealed by two-way ANOVA with repeated measures, AZT treatment (□) for 5 days significantly impaired endothelium-dependent vessel relaxation compared with the controls (○; p < 0.05). Coadministration of 1 mg/kg MnTMPyP (▪) attenuated the AZT-induced endothelial dysfunction (p < 0.05). (B) Indinavir (IDV) treatment (□) for 5 days did not alter endothelium-dependent vessel relaxation compared with controls (○). (C) AZT + IDV (□) significantly impaired endothelium-dependent relaxation (p < 0.05) compared with controls (○), and the MnTMPyP coadministration (▪) attenuated this effect (p < 0.05). In addition, endothelium-dependent vasorelaxation measured after treatment with AZT + IDV was significantly decreased compared with treatment with AZT treatment alone (p < 0.001).
FIG. 2.
Effects of antiretroviral treatment on endothelium-independent vasorelaxation. Aortic rings from animals treated for 5 days with antiretrovirals were precontracted with 10−7M phenylephrine, and the percent relaxation was determined after addition of increasing concentrations of SNP. Two-way ANOVA revealed no effect of treatment with (A) AZT, (B) indinavir (IDV), or (C) AZT + IDV.
Antiretroviral Treatment Leads to an Inactivation of Aconitase
Aconitase is an enzyme known highly susceptible to inactivation by ROS and is thus commonly used as a marker for ROS production (Gardner, 2002). Aconitase is localized both in the cytosol and in the mitochondrion. However, according to literature reports, mitochondrial aconitase represents 67–72% of total activity in endothelial cells (Kotamraju et al., 2003; Quijano et al., 2007) and 82% of total activity in the heart (Gottipolu et al., 2009). To validate these literature reports, we first created a homogenate of a mouse aorta, separated cytosol from mitochondria using a cell fractionation kit from Mitosciences (Eugene, OR), and then measured aconitase activity. We found that the mitochondrial fraction represented 73% of total activity, with 23.6 U/mg detected in the cytosol and 67.3 U/mg in the mitochondrial fraction (data not shown).
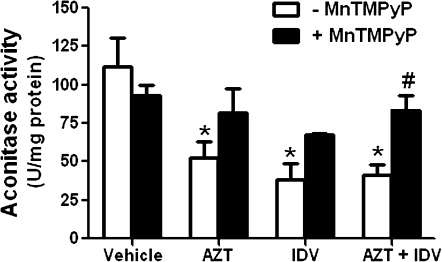
We then measured aconitase activity in segments of aorta from the animals treated for 5 days with antiretrovirals ± MnTMPyP. In animals treated with AZT, indinavir, or AZT + indinavir, aortic aconitase activity was significantly reduced by 50–60% (Fig. 3). MnTMPyP treatment alone did not alter aconitase activity, but it protected against antiretroviral-mediated decreases in levels of aconitase activity. Specifically, in animals treated with MnTMPyP + either AZT, indinavir, or AZT + indinavir, aconitase activity levels were not significantly different from controls (Fig. 3).
FIG. 3.
Effects of antiretroviral treatment on vascular aconitase activity. The enzymatic activity of aconitase was determined in segments of aortic tissue from animals treated for 5 days with antiretrovirals. Results are means ± SEM. ANOVA with Dunnett's multiple comparison test revealed that compared with controls, antiretroviral treatment alone significantly decreased aconitase activity. Treatment with MnTMPyP alone or MnTMPyP + antiretrovirals did not alter aconitase activities compared with controls. Asterisk denotes significance compared with controls.
Antiretrovirals Exacerbate Neointimal Hyperplasia
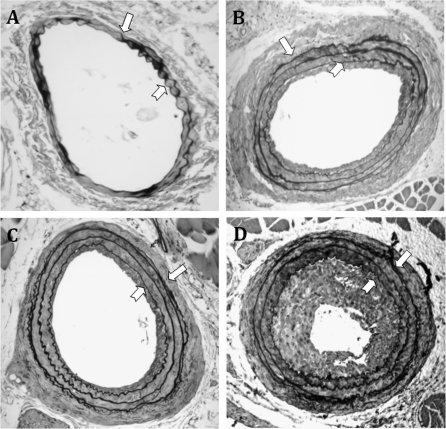
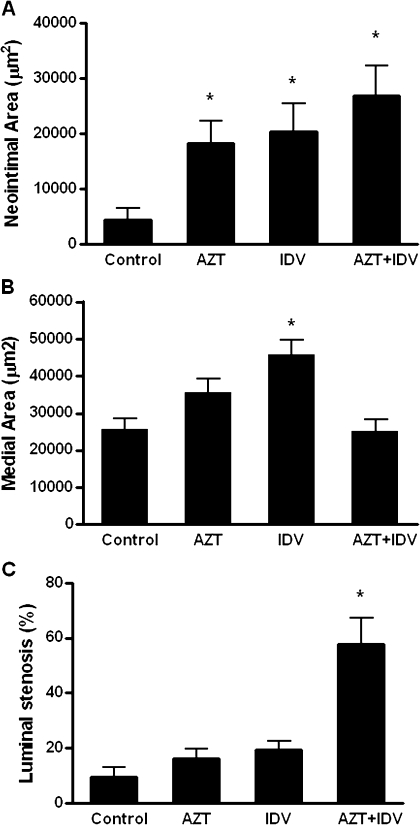
Treatment with AZT, indinavir, or AZT + indinavir for 4 weeks did not alter plasma levels of total cholesterol (∼140 to 170 mg/dl) or triglycerides (50–100 mg/dl). Probe-induced carotid artery injury results in a prominent neointimal formation in the common carotid arteries after 14 days. By examining Verhoeff's van Gieson–stained cross-sections, neointimal areas were calculated by subtraction of lumen area from that encircled by the IEL. In the control mice, neointimal layers were barely detectable (4355 μm2) (Figs. 4 and 5A). Antiretroviral treatment markedly increased neointimal areas compared with controls. Specifically, the average neointimal area in AZT-treated animals was 18,326 μm2 and that of indinavir-treated and AZT + indinavir–treated animals were 20,360 μm2 and 26,839 μm2, respectively (Fig. 5A). Examination of the contralateral uninjured carotid arteries in the same animals did not reveal any neointimal formation (data not shown).
