Abstract
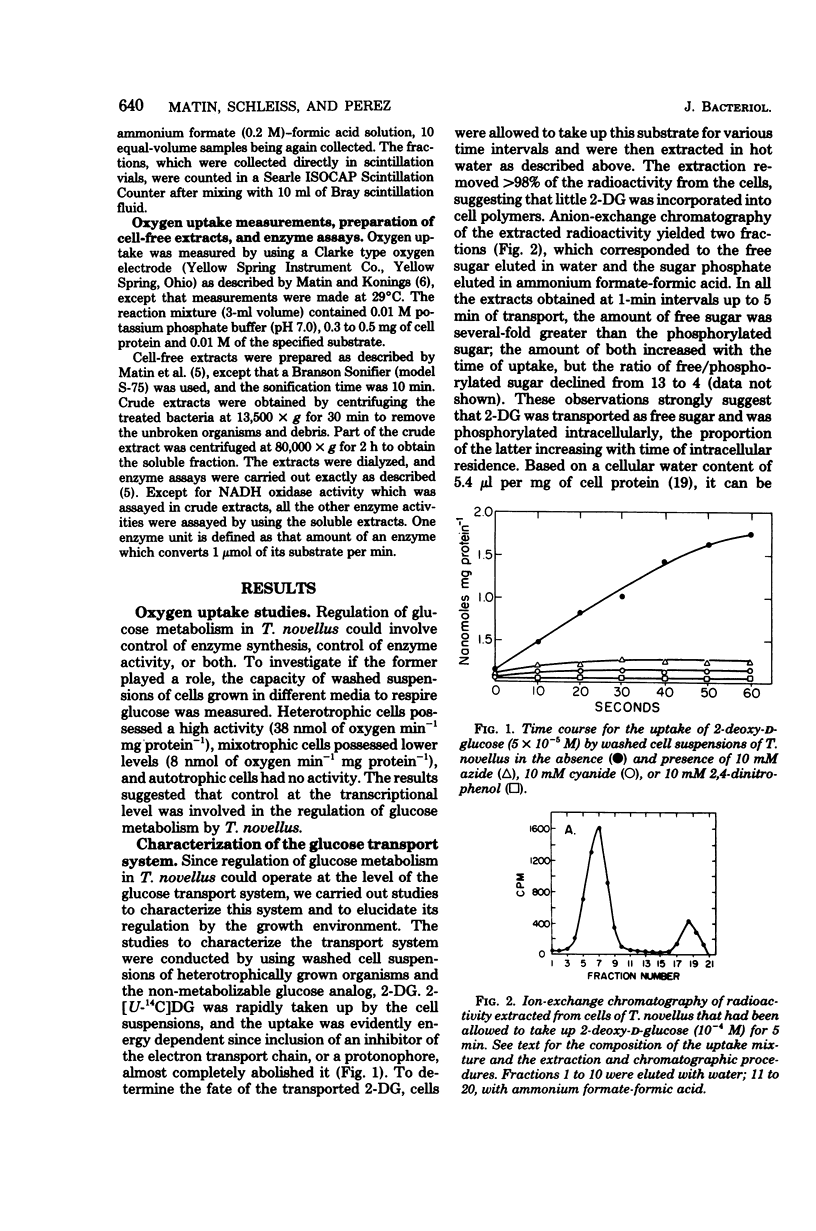
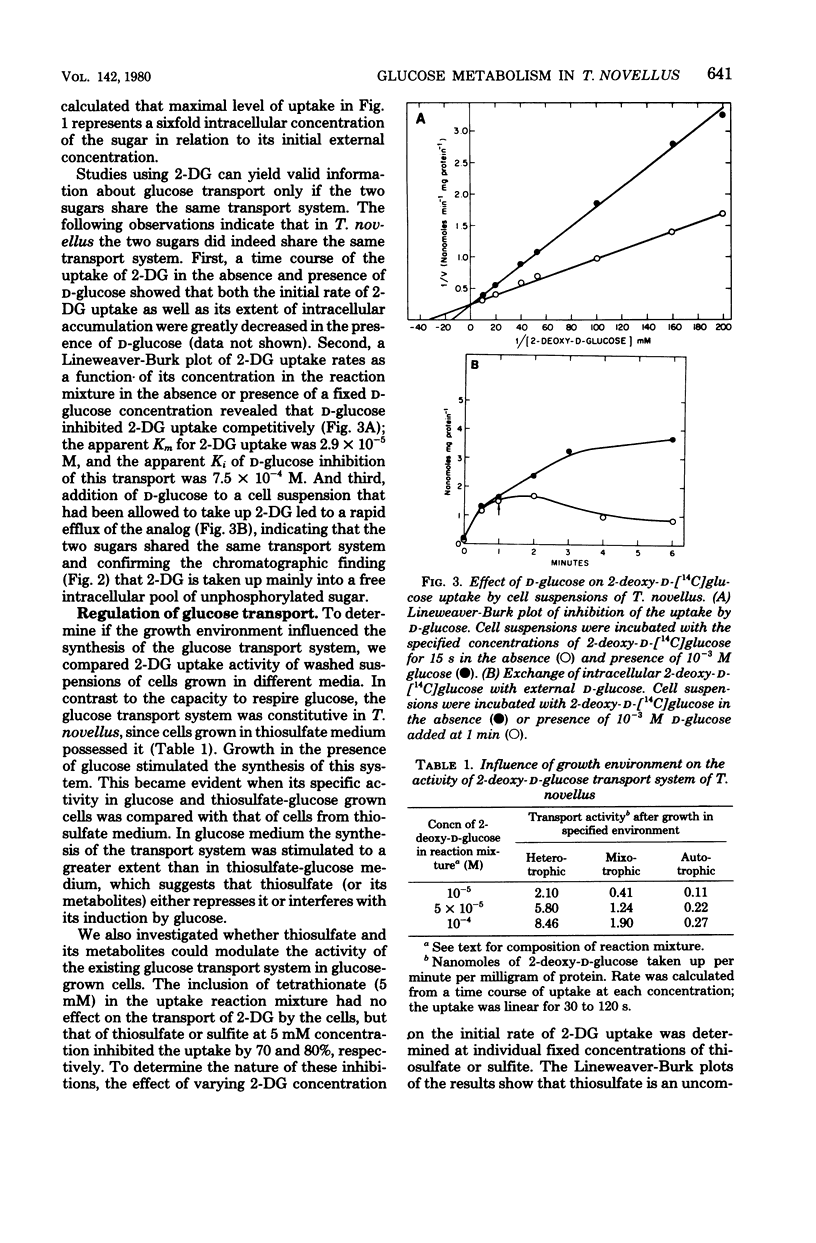
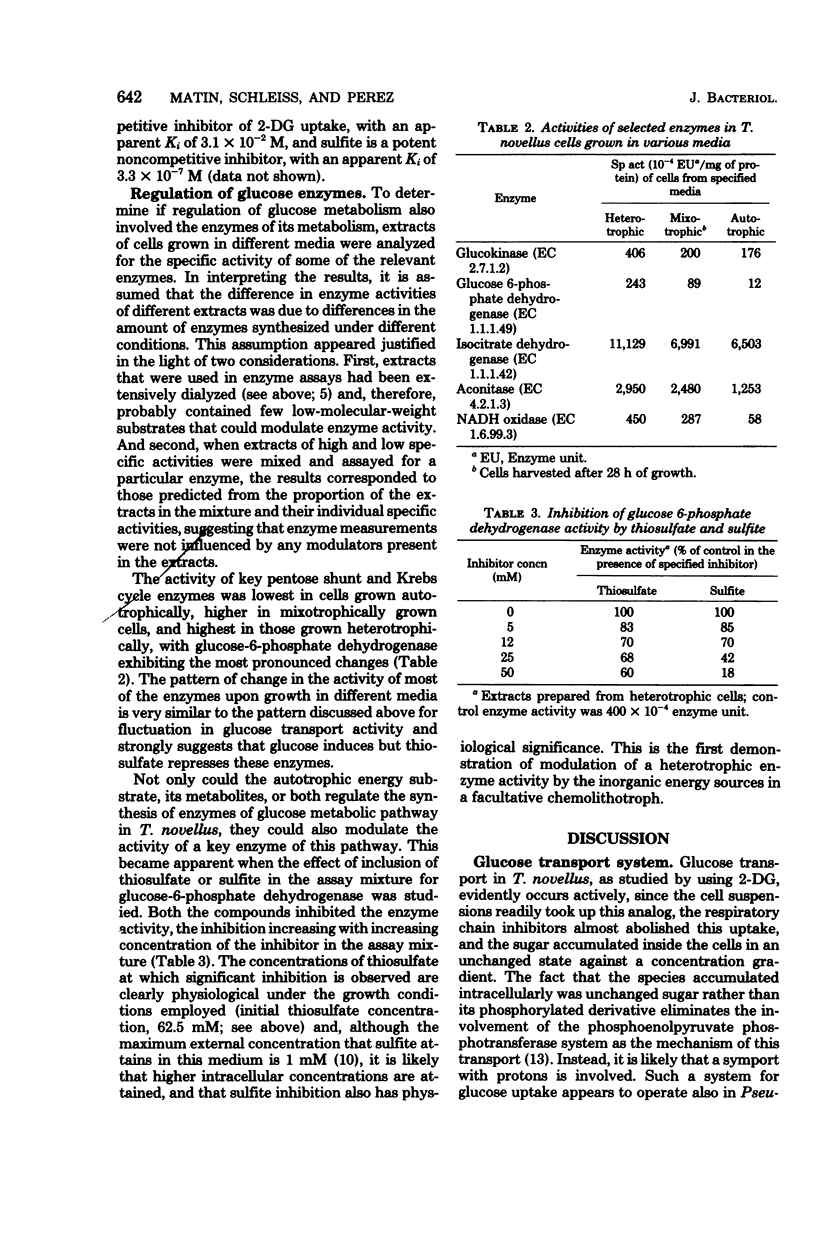
To investigate the physiological basis of decreased rate of glucose utilization by Thiobacillus novellus in a mixotrophic environment (R. C. Perez and A. Matin, J. Bacteriol. 142:633–638, 1980), its glucose transport system was characterized and the modulation of this system as well as enzymes of glucose metabolism by the growth environment was examined. Uptake of 2-deoxy-d-glucose by cell suspensions was almost abolished by respiratory chain inhibitors, and the sugar accumulated unchanged inside the cells against a concentration gradient: its transport is probably linked to the proton electrochemical gradient. The glucose transport system, as well as several enzymes of glucose metabolism, had a high specific activity in heterotrophic cells, intermediate activity in mixotrophic cells, and low activity in autotrophic cells; thus, they are induced by glucose but repressed by thiosulfate, its metabolites, or both. Thiosulfate and sulfite inhibited the glucose transport system uncompetitively and noncompetitively, respectively (apparent Ki = 3.1 × 10−2 M and 3.3 × 10−7 M, respectively) and also inhibited glucose-6-phosphate dehydrogenase activity. Thus, the rate of glucose utilization in mixotrophic environments decreased because