Abstract
Brain-derived neurotrophic factor (BDNF) is a neurotrophin essential for neuronal survival and differentiation. We examined the concentration of BDNF in cord serum from newborns exposed to methylmercury (MeHg) and polychlorinated biphenyls (PCB) in utero by maternal consumption of whale meat. The cohort consisted of 395 singleton births (206 boys and 189 girls), gestational age ranging from 38 to 42 weeks. Serum BDNF was measured by sandwich ELISA. Maternal smoking habits and other relevant factors were obtained by interviewing the mothers. The exposure to MeHg was estimated from Hg concentrations in cord blood, whereas exposure to PCB was estimated based on maternal serum concentrations. Only MeHg exposure affected the serum BDNF, which decreased in a concentration-dependent manner in girls born to nonsmoking mothers. Maternal smoking significantly increased BNDF in girls but not in boys. For further statistical analyses, we used the serum BDNF concentration as a continuous outcome variable in supervised regression models. Serum BDNF concentration increased with gestational age, increased by maternal smoking, decreased slightly with MeHg exposure, and maternal smoking enhanced the decrease in serum BDNF induced by MeHg exposure. Cord blood BDNF has been reported to increase in association with perinatal brain injuries and has been proposed as a possible predictive marker of neurodevelopmental outcomes. The negative effect that MeHg seems to exert on cord blood BDNF concentration could endanger compensatory responses to an adverse impact and therefore deserves attention.
Keywords: environmental pollutant, environmental exposure, developmental neurotoxicity, early marker of neurotoxicity
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors that has essential roles in neuronal survival and differentiation (Greenberg et al., 2009; Huang and Reichardt, 2001). Lower concentrations of BDNF in the serum have been associated with clinical conditions, such as depression (Castrén et al., 2007) and schizophrenia (Weickert et al., 2003), whereas increased serum concentrations are thought to be compensatory in early stages of Alzheimer’s disease (Laske et al., 2006).
BDNF concentrations in plasma are low, but high concentrations are found in serum and in blood platelets, from which it is released upon platelet activation (Fujimura et al., 2002; Karege et al., 2005). It is currently unclear whether and to which extent circulating BDNF can pass across the blood-brain barrier (Pan et al., 1998; Poduslo and Curran, 1996; Zhang and Pardridge, 2006) and to which extent serum and platelet BDNF levels reflect the brain production. Nevertheless, there is evidence that interventions influencing BDNF production in brain have parallel effects on serum BDNF concentrations (Chen et al., 2001; Sen et al., 2008; Shimizu et al., 2003), and the serum BDNF concentration was shown to correlate with cognitive performance and hippocampal volume in elderly human subjects (Erickson et al., 2010; Komulainen et al., 2008). The serum BDNF concentration therefore deserves to be examined as a possible marker of neurotoxicity.
Epidemiological studies have provided evidence for the neurodevelopmental toxicity of methylmercury (MeHg) even at low level of exposure via dietary intake of fish and seafood (see Castoldi et al., 2008, for review). In a major study on the Faroe Islands population, the umbilical cord blood levels of Hg at birth were found to be associated with adverse effects on cognitive performance in childhood (Debes et al., 2006; Grandjean et al., 1997). In contrast, the Seychelles Islands population yielded virtually no negative effect of prenatal exposure to MeHg on the neuropsychiatric development (Davidson et al., 2006; Myers et al., 2003). There are no obvious explanations for the disparities between the studies (see Castoldi et al., 2008; Spurgeon, 2006).
The developmental neurotoxicity of MeHg is extensively supported by a large number of experimental studies that have shown that intrauterine exposure to this environmental contaminant MeHg is associated with neurodevelopmental deficits (Debes et al., 2006; Johansson et al., 2007; Marques et al., 2009). A possible underlying mechanism might be the decrease in BDNF gene expression, which has been shown to occur as a result of developmental (Onishchenko et al., 2008) as well as after acute exposure to MeHg in adult animals (Andersson et al., 1997).