FIG. 4.
Representative Verhoeff's van Gieson staining of the injured carotid arteries from C57BL/6 mice treated with (A) control, (B) AZT, (C) indinavir (IDV), or (D) AZT + IDV. Digitalized images were acquired, and the neointimal and medial areas were analyzed as described in Materials and Methods. The block arrows indicate the EEL, and the notched arrows indicate IEL. The images were taken at a magnification of ×100.
FIG. 5.
Morphometric quantitation of the carotid arteries of antiretroviral-treated mice, measured 14 days after the endothelial injury procedure and treatment ± AZT, indinavir (IDV), or AZT + IDV. Paraffin-embedded tissues were cut into 5-μm thick cross-sections and were stained with Verhoeff's van Gieson. Digitized images were acquired and (A) neointimal areas or (B) medial areas were calculated by subtraction of lumen area or IEL area from EEL area. (C) Luminal stenosis was calculated as the percentage of the area inside of the IEL that is occupied by the intima. The data represent means ± SE. ANOVA revealed a significant effect of treatment. Asterisk indicates statistical significance compared with controls, at p < 0.05.
In addition to neointimal hyperplasia, compared with the control mice, treatment with indinavir produced a 78% increase in vascular medial area in the injured carotid arteries (Fig. 5B). However, treatment with AZT alone increased vascular medial area by ∼38%, though this increase did not achieve statistical significance. Interestingly, coadministration of AZT + indinavir did not produce an increase in medial area compared with controls.
An index for luminal stenosis was calculated from the measured areas of the neointima and intima + lumen area (intimal area/area inside the IEL × 100). Compared with controls, treatment with AZT + indinavir markedly increased luminal stenosis in the injured carotid arteries of the C57BL/6 mice (Fig. 5C). However, treatments with AZT or indinavir alone did not significantly increase luminal stenosis compared with the controls.
Antiretrovirals Increase Smooth Muscle Cells in the Neointima
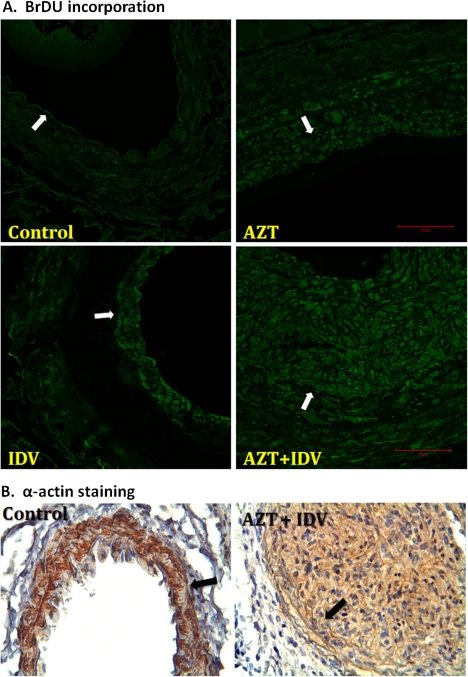
To determine whether the observed neointimal hyperplasia was because of increased cell proliferation, the animals were administered 25 mg/kg BrdU, i.p., 2 h before sacrifice, and the tissue cross-sections were stained with anti-BrdU antibody. As shown in Figure 6A, BrdU fluorescence staining of the neointimal layer of the injured carotid arteries was more pronounced than other layers within the artery wall, indicating a massive cell proliferation. To determine whether this proliferating layer of cells was vascular smooth muscle, in a separate subset of animals, mice were treated with and without AZT + indinavir, and the carotid arteries were stained for smooth muscle α-actin. In these sections, brown staining was evident across both the media and neointimal areas, confirming that the proliferated cells within these areas were mainly smooth muscle cells (Fig. 6B).
FIG. 6.
BrdU fluorescence (A) compared with smooth muscle α-actin staining (B) of proliferating cells in injured carotid arteries from C57BL/6 mice treated with antiretrovirals. Paraffin-embedded tissues were obtained 14 days after the endothelial injury procedure. For BrDU incorporation, injured artery cross-sections were incubated with an anti-BrdU primary antibody and then a secondary antibody conjugated to a fluorophore. The images were visualized at 488 nm. α-Actin was visualized using immunohistochemistry with hemotoxylin and eosin counterstaining. Brown staining thus indicates smooth muscle. The arrows denote IEL. The scale bar indicates 50 μm.