thiosulfate and its metabolites repress as well as inhibit the glucose transport system and enzymes of glucose metabolism. The significance of this and other regulatory phenomena that come into play in such environments is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr Multiple sites for coupling of glucose transport to the respiratory chain of membrane vesicles from Azotobacter vinelandii. J Biol Chem. 1973 Dec 10;248(23):8120–8124. [PubMed] [Google Scholar]
- Matin A., Grootjans A., Hogenhuis H. Influence of dilution rate on enzymes of intermediary metabolism in two freshwater bacteria grown in continuous culture. J Gen Microbiol. 1976 Jun;94(2):323–332. doi: 10.1099/00221287-94-2-323. [DOI] [PubMed] [Google Scholar]
- Matin A., Konings W. N. Transport of lactate and succinate by membrane vesicles of Escherichia coli, Bacillus subtilis and a pseudomonas species. Eur J Biochem. 1973 Apr 2;34(1):58–67. doi: 10.1111/j.1432-1033.1973.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Matin A. Organic nutrition of chemolithotrophic bacteria. Annu Rev Microbiol. 1978;32:433–468. doi: 10.1146/annurev.mi.32.100178.002245. [DOI] [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Enzymes of carbohydrate metabolism in Thiobacillus species. J Bacteriol. 1971 Jul;107(1):179–186. doi: 10.1128/jb.107.1.179-186.