BDNF can be measured in umbilical cord blood and has been shown to increase with the gestational age (Chouthai et al., 2003; Malamitsi-Puchner et al., 2004). Interestingly, a decrease in cord blood BDNF concentration in response to birth hypoxia has been associated to schizophrenia (Cannon et al., 2008). So far, no information is available whether cord blood concentrations of BDNF are affected by prenatal exposures to neurotoxicants. Therefore, to explore whether peripheral BDNF may reflect developmental exposure to environmental contaminants, such as MeHg and polychlorinated biphenyls (PCBs), we measured BDNF in the cord blood of children exposed to a wide range of MeHg and PCB concentrations during intrauterine development.
MATERIALS AND METHODS
Subjects.
The Faroe Islands constitute a Nordic fishing community located in the North Atlantic between Shetland and Iceland. The exposure to environmental pollutants (MeHg and PCBs) is increased above European levels because of traditional consumption of pilot whale meat and blubber. A birth cohort was formed from consecutive births during 1999–2001. All procedures were approved by the Faroese Ethical Review Committee. Informed consent was obtained from a total of 656 mothers in connection with consecutive spontaneous singleton birth. Blood from the umbilical cord was obtained immediately following delivery, and serum was isolated and kept frozen until analysis. In the Faroes, the government offers free medical care. The study protocol was approved by the ethical review committee serving the Faroe Islands and by the institutional review board at Harvard School of Public Health. The original cohort from which our study group was derived is described elsewhere (Heilmann et al., 2010). The serum BDNF was measured in 581 serum samples with sufficient volume for the assay, out of which measurement of PCB was available for 425 and of Hg for 571. After excluding preterm and late deliveries (gestational age below 38 or above 42 weeks as estimated by ultrasound measurements), the final cohort consisted of 395 children (206 boys and 189 girls) (Table 1).
TABLE 1.
Descriptive Data on 395 Singleton Term Births in the Faroe Islands, from Which Cord Serum was Available for BDNF Analysis
| Total | Boys | Girls | p | |
| N | 395 | 206 | 189 | |
| Birth weight (g) | 3756.3 (4.9) | 3844.7 (527.9) | 3659.9 (416.4) | < 0.001 |
| Gestational age (weeks) | 39.8 (1.1) | 39.8 (1.1) | 39.8 (1.1) | 0.512 |
| Maternal smoking (%) | 30.9 | 34.5 | 27.0 | 0.054 |
| Hg in maternal hair (μg/g) | 3.1 (3) | 3.0 (2.8) | 3.2 (3.2) | 0.659 |
| Hg in cord blood (μg/l) | 16.9 (15.5) | 16.8 (15.4) | 17.0 (15.6) | 0.910 |
| PCB total (μg/g lipid) | 1.5 (1.0) | 1.5 (1.1) | 1.4 (0.9) | 0.459 |
| BDNF (ng/ml) | 9.3 (4.9) | 8.6 (4.4) | 10.1 (5.3) | 0.004 |
Note. Data Shown as Mean (SEM).
Samples and measurements.
Obstetrical parameters (gestational age, birth weight, and Apgar scores at 0 and 5 min after birth) were retrieved from medical records. The smoking habits of the mother and the alcohol consumption during pregnancy were obtained by interview of the mother. Maternal smoking was scored with increasing values for every additional five cigarettes smoked per day.
In connection with the parturition, a sample of maternal hair was collected for mercury analysis to estimate the long-term exposure to MeHg. Immediately after birth, a blood sample was obtained from the umbilical vein, and whole blood (for mercury analysis) and serum were stored at −80°C until analysis. The exposure to PCB was estimated by measuring the serum PCB concentration in a sample of maternal blood obtained at the last antenatal examination at pregnancy week 32.
Exposures to marine contaminants were assessed from analysis of biological samples. Maternal serum samples were analyzed by gas chromatography with electron capture detection at the University of Southern Denmark. As described earlier (Heilmann et al., 2006), the accuracy and reliability of the data were ensured by including quality control serum samples (excess serum samples from the German External Quality Assessment Scheme round-robin program as well as spiked serum pools) in each analytical batch of samples, calibration standards, along with reagent and serum blanks. A simplified total PCB concentration was calculated (the sum of congeners CB-138, CB-153, and CB-180 multiplied by 2) in order to account for the congeners not assessed or having concentrations below the detection limit (Grandjean et al., 1995). The total PCB concentration was expressed in relation to the total lipid concentration determined using the Cypress Diagnostics kit (Langdorp, Belgium).