Effects of Antiretrovirals on Plasma Lipids in C57BL/6 Mice Fed a Western Diet
In an experiment to determine antiretroviral-induced effects on neointimal hyperplasia, C57BL/6 mice were administered AZT, indinavir, or AZT + indinavir in their drinking water for 28 days, along with a Western diet for the latter 3 weeks. Plasma levels of cholesterol and triglycerides were determined after tissue harvest. For these experiments, neither treatment with AZT or indinavir alone nor AZT + indinavir in combination for 4 weeks significantly altered plasma levels of total cholesterol and triglycerides compared with controls (data not shown).
Effects of Antiretrovirals on Plasma ADMA Level
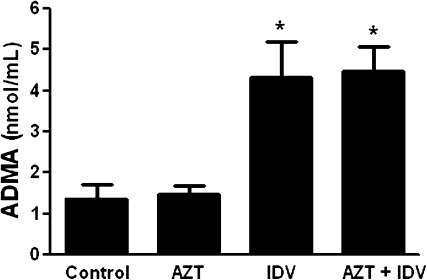
ADMA, the methyl derivate of the amino acid arginine, is an endogenous competitive inhibitor of nitric oxide synthase. Elevated plasma levels of ADMA have been shown correlated with endothelial dysfunction and major cardiovascular events (Boger, 2005). Recent studies suggest that plasma ADMA levels predict the progression of vascular lesion formation (Wu et al., 2009), and that its synthesis and release from endothelial cells are increased when the cells become dysfunctional (Boger et al., 2000). We thus measured plasma levels of ADMA in the same mice that underwent the carotid artery injury procedure. As shown in Figure 7, treatment with indinavir or AZT + indinavir increased plasma levels of ADMA two- to threefold (p < 0.05). However, AZT treatment alone had no effect on plasma ADMA levels.
FIG. 7.
Effects of antiretroviral treatment on plasma ADMA and orthinine/arginine ratio. Plasma ADMA, orthinine, and argnine levels were determined 2 weeks after the endothelial injury. The data represent means ± SE. Asterisk indicates a significant difference compared with controls group, as revealed by ANOVA.
Antiretrovirals Increase VCAM-1 Expression
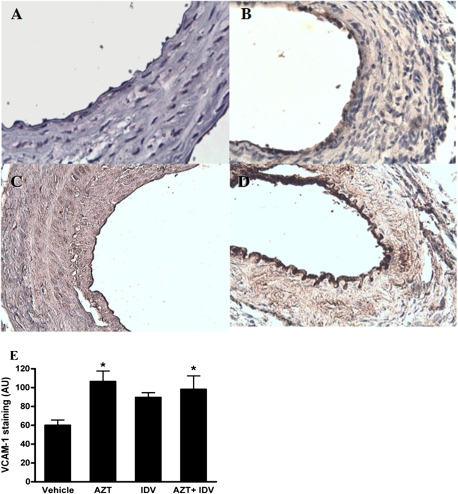
Prior reports have demonstrated that the expression of VCAM-1 staining is associated with endothelial dysfunction in the regenerated endothelium after balloon injury (Krejcy et al., 1996). We thus assessed VCAM-1 expression in the carotid arteries of animals that underwent the injury procedure to further delineate whether the observed increase in proliferative response induced by the antiretroviral treatment was associated with a drug-induced endothelial dysfunction. To our surprise, significant VCAM-1 immunostaining was not apparent in the injured carotid arteries of control animals at 14 days after the procedure (Fig. 8A). However, treatment with AZT alone increased VCAM-1 staining in the endothelium of the injured arteries (Figs. 8B and 8E), whereas treatment with indinavir alone did not (Figs. 8C and 8E). The most pronounced staining was observed for the AZT + indinavir–treated group (Figs. 8D and 8E). In these tissues, VCAM-1 staining was apparent across the vessel, though staining was again most pronounced in the endothelial layer.
FIG. 8.
Representative immnuhistochemical staining for VCAM-1. Paraffin-embedded cross-sections from the animals that underwent the injury procedure were stained with a polyclonal antibody against mouse VCAM-1. Images represent immunostaining observed for the (A) control, (B) AZT-, (C) indinavir (IDV)-, (D) AZT + IDV–treated groups. Images were taken at a magnification of ×20. (E) Endothelial staining intensities for VCAM. Data are means ± SE. One-way ANOVA revealed a significant effect of treatment. Asterisk indicates p < 0.05.
DISCUSSION
Antiretroviral-associated endothelial dysfunction has been widely described in both clinical observations and experimental studies (Dube et al., 2008; Shankar et al., 2005; Stein et al., 2001) and may be associated with the increased incidence of cardiovascular diseases in HIV-infected patients. Data presented here demonstrate that in mice, the NRTI AZT induces endothelial injury only after 5 days treatment. This finding supports our prior studies in rats (Jiang et al., 2006) and a recent retrospective/prospective observational study (Vaughn and Detels, 2007). AZT was studied as a representative NRTI; however, it should be noted that an induction of endothelial injury may not be limited to AZT, as our recent unpublished studies suggest that other NRTI, including lamivudine, stavudine, and didanisone, may also have a deleterious effect on the endothelium.