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Regulation of glucose metabolism in Thiobacillus intermedius. J Bacteriol. 1970 Oct;104(1):239–246. doi: 10.1128/jb.104.1.239-246.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Utilization of glucose in heterotrophic media by Thiobacillus intermedius. J Bacteriol. 1970 Oct;104(1):234–238. doi: 10.1128/jb.104.1.234-238.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez R. C., Matin A. Growth of Thiobacillus novellus on mixed substrates (mixotrophic growth). J Bacteriol. 1980 May;142(2):633–638. doi: 10.1128/jb.142.2.633-638.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Van Vranken N. J., Preisand P., Brustolon M. Regulation of the Thiobacillus intermedius glucose uptake system by thiosulfate. J Bacteriol. 1975 Feb;121(2):577–582. doi: 10.1128/jb.121.2.577-582.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Guymon L. F., Eagon R. G. A novel technique for the preparation of transport-active membrane vesicles from Pseudomonas aeruginosa: observations on gluconate transport. Biochem Biophys Res Commun. 1973 May 1;52(1):285–290. doi: 10.1016/0006-291x(73)90985-6. [DOI] [PubMed] [Google Scholar]
- Williams R. A., Hoare D. S. Physiology of a new facultatively autotrophic thermophilic thiobacillus. J Gen Microbiol. 1972 May;70(3):555–566. doi: 10.1099/00221287-70-3-555. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]