Total mercury concentrations (as a measure of MeHg exposure) in whole blood from the cord and maternal hair were measured by atomic absorption technique (Grandjean et al., 2003). The quality of this analysis was monitored by successful participation in the Canadian quality assurance program.
Serum levels of BDNF were measured using a two-site ELISA. The samples (diluted 1:15 into dilution buffer) and standards (62.5–2000 pg/ml) were run in duplicate in Nunc Maxisorb 96-well plates. Standard curve was made using human recombinant BDNF (Peprotech; R2 ≥ 0.99 in all plates). All samples added yielded absorbance values within the linear range of standard curve (absorbance after background substraction ≥ 0.08). All values were normalized to an internal control included in all plates.
Statistics.
Preliminary analyses indicated sex- and gestational age–related differences in most of the outcome variables. Therefore, all subsequent analyses considered boys and girls separately and were corrected for the gestational age.
Birth weight and BDNF levels were analyzed by ANCOVA using sex and gestational age as categorical predictors and cord blood Hg, total PCB, and maternal smoking as metric independent variables (models described in Table 3). Partial correlations were performed for all measurements included in analyses in order to check for multicollinearity.
TABLE 3.
ANCOVA Models for Birth Weight and BDNF
| Model 1 |
Model 2 |
|||||||
| df | F | Effect size | Estimated p | df | F | Effect size | Estimated p | |
| Birth weight | ||||||||
| Intercept | 1 | 6894.2 | 1.000 | < 0.001 | 1 | 7182.4 | 1.000 | < 0.001 |
| Hg in cord blood | 1 | 0.703 | 0.083 | 0.594 | 1 | 1.529 | 0.153 | 0.352 |
| Total PCB | 1 | 1.110 | 0.056 | 0.821 | 1 | 0.792 | 0.070 | 0.674 |
| Maternal smokinga | 1 | 23.475 | 0.998 | < 0.001 | ||||
| Sex | 1 | 13.018 | 0.944 | < 0.001 | 1 | 17.133 | 0.983 | < 0.001 |
| Gestational ageb | 4 | 12.781 | 0.999 | < 0.001 | 4 | 12.437 | 0.999 | < 0.001 |
| Sex × gestational age | 4 | 1.056 | 0.311 | 0.416 | 4 | 0.876 | 0.226 | 0.527 |
| BDNF | ||||||||
| Intercept | 1 | 364.1 | 1.000 | < 0.001 | 1 | 338.7 | 1.000 | < 0.001 |
| Hg in cord blood | 1 | 0.355 | 0.075 | 0.645 | 1 | 0.564 | 0.095 | 0.535 |
| Total PCB | 1 | 0.536 | 0.054 | 0.856 | 1 | 0.428 | 0.051 | 0.917 |
| Maternal smokinga | 1 | 3.985 | 0.521 | 0.044 | ||||
| Sex | 1 | 1.490 | 0.223 | 0.232 | 1 | 1.966 | 0.281 | 0.168 |
| Gestational ageb | 4 | 3.729 | 0.890 | 0.005 | 4 | 3.985 | 0.912 | 0.003 |
| Sex × gestational age | 4 | 2.703 | 0.745 | 0.031 | 4 | 2.877 | 0.775 | 0.023 |
Maternal smoking was scored with increasing values for every additional five cigarettes smoked per day (i.e., 0 = nonsmoker, 1 = 1–5 cigarettes per day, 2 = 5–10 cigarettes per day, etc.).
Based on the inclusion criteria, the gestational age at delivery was divided into five categories, i.e., gestational weeks 38, 39, 40, 41, and 42.
We used serum BDNF concentration as continuous outcome variable in all regression models and fitted the data swarm separately for boys, girls, and for pooled sexes. We tested several linear and nonlinear multivariate models using combinations of all other measurements (continuous or categorical) as predictors. The criteria for selecting relevant models were the percentage of variance explained to be above 10% (corrected R2 > 0.10) and the value of the intercept to differ by no more than 1 SD from the average serum BDNF concentration measured in our cohort. First, we ran multiple regression analysis with forward stepwise inclusion of variables in the model (not shown) but found no model to fit the relevance criteria. Second, we estimated supervised models starting from the variables found to have significant effects in the multiple regression models.