In our prior studies in rats, we observed no effect of indinavir on endothelium-dependent vasorelaxation (Jiang et al., 2006). However, in these experiments, we compared AZT-induced endothelial toxicity in mice side-by-side to that exhibited by the early generation PI indinavir and found that although indinavir treatment alone had no effect on vasomotor function, it exacerbated AZT-mediated reductions in endothelium-dependent vasorelaxation (p < 0.001). In addition, it increased plasma ADMA levels. Recent studies suggest that plasma ADMA levels predict the progression of vascular lesion formation (Wu et al., 2009). ADMA is formed in endothelial cells first by methylation of arginine residues within proteins through the enzymatic action of S-adenosylmethionine–dependent type I protein arginine N-methyltransferases, then by release of the methylated residues through proteolysis (Surdacki, 2008). What's more, ADMA release from endothelial cells is increased when the cells become dysfunctional. In particular, exposure of endothelial cells to native or oxidized low-density lipoprotein cholesterol promotes PMRT expression, as well as ADMA synthesis and release (Boger et al., 2000). Plasma ADMA levels have also been shown correlated with endothelial dysfunction and with major cardiovascular events (Boger, 2005; Cooke, 2000). Thus, our observations of indinavir-induced increases in plasma ADMA levels, taken together with its potentiation of AZT-induced impairment in vasorelaxation, suggest that indinavir elicits an endothelial dysfunction not readily identified by only one index for endothelial injury. Furthermore, in association with these short-term effects of drug-induced endothelial dysfunction, treatments with either AZT, indinavir, or AZT + indinavir exacerbated neointimal hyperplasia.
Because HIV treatment generally involves long-term administration of antiretrovirals, our data suggest that chronic endothelial injury from exposure to either AZT, indinavir, or AZT + indinavir may place HIV-infected patients at a greater and earlier risk for developing advanced atherosclerotic lesions. In addition, because we observed an exacerbation of vascular lesion development in an injury model, this could imply that chronic exposure to antiretrovirals may exacerbate lesion progression in patients with preexisting vascular disease. Furthermore, because we observed no alterations in plasma cholesterol and triglyceride levels after antiretroviral treatment, it is thus impractical to ascribe the antiretroviral-mediated vasomotor functional impairment and endothelial dysfunction/activation to PI–associated hyperlipidemia. Therefore, these data and our prior work support that antiretrovirals induce a direct endothelial injury (Jiang et al., 2006, 2007). As for the mechanisms of action of the two drugs tested here, although both antiretrovirals induce what we would describe as an endothelial dysfunction, given the observed differences in the presentation of endothelial injury, the mechanism of action and etiology of this dysfunction are likely different. Further support for this interpretation was that indinavir cotreatment potentiated AZT-induced effects on vasomotor function. From a toxicological point of view, this is further evidence of diverging mechanisms of action; otherwise, the cotreatment would have resulted in similar or additive responses.
It is interesting that both AZT and indinavir were shown to elicit cellular oxidant production and oxidative stress in cultured human endothelial cells (Jiang et al., 2007), and in these studies, both antiretrovirals promoted vascular ROS production (Fig. 3). However, AZT, but not indinavir, directly impaired endothelium-dependent vasorelaxation in rats (Jiang et al., 2006) and here, indinavir only exhibited effects on some of the indices of endothelial dysfunction we tested. This discrepancy could be because of (1) in vivo drug metabolism. Indinavir is rapidly absorbed and metabolized in vivo. Six oxidative metabolites and one glucuronide conjugate are formed and excreted in urine and feces (Lin et al., 1996). Therefore, a lack of a pronounced in vivo effect could be because of hepatic detoxification of indinavir. (2) Species differences. Shankar et al. (2005) and Dube et al. (2008) described indinavir-mediated impairment in endothelium-dependent vasorelaxation in humans. However, the work of Chai et al. (2005a) suggested no endothelial vasomotor impairment in porcine coronary arteries. Therefore, humans may be more sensitive to the effects of indinavir. (3) Indinavir-associated endothelial injury may not directly interfere with Ach-mediated vasorelaxation.
Endothelial dysfunction is a broad term that includes several phenotypic changes that can result from injury or injurious stimuli. These changes can include enhanced permeability to plasma lipoproteins, increased secretion of adhesion molecules that are hyperadhesive for blood leukocytes, functional imbalances in local pro- and antithrombotic factors, and the release of growth stimulators, inhibitors, and vasoactive substances, i.e., those that induce relaxation or constriction. Together, all of these alterations in endothelial function can critically contribute to the pathogenesis of vascular diseases. We observed in our in vitro studies that both AZT and indinavir elicited oxidant production and oxidative stress at a cellular level; however, the localization of these oxidants may not be identical, such that divergent signal transduction pathways are promoted, ultimately manifesting as differing phenotypic changes. As is highlighted in this report, AZT-induced oxidants impaired endothelial relaxation (Fig. 1), whereas indinavir-associated oxidants result in ADMA release (Fig. 7) and perhaps other endothelium-dependent events that we have yet not investigated. Although lacking of vasomotor impairment, these parameters certainly also indicate endothelial injury. The complexity of the results observed here perhaps begins to explain the conflicting reports of indinavir-induced effects on the vascular endothelium.