The parameters of the models were estimated using the Levenberg-Marquardt algorithm with least squares loss function. All analyses were performed using Statistica version 8 (Statsoft Inc., Tulsa, OK).
RESULTS
Basic statistics of the cohort are shown in Table 1. Partial linear correlations between the variables analyzed are presented in Table 2. Hair and cord blood Hg concentrations correlated very well (r = 0.84). The birth weight increased with the gestational age, was decreased by maternal smoking, and neither Hg nor PCBs (alone or in combination) had any effect after correcting for gestational age (Table 3).
TABLE 2.
Partial Linear Correlations for Pooled Sexes
| BDNF | Hg in cord blood | Total PCB | Maternal smoking | Gestational age | |
| Hg in cord blood | −0.008 | ||||
| Total PCB | 0.035 | 0.244 | |||
| Maternal smoking | 0.064 | 0.078 | 0.056 | ||
| Gestational age | 0.143 | 0.046 | 0.022 | −0.032 | |
| Birth weight | −0.105 | 0.042 | −0.021 | −0.219 | 0.338 |
Note. Significant correlations in bold typeface.
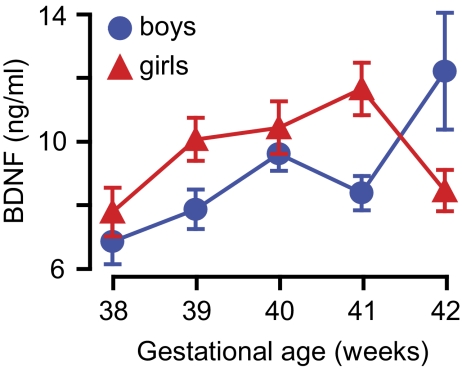
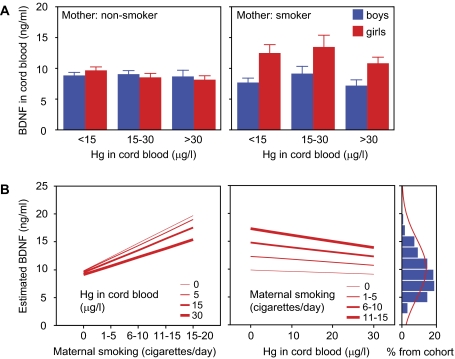
BDNF concentration in the cord blood was higher in girls than in boys, and it increased with the gestational age. We also found a significant interaction effect for sex and gestational age (Table 3, Fig. 1). There was a negative correlation between prenatal MeHg exposure and serum BDNF only in girls born to nonsmoking mothers (Pearson r = −0.149, p = 0.04). Similarly, smoking significantly increased BNDF only in girls. The increase was reduced in the presence of cord blood Hg concentration above 30 μg/l (Table 3, Fig. 2A). We found no significant effect of exposure to PCB and serum BDNF concentration in cord blood.
FIG. 1.
Serum BDNF concentration in the cord blood in relation to gestational age at delivery. Values corrected for exposure to environmental toxicants and maternal smoking.
FIG. 2.
Effect of prenatal exposure to MeHg on BDNF levels. (A) No effect of either Hg or maternal smoking is detected in boys, whereas maternal smoking increases BDNF serum concentration in girls. (B) Predicted trends for gestational week 40 derived from the supervised model (see Table 4). Note that the negative effect of prenatal exposure to MeHg on BDNF serum concentration increases with the number of cigarettes per day the mother smokes. Also note that the model does not predict extreme BDNF concentrations (panel furthest to the right) but explains variations around the average.
Only one model for serum BDNF concentration met the relevance criteria for girls, but not for boys, nor for pooled sexes. The highest predictive power was achieved using a nonlinear model, which included the main predictors found significant in the preliminary analyses (gestational age, cord blood Hg concentration, and maternal smoking), as well as a potentiating effect of maternal smoking on Hg exposure (Table 4). Thus, the explanatory power of the model improved above relevance threshold only after adding an explicit term for interaction between smoking and exposure to MeHg. This model predicts that serum BDNF concentration increases with gestational age at delivery, is increased by maternal smoking, is decreased by MeHg exposure, and combined exposure to maternal smoking and MeHg enhances the decrease in serum BDNF concentration induced by MeHg (Table 4, Fig. 2).
TABLE 4.
Parameter Estimates for Supervised Regression Model for Serum BDNF Concentration in Cord Blood
| Estimate | Metric | Standard | p | Description |
| R2 | 0.103 | < 0.001 | Proportion of global variance explained by the model | |
| b0 | 8.615 | < 0.001 | Expected value when all predictors are absent | |
| b1 | 0.711 | 0.145 | 0.037 | Effect of the gestational age |
| b2 | −0.036 | −0.010 | 0.233 | Effect of the exposure to MeHg in utero |
| b3 | 2.126 | 0.138 | 0.002 | Effect of the maternal smoking |
| b4 | −0.015 | −0.085 | 0.512 | Effect of combined exposure to maternal smoking and MeHg in utero |
 |
DISCUSSION
We found that the BDNF concentration in cord blood in term deliveries varies with gestational age, as anticipated, and that the variation is sex dependent. We also found sex-related differences in serum BDNF concentrations in relation to prenatal exposure to MeHg, with significant alterations present in girls only. Serum BDNF concentration was decreased by MeHg exposure in a dose-dependent fashion, whereas it was increased in response to maternal smoking during pregnancy. The negative effect of MeHg on the BDNF concentration was enhanced by maternal smoking. Thus, the in utero exposure to MeHg blunted the increase in BDNF associated with maternal smoking.
We found that exposure to environmental pollutants (i.e., MeHg and PCBs) had little, if any, effect on intrauterine growth. Conversely, in agreement with earlier reports (Ong et al., 2002), maternal smoking negatively affected birth weight and to a larger extent in girls than in boys. Our cohort included very few children small for gestational age at birth (see Marsál et al., 1996, for criteria). All cases occurred at the lower end of prenatal exposure range for both MeHg and PCBs, and their exclusion from the cohort did not affect the results. Therefore, in utero growth retardation was not considered in subsequent analyses.
Sex-related differences in the neurotoxic effects of exposure to MeHg have been reported in epidemiological (Grandjean et al., 1998; McKeown-Eyssen et al., 1983) and experimental studies (Onishchenko et al., 2007; Rossi et al., 1997), which indicate that prenatal exposure to MeHg has larger developmental effects in males than in females (see also Vahter et al., 2007, for review). Interestingly, we have recently shown that the long-term effects of developmental exposure to MeHg in mice are associated with epigenetic alterations that decrease the expression of BDNF (Onishchenko et al., 2008).
In healthy human adults, BDNF concentration in the peripheral blood has been reported to be higher in females than in males (Lommatzsch et al., 2005; Piccinni et al., 2008), and adverse health outcomes in the elderly have been associated with alterations in plasma BDNF primarily in women (Komulainen et al., 2008; Krabbe et al., 2009). Regarding healthy infants and neonates, the data on sex-related differences in BDNF are too limited to draw any conclusion (Chouthai et al., 2003). We found that maternal smoking resulted in higher serum BDNF concentration in girls only. This is relevant in light of the increased BDNF concentrations found in response to hypoxia/asphyxia in infants (Cannon et al., 2008; Korhonen et al., 1998). The long-term persistence of developmental outcomes of prenatal exposure to maternal smoking is uncertain (MacArthur et al., 2001). However, higher cord blood BDNF was associated with more favorable outcome in a variety of perinatal clinical conditions (Chouthai et al., 2003), and failure to increase cord blood BDNF concentration in response to perinatal hypoxia has been associated with adult onset of psychotic disorders (Cannon et al., 2008). Other toxicants associated with cigarette smoking, such as carbon monoxide or cadmium, may contribute to the neurodevelopmental effects of maternal smoking (see Zdravkovic et al., 2005, for review). The increase in cord blood BDNF concentration that we observed associated to maternal smoking may be an active reaction to hypoxia perhaps subsequent to an impaired placenta function. Importantly, the model we used for predicting the serum BDNF concentration indicates a negative effect of MeHg on maternal smoking–induced increase in BDNF concentration. Altogether, our data suggest that the compensatory increase in cord blood BDNF in relation to maternal smoking is blunted by MeHg exposure. The negative effect of MeHg may thus hamper a compensatory response to an adverse milieu, possibly with latent detrimental consequences for the developing nervous system. The mechanism(s) behind the observed effects of MeHg on cord blood BDNF remains to be elucidated. Based on our previous data (Onishchenko et al., 2008), it is tempting to speculate that epigenetic changes may be involved.