MnTMPyP is a stable manganese-porphyrin complex that has been shown to act as a superoxide dismutase mimic, converting superoxide radicals to hydrogen peroxide (H2O2) (Faulkner et al., 1994). Studies have also suggested that MnTMPyP possesses catalase activity, converting H2O2 to H2O, and can protect endothelial cells against H2O2-mediated injury (Day et al., 1997). Here we showed that administration of 1 mg/kg MnTMPyP, i.p., alone for 5 consecutive days did not affect vascular reactivity, including vessel contraction induced by phenylephrine or vasorelaxation in response to either Ach (Fig. 1) or SNP (Fig. 2). It also did not alter basal levels of ROS production, as indirectly indicated by lack of change in levels of vascular aconitase activity (Fig. 3). However, coadministration of mice with MnTMPyP significantly attenuated the AZT-mediated and AZT + indinavir–mediated impairment in endothelium-dependent relaxation (Fig. 1), and at the same time inhibited vascular ROS production (Fig. 3). These animal studies thus provided direct evidence that oxidant injury may have a causal role in AZT-induced and AZT + indinavir–induced endothelial dysfunction.
Our findings with respect to antiretroviral-mediated decreases in aconitase activity may furthemore provide clues as to the source of ROS production. Although aconitase is expressed both in the cytosol and in mitochondria, in these experiments, vascular aconitase activity was reduced by 53–66% across each of the antiretroviral treatment groups (Fig. 3). Although mitochondrial aconitase reportedly represents 67–82% of total activity (Gottipolu et al., 2009; Kotamraju et al., 2003; Quijano et al., 2007), in these studies, we found that the mitochondrial fraction represented 73% of total activity (not shown). Considering the profound decrease in aconitase activity, we measured after antiretroviral treatment (53–66%), and that the mitochondrial fraction represents the vast majority of the total activity, it seems logical to conclude that a significant portion of the mitochondrial activity is lost. This may suggest that the mitochondrion is at least one source of ROS production, in agreement with our prior in vitro findings (Jiang et al., 2007). However, other sources of ROS production are also possible. For example, in prior reports, AZT was shown to activate cardiac NADPH oxidase in rats treated for 8 months (Papparella et al., 2007), and the PIs ritonavir, indinavir, and atazanavir were shown to activate endoplasmic reticulum (ER) stress in macrophages (Zhou et al., 2005). The latter is of particular interest, because ER stress is known highly correlated with an induction of ROS production, either upstream or downstream of the unfolded protein response, and at least one study suggests the ROS evolving from ER stress are derived from pertubations of mitochondrial function (Santos et al., 2009). Thus, a number of cellular sources for antiretroviral-mediated ROS production are possible.
Neointimal hyperplasia represents another important component of atherogenesis. The cellular basis of this pathological event is the migration and proliferation of smooth muscle cells. In a healthy vessel wall, VSMC exhibit a contractile, nonproliferative phenotype. The endothelium has an inhibitory effect on VSMC proliferation, maintaining vascular homeostasis. Upon endothelial disruption and injury to the smooth muscle layer, VSMC are exposed directly to the circulation. As a result, they begin to migrate across the IEL and proliferate, thus initiating neointimal hyperplasia. Regrowth of the endothelium over the area of injury, a process known as re-endothelialization, is important in limiting this VSMC migration and proliferation, and a functional endothelium is vital to successful endothelial re-growth.
The present studies demonstrated that both AZT and indinavir markedly promote neointima formation in C57BL/6 mice. Coadministration with AZT and indinavir additively increased neointimal hyperplasia. This is even more prominent for the luminal restenosis calculation, which showed a dramatic increase (almost ∼70% occlusion) in the combination treatment group. Luminal stenosis did not reach statistical significance after treatments with AZT or indinavir alone, but this may simply be because of a compensatory expansion of the injured arteries. In addition, BrdU-labeled and α-actin–stained cells in the intimal layer indicate a massive proliferation of smooth muscle cells following injury, but not deposition of lipids or fibrotic extracellular matrix. These proliferative effects were coupled to increases in plasma levels of ADMA and endothelial staining for VCAM-1, both markers for endothelial dysfunction, suggesting that endothelial dysfunction may be a mechanism involved in the observed drug-induced neointimal expansion.
Our observations of antiretroviral-mediated neointimal hyperplasia are consistent with our prior in vitro findings. In these earlier studies, although direct treatment of VSMC with antiretrovirals did not alter VSMC proliferation, both AZT and indinavir promoted VSMC proliferation when cocultured with endothelial cells (Hebert et al., 2004). Mechanisms for AZT or indinavir-mediated neointimal hyperplasia may include an upregulation of mitogenic growth factors from injured endothelial cells after antiretroviral treatment. For example, in our in vitro studies, an endothelium-derived growth factor, endothelin-1, was shown released by drug-treated endothelial cells, so as to mediate antiretroviral-induced VSMC proliferation (Hebert et al., 2004). Thus, taken together with the current findings, we propose that the initial insult to the vascular endothelium has a causative role in promoting VSMC proliferation that, in turn, leads to the development of neointimal hyperplasia. Another explanation for antiretroviral-induced neointimal hyperplasia may be that antiretrovirals promote platelet adhesion and degranulation after endothelial injury, thus resulting in the activation of platelets and release of PDGF that in turn promotes neointimal hyperplasia. In support of this hypothesis, one report demonstrated that indinavir enhances platelet activation (Holme et al., 1998).