Interestingly, only female nonhuman primates display a selective increase in peripheral BDNF in response to early postnatal adversity (Cirulli et al., 2009). Similarly, we found that serum concentration of BDNF was higher in girls as compared with boys, and also, the increase in response to maternal smoking occurred only in girls. Altogether, the data may be interpreted in light of a greater reserve in girls compared with boys, who may be more vulnerable to changes in BDNF, perhaps even changes too small to be detected in this study. Further studies should be designed to explore the role of BDNF in sex-related susceptibility to MeHg neurotoxicity and other neurodevelopmental disorders.
In conclusion, our findings suggest that the BDNF concentration in cord serum may be a promising indicator of exposure to potential insults occurring in utero, which may result in alterations in neurodevelopmental processes. Serum sampling is difficult to standardize in regard to cord blood sampling in connection with childbirth, and a certain degree of variability must be taken into account when cord serum is used. Moreover, it should be kept in mind that serum BDNF may originate from various sources, including release from thrombocytes, and may not directly reflect brain BDNF. Nonetheless, the associations observed in the present study appear meaningful, and the results suggest that this parameter may be affected by noxious factors, such as maternal smoking and exposure to MeHg in a sex-related manner. The long-term significance of altered cord serum BDNF concentrations is unknown at present but calls for further research aimed at establishing a correlation with future neurodevelopmental outcome.
FUNDING
Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS); Sigrid Jusélius Foundation; The Academy of Finland; National Institute of Environmental Health Sciences (ES12199 and ES09797).
References
- Andersson H, Lindqvist E, Olson L. Downregulation of brain-derived neurotrophic factor mRNA in adult rat brain after acute administration of methylmercury. Mol. Chem. Neuropathol. 1997;31:225–233. doi: 10.1007/BF02815126. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Yolken R, Buka S, Torrey EF. Decreased neurotrophic response to birth hypoxia in the etiology of schizophrenia. Biol. Psychiatry. 2008;64:797–802. doi: 10.1016/j.biopsych.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi AF, Johansson C, Onishchenko N, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo L. Human developmental neurotoxicity of methylmercury: impact of variables and risk modifiers. Regul. Toxicol. Pharmacol. 2008;51:201–214. doi: 10.1016/j.yrtph.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Castrén E, Võikar V, Rantamäki T. Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 2007;7:18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chouthai NS, Sampers J, Desai N, Smith GM. Changes in neurotrophin levels in umbilical cord blood from infants with different gestational ages and clinical conditions. Pediatr. Res. 2003;53:965–969. doi: 10.1203/01.PDR.0000061588.39652.26. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Branchi I, Antonucci MT, Aloe L, Suomi SJ, Alleva E. Changes in plasma levels of BDNF and NGF reveal a gender-selective vulnerability to early adversity in rhesus macaques. Psychoneuroendocrinology. 2009;34:172–180. doi: 10.1016/j.psyneuen.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Weiss B, Shamlaye CF, Cox C. Prenatal methyl mercury exposure from fish consumption and child development: a review of evidence and perspectives from the Seychelles Child Development Study. Neurotoxicology. 2006;27:1106–1109. doi: 10.1016/j.neuro.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jørgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002;87:728–734. [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DGJ, Sampson EJ, Jørgensen PJ, Vahter M. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ. Res. 1995;71:29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ. Res. 1998;77:165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sørensen N, Dahl R, Jørgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Grandjean P, White RF, Weihe P, Jørgensen PJ. Neurotoxic risk caused by stable and variable exposure to methylmercury from seafood. Ambul. Pediatr. 2003;3:18–23. doi: 10.1367/1539-4409(2003)003<0018:nrcbsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C, Budtz-Joergensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ. Health. Perspect. 2010 doi: 10.1289/ehp.1001975. Advance Access published on June 20, 2010; doi:10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jørgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3:e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Castoldi AF, Onishchenko N, Manzo L, Vahter M, Ceccatelli S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox. Res. 2007;11:241–260. doi: 10.1007/BF03033570. [DOI] [PubMed] [Google Scholar]
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry J, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Komulainen P, Pedersen M, Hänninen T, Bruunsgaard H, Lakka TA, Kivipelto M, Hassinen M, Rauramaa TH, Pedersen BK, Rauramaa R. BDNF is a novel marker of cognitive function in ageing women: the DR’s EXTRA Study. Neurobiol. Learn. Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Korhonen L, Riikonen R, Nawa H, Lindholm D. Brain derived neurotrophic factor is increased in cerebrospinal fluid of children suffering from asphyxia. Neurosci. Lett. 1998;240:151–154. doi: 10.1016/s0304-3940(97)00937-3. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Mortensen EL, Avlund K, Pedersen AN, Pedersen BK, Jørgensen T, Bruunsgaard H. Brain-derived neurotrophic factor predicts mortality risk in older women. J. Am. Geriatr. Soc. 2009;57:1447–1452. doi: 10.1111/j.1532-5415.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G, Schott K. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J. Neural Transm. 2006;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- MacArthur C, Knox EG, Lancashire RJ. Effects at age nine of maternal smoking in pregnancy: experimental and observational findings. BJOG. 2001;108:67–73. doi: 10.1111/j.1471-0528.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- Malamitsi-Puchner A, Economou E, Rigopoulou O, Boutsikou T. Perinatal changes of brain-derived neurotrophic factor in pre- and fullterm neonates. Early Hum. Dev. 2004;76:17–22. doi: 10.1016/j.earlhumdev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Marques RC, Dórea JG, Bernardi JVE, Bastos WR, Malm O. Prenatal and postnatal mercury exposure, breastfeeding and neurodevelopment during the first 5 years. Cogn. Behav. Neurol. 2009;22:134–141. doi: 10.1097/WNN.0b013e3181a72248. [DOI] [PubMed] [Google Scholar]
- Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843–848. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- McKeown-Eyssen GE, Ruedy J, Neims A. Methyl mercury exposure in northern Quebec. II. Neurologic findings in children. Am. J. Epidemiol. 1983;118:470–479. doi: 10.1093/oxfordjournals.aje.a113652. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang L, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Ong KKL, Preece MA, Emmett PM, Ahmed ML, Dunger DB. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: longitudinal birth cohort study and analysis. Pediatr. Res. 2002;52:863–867. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castrén E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. J. Neurochem. 2008;106:1378–1387. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Tamm C, Vahter M, Hökfelt T, Johnson JA, Johnson DA, Ceccatelli S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol. Sci. 2007;97:428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Piccinni A, Marazziti D, Del Debbio A, Bianchi C, Roncaglia I, Mannari C, Origlia N, Catena Dell’Osso M, Massimetti G, Domenici L, et al. Diurnal variation of plasma brain-derived neurotrophic factor (BDNF) in humans: an analysis of sex differences. Chronobiol. Int. 2008;25:819–826. doi: 10.1080/07420520802387773. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res. Mol. Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Exp. Brain Res. 1997;117:428–436. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol. Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Spurgeon A. Prenatal methylmercury exposure and developmental outcomes: review of the evidence and discussion of future directions. Environ. Health Perspect. 2006;114:307–312. doi: 10.1289/ehp.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahter M, Akesson A, Lidén C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ. Res. 2007;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol. Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Zdravkovic T, Genbacev O, McMaster MT, Fisher SJ. The adverse effects of maternal smoking on the human placenta: a review. Placenta. 2005;26(Suppl. A):S81–S86. doi: 10.1016/j.placenta.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Blood-brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion. Brain Res. 2006;1111:227–229. doi: 10.1016/j.brainres.2006.07.005. [DOI] [PubMed] [Google Scholar]