One limitation of this work is our inability to conclusively link antiretroviral-induced ROS production in endothelial cells with antiretroviral-mediated exacerbation of neointimal hyperplasia. To reiterate, our central hypothesis was that antiretroviral-mediated exacerbation of neointimal thickening is because of an antiretroviral-induced endothelial dysfunction and ROS production. In the absence of an endothelial-specific overexpression of an antioxidant enzyme, it would be difficult to design an experiment to conclusively test this hypothesis. This is because ROS production in VSMC themselves has a direct role in modulating their proliferation and thus, neointimal hyperplasia. For example, Szocs et al. (2002) showed that VSMC proliferation comprising the proliferative response involved in neointimal hyperplasia was associated with the activation of NADPH oxidase in both VSMC and in fibroblasts. Thus, cotreatment with antiretrovirals + an antioxidant would likely produce a profound decrease in neointimal hyperplasia, as has been shown previously (Jagadeesha et al., 2009), but it would be difficult to distinguish between direct effects of the antioxidant on the redox state of VSMC and the effects of the antioxidant on antiretroviral-mediated ROS production in endothelial cells.
Another limitation of this study is that here we explored the role of only the antiretrovirals in initiating/exacerbating atherosclerosis. However, the HIV virus itself may also promote a chronic inflammatory condition, and chronic inflammation is certainly a recognized risk factor for atherosclerosis. In addition, it is possible that proteins released by the virus may promote endothelial dysfunction. For example, treatment of endothelial cells with HIV Tat protein induces the release of IL-1β, MCP-1, VCAM-1, and E-selectin. Tat thus increases leukocyte adhesion to endothelial cells (Matzen and Dirkx, 2004). HIV itself can enter the endothelial cell either through CD4 or galactosyl-ceramide receptors (Chi et al., 2000) or through certain chemokine receptors (Berger et al., 1999), and thus, it is entirely possible that infected endothelial cells may become activated. HIV-induced endothelial activation may also be the result of increased circulating cytokines because of the infection of monocytes-macrophages (Chi et al., 2000). Thus, through many possible pathways, the vascular endothelium may become activated by HIV. If this is the case, then one would expect that the effect of the virus in initiating activation and the effects of antiretrovirals in promoting endothelial dysfunction would be additive, leading to a profoundly dysfunctional endothelium and a vastly accelerated rate of atherogenesis. Studies addressed here answer one controllable part of this complex problem—the drug therapy. Presumably, effective viral titer reduction through antiretroviral therapy should reduce the inflammatory component of HIV, but direct effects of the antiretrovirals on vascular endothelial dysfunction/activation would remain. Thus, there is a clinical need for therapies that both reduce viral titers and exhibit diminished toxicity to the vascular endothelium.
In conclusion, data generated from these studies demonstrated that the antiretroviral AZT induced an impairment of endothelial relaxation in mice, and an oxidant-mediated pathway may play a causal role in this injury. Indinavir treatment alone did not alter vasomotor function, but potentiated AZT-induced endothelial injury. In addition, indinavir increased plasma ADMA levels, further suggestive of endothelial dysfunction. Following this endothelial injury, AZT, indinavir, and AZT + indinavir treatments stimulated smooth muscle cell migration and proliferation, accounting for the observed neointimal hyperplasia. Together, drug-induced effects on these two events may contribute to antiretroviral-associated atherosclerosis and perhaps other cardiovascular diseases in HIV-1–infected patients.
FUNDING
National Heart, Lung, and Blood Institute (HL082472 to T.R.D.).
References
- Barbaro G, Di Lorenzo G, Cirelli A, Grisorio B, Lucchini A, Hazra C, Barbarini G. An open-label, prospective, observational study of the incidence of coronary artery disease in patients with HIV infection receiving highly active antiretroviral therapy. Clin. Ther. 2003;25:2405–2418. doi: 10.1016/s0149-2918(03)80283-7. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Bogaards JJ, Bertrand M, Jackson P, Oudshoorn MJ, Weaver RJ, van Bladeren PJ, Walther B. Determining the best animal model for human cytochrome P450 activities: a comparison of mouse, rat, rabbit, dog, micropig, monkey and man. Xenobiotica. 2000;30:1131–1152. doi: 10.1080/00498250010021684. [DOI] [PubMed] [Google Scholar]
- Boger RH. Asymmetric dimethylarginine (ADMA) and cardiovascular disease: insights from prospective clinical trials. Vasc. Med. 2005;10(Suppl. 1):S19–S25. doi: 10.1177/1358836X0501000104. [DOI] [PubMed] [Google Scholar]
- Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ. Res. 2000;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- Caldwell J. The current status of attempts to predict species differences in drug metabolism. Drug Metab. Rev. 1981;12:221–237. doi: 10.3109/03602538108994030. [DOI] [PubMed] [Google Scholar]
- Chai H, Yang H, Yan S, Li M, Lin PH, Lumsden AB, Yao Q, Chen C. Effects of 5 HIV protease inhibitors on vasomotor function and superoxide anion production in porcine coronary arteries. J. Acquir. Immune Defic. Syndr. 2005a;40:12–19. doi: 10.1097/01.qai.0000172368.05327.7b. [DOI] [PubMed] [Google Scholar]
- Chai H, Zhou W, Lin P, Lumsden A, Yao Q, Chen C. Ginsenosides block HIV protease inhibitor ritonavir-induced vascular dysfunction of porcine coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 2005b;288:H2965–H2971. doi: 10.1152/ajpheart.01271.2004. [DOI] [PubMed] [Google Scholar]
- Charakida M, Donald AE, Green H, Storry C, Clapson M, Caslake M, Dunn DT, Halcox JP, Gibb DM, Klein NJ, et al. Early structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapy. Circulation. 2005;112:103–109. doi: 10.1161/CIRCULATIONAHA.104.517144. [DOI] [PubMed] [Google Scholar]
- Chi D, Henry J, Kelley J, Thorpe R, Smith JK, Krishnaswamy G. The effects of HIV infection on endothelial function. Endothelium. 2000;7:223–242. doi: 10.3109/10623320009072210. [DOI] [PubMed] [Google Scholar]
- Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler. Thromb. Vasc. Biol. 2000;20:2032–2037. doi: 10.1161/01.atv.20.9.2032. [DOI] [PubMed] [Google Scholar]
- Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- de Gaetano Donati K, Rabagliati R, Tumbarello M, Tacconelli E, Amore C, Cauda R, Lacoviello L. Increased soluble markers of endothelial dysfunction in HIV-positive patients under highly active antiretroviral therapy. Aids. 2003;17:765–768. doi: 10.1097/00002030-200303280-00020. [DOI] [PubMed] [Google Scholar]
- Dube MP, Gorski JC, Shen C. Severe impairment of endothelial function with the HIV-1 protease inhibitor indinavir is not mediated by insulin resistance in healthy subjects. Cardiovasc. Toxicol. 2008;8:15–22. doi: 10.1007/s12012-007-9010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner KM, Liochev SI, Fridovich I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- Gardner PR. Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- Gottipolu RR, Wallenborn JG, Karoly ED, Schladweiller MC, Ledbetter AD, Krantz T, Linak WP, Nyska A, Johnson JA, Thomas R. One-month diesel exhaust inhalation produces hypertensive gene expression pattern in healthy rats. Environ. Health Perspect. 2009;117:38–46. doi: 10.1289/ehp.11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert VY, Crenshaw BL, Romanoff RL, Ekshyyan VP, Dugas TR. Effects of HIV drug combinations on endothelin-1 and vascular cell proliferation. Cardiovasc. Toxicol. 2004;4:117–131. doi: 10.1385/ct:4:2:117. [DOI] [PubMed] [Google Scholar]
- Heresztyn T, Worthley MI, Horowitz JD. Determination of l-arginine and NG, NG - and NG, NG' -dimethyl-L-arginine in plasma by liquid chromatography as AccQ-Fluor fluorescent derivatives. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2004;805:325–329. doi: 10.1016/j.jchromb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Holme PA, Muller F, Solum NO, Brosstad F, Froland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 1998;12:79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- Jagadeesha DK, Miller FJ, Jr, Bhalla RC. Inhibition of apoptotic signaling and neointimal hyperplasia by tempol and nitric oxide synthase following vascular injury. J. Vasc. Res. 2009;46:109–118. doi: 10.1159/000151444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Hebert VY, Khandelwal AR, Stokes KY, Dugas TR. HIV-1 antiretrovirals induce oxidant injury and increase intima-media thickness in an atherogenic mouse model. Toxicol. Lett. 2009;187:164–171. doi: 10.1016/j.toxlet.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Hebert VY, Li Y, Mathis JM, Alexander JS, Dugas TR. HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis. Toxicol. Appl. Pharmacol. 2007;224:60–71. doi: 10.1016/j.taap.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Jiang B, Hebert VY, Zavecz JH, Dugas TR. Antiretrovirals induce direct endothelial dysfunction in vivo. J. Acquir. Immune Defic. Syndr. 2006;42:391–395. doi: 10.1097/01.qai.0000228790.40235.0c. [DOI] [PubMed] [Google Scholar]
- Kotamraju S, Tampo Y, Keszler A, Chitambar CR, Joseph J, Haas AL, Kalyanaraman B. Nitric oxide inhibits H2O2-induced transferrin receptor-dependent apoptosis in endothelial cells: role of ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci U.S.A. 2003;100:10653–10658. doi: 10.1073/pnas.1933581100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejcy K, Schwarzacher S, Ferber W, Plesch C, Cybulsky MI, Weidinger FF. Expression of VCAM-1 in rabbit iliac arteries is associated with vasodilator dysfunction of regenerated endothelium following balloon injury. Atherosclerosis. 1996;122:59–67. doi: 10.1016/0021-9150(95)05747-1. [DOI] [PubMed] [Google Scholar]
- Lee GA, Rao MN, Grunfeld C. The effects of HIV protease inhibitors on carbohydrate and lipid metabolism. Curr. Infect. Dis. Rep. 2004;6:471–482. doi: 10.1007/s11908-004-0067-5. [DOI] [PubMed] [Google Scholar]
- Lin JH, Chiba M, Balani SK, Chen IW, Kwie GY, Vastag KJ, Nishime JA. Species differences in the pharmacokinetics and metabolism of indinavir, a potent human immunodeficiency virus protease inhibitor. J. Pharmacol. Exp. Ther. 1996;24:1111–1120. [PubMed] [Google Scholar]
- Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel M, von Kegler S, Ruhkamp D, Steinmetz H, Sitzer M. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Matzen K, Dirkx AE, oude Egbrink MG, Speth C, Gotte M, Ascherl G, Grimm T, Griffioen AW, Sturzl M. HIV-1 Tat increases the adhesion of monocytes and T-cells to the endothelium in vitro and in vivo: implications for AIDS-associated vasculopathy. Virus Res. 2004;104:145–155. doi: 10.1016/j.virusres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Note R, Maisonneuve C, Letteron P, Peytavin G, Djouadi F, Igoudjil A, Guimont MC, Biour M, Pessayre D, Fromenty B. Mitochondrial and metabolic effects of nucleoside reverse transcriptase inhibitors (NRTIs) in mice receiving one of five single- and three dual-NRTI treatments. Antimicrob. Agents Chemother. 2003;47:3384–3392. doi: 10.1128/AAC.47.11.3384-3392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papparella I, Ceolotto G, Berto L, Cavalli M, Bova S, Cargnelli G, Ruga E, Milanesi O, Franco L, Mazzoni M, et al. Vitamin C prevents zidovudine-induced NADPH oxidase activation and hypertension in the rat. Cardiovasc. Res. 2007;72:432–438. doi: 10.1016/j.cardiores.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Peng SX, Rockafellow BA, Skedzielewski TM, Huebert ND, Hageman W. Improved pharmacokinetic and bioavailability support of drug discovery using serial blood sampling in mice. J. Pharm. Sci. 2009;98:1877–1884. doi: 10.1002/jps.21533. [DOI] [PubMed] [Google Scholar]
- Quijano C, Castro L, Peluffo G, Valez V, Radi R. Enhanced mitochondrial superoxide in hyperglycemic endothelial cells: direct measurements and formation of hydrogen peroxide and peroxynitrite. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3404–H3414. doi: 10.1152/ajpheart.00761.2007. [DOI] [PubMed] [Google Scholar]
- Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, De Wit S, Law M, D'Arminio Monforte A, Friis-Moller N, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the DAD study: a multi-cohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankatsing RR, Wit FW, Vogel M, de Groot E, Brinkman K, Rockstroh JK, Kastelein JJ, Stroes ES, Reiss P. Increased carotid intima-media thickness in HIV patients treated with protease inhibitors as compared to non-nucleoside reverse transcriptase inhibitors. Atherosclerosis. 2009;202:589–595. doi: 10.1016/j.atherosclerosis.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Santos CX, Tanaka LY, Wosniak J, Jr, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antiox. Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- Shankar SS, Dube MP, Gorski JC, Klaunig JE, Steinberg HO. Indinavir impairs endothelial function in healthy HIV-negative men. Am. Heart J. 2005;150:933. doi: 10.1016/j.ahj.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Shi W, Pei H, Fischer JJ, James JC, Angle JF, Matsumoto AH, Helm GA, Sarembock IJ. Neointimal formation in two apolipoprotein E-deficient mouse strains with different atherosclerosis susceptibility. J. Lipid Res. 2004;45:2008–2014. doi: 10.1194/jlr.M400254-JLR200. [DOI] [PubMed] [Google Scholar]
- Soriano V, Puoti M, Peters M, Benhamou Y, Sulkowski M, Zoulim F, Mauss S, Rockstroh J. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B Virus International Panel. AIDS. 2008;22:1399–1410. doi: 10.1097/QAD.0b013e3282f8b46f. [DOI] [PubMed] [Google Scholar]
- Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- Surdacki A. L-arginine analogs—inactive markers or active agents in atherogenesis? Cardiovasc. Hematol. Agents Med. Chem. 2008;6:302–311. doi: 10.2174/187152508785909429. [DOI] [PubMed] [Google Scholar]
- Sutliff RL, Dikalov S, Weiss D, Parker J, Raidel S, Racine AK, Russ R, Haase CP, Taylor WR, Lewis W. Nucleoside reverse transcriptase inhibitors impair endothelium-dependent relaxation by increasing superoxide. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H2363–H2370. doi: 10.1152/ajpheart.00151.2002. [DOI] [PubMed] [Google Scholar]
- Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NADPH oxidases in restenosis after carotid injury. Arterioscler. Thromb. Vasc. Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- Vaughn G, Detels R. Protease inhibitors and cardiovascular disease: analysis of the Los Angeles County adult spectrum of disease cohort. AIDS Care. 2007;19:492–499. doi: 10.1080/09540120701203329. [DOI] [PubMed] [Google Scholar]
- Wu CC, Wen SC, Yang CW, Pu SY, Tsai KC, Chen JW. Plasma ADMA predicts restenosis of arteriovenous fistula. J. Am. Soc. Nephrol. 2009;20:213–222. doi: 10.1681/ASN.2008050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WZ, Kaye DM. Simultaneous determination of arginine and seven metabolites in plasma by reversed-phase liquid chromatography with a time-controlled ortho-phthaldialdehyde precolumn derivatization. Anal. Biochem. 2004;326:87–92. doi: 10.1016/j.ab.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Zhou H, Pandak WM, Jr, Lyall V, Natarajan R, Hylemon PB. HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol. Pharmacol. 2005;68:690–700. doi: 10.1124/mol.105.012898. [DOI] [PubMed] [Google Scholar]
- Zhu B, Kuhel DG, Witte DP, Hui DY. Apolipoprotein E inhibits neointimal hyperplasia after arterial injury in mice. Am. J. Pathol. 2000;157:1839–1848. doi: 10.1016/S0002-9440(10)64823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]