Abstract
National Health and Nutrition Examination Survey data show an association between hypertension and exposure to dioxin-like halogenated aromatic hydrocarbons (HAHs). Furthermore, chronic exposure of mice to the prototypical HAH, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), induces reactive oxygen species (ROS), endothelial dysfunction, and hypertension. Because TCDD induces cytochrome P4501A1 (CYP1A1) and CYP1A1 can increase ROS, we tested the hypothesis that TCDD-induced endothelial dysfunction and hypertension are mediated by CYP1A1. CYP1A1 wild-type (WT) and knockout (KO) mice were fed one control or TCDD-containing pill (180 ng TCDD/kg, 5 days/week) for 35 days (n = 10–14/genotype/treatment). Blood pressure was monitored by radiotelemetry, and liver TCDD concentration, CYP1A1 induction, ROS, and aortic reactivity were measured at 35 days. TCDD accumulated to similar levels in livers of both genotypes. TCDD induced CYP1A1 in endothelium of aorta and mesentery without detectable expression in the vessel wall. TCDD also induced superoxide anion production, measured by NADPH-dependent lucigenin luminescence, in aorta, heart, and kidney of CYP1A1 WT mice but not KO mice. In contrast, TCDD induced hydrogen peroxide, measured by amplex red assay, to similar levels in aorta of CYP1A1 WT and KO mice but not in heart or kidney. TCDD reduced acetylcholine-dependent vasorelaxation in aortic rings of CYP1A1 WT mice but not in KO mice. Finally, TCDD steadily increased blood pressure after 15 days, which plateaued after 25 days (+20 mmHg) in CYP1A1 WT mice but failed to alter blood pressure in KO mice. These results demonstrate that CYP1A1 is required for TCDD-induced cardiovascular superoxide anion production, endothelial dysfunction, and hypertension.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; cytochrome P4501A1; hypertension; reactive oxygen species; endothelial dysfunction
Hypertension occurs in one of three U.S. adults and represents a significant risk factor for life-threatening cardiovascular diseases. It is well established that environmental modifiers, such as diet and exercise, can significantly impact cardiovascular disease risk, but accumulating evidence also suggests a role for the contribution of environmental pollutants to the overall cardiovascular disease burden (O'Toole et al., 2008). Nonetheless, only a limited number of studies have investigated the impact of environmental pollutant exposure on the incidence of hypertension.
For one class of environmental pollutants, dioxin-like halogenated aromatic hydrocarbons (HAHs), epidemiology studies have linked their exposure to human hypertension. These pollutants include polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and biphenyls (PCBs) that are structurally similar to the most potent congener, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Exposure of Vietnam veterans to TCDD via the defoliant Agent Orange has been associated with a significantly higher incidence of hypertension (Air Force Health Study, 2005; Kang et al., 2006). Although Vietnam veterans were exposed to high levels of TCDD, more recent National Health and Nutrition Examination Survey data have shown an association between the prevalence of hypertension and exposure to background levels of dioxin-like PCDD/Fs and PCBs in the general U.S. population (Everett et al., 2008a,b; Lee et al., 2007). In all these studies, odds ratios remained significant after adjusting for age, gender, race, smoking status, and body mass index. Similar associations have been reported among the general population in Japan (Uemura et al., 2009).
Results from a limited number of animal studies are consistent with the epidemiology studies. Acute or chronic exposure of rodents to TCDD or TCDD-like HAHs significantly increases blood pressure (Dalton et al., 2001; Kopf et al., 2008; Lind et al., 2004). Additionally, hypertension induced by chronic TCDD exposure is associated with an increase in cardiovascular reactive oxygen species (ROS), endothelial dysfunction, and cardiac hypertrophy (Kopf et al., 2008). One mechanism by which TCDD may induce these effects is by the sustained induction of cytochrome P4501A1 (CYP1A1) via activation of the aryl hydrocarbon receptor (AHR). CYP1A1 is highly induced in vascular endothelium (Garrick et al., 2005; Guiney et al., 1997; Schlezinger and Stegeman, 2000; Smolowitz et al., 1991; Stegeman et al., 1991), and over expression is associated with the production of ROS, including superoxide anion and hydrogen peroxide (H2O2) (Kopf and Walker, 2010; Zangar et al., 2004). ROS can be elevated in human, and experimental hypertension (Touyz, 2004) and antioxidants can improve vascular function and normalize hypertension in some animal models (Wilcox and Pearlman, 2008).
Furthermore, linkage analysis and genome-wide scans have mapped the Cyp1a1 gene to a quantitative trait locus associated with hypertension (Krushkal et al., 1999; Stoll et al., 2000), and Cyp1a1 polymorphisms that increase basal and inducible expression are associated with human hypertension (Gambier et al., 2006). Thus, in our study, we used CYP1A1 wild-type (WT) and knockout (KO) mice to test the hypothesis that TCDD-induced ROS, endothelial dysfunction, and hypertension are mediated by CYP1A1 induction.
MATERIALS AND METHODS
Animals.
Male CYP1A1 KO mice, backcrossed more than eight generations onto the C57Bl/6 background, were generously provided by Dr Daniel Nebert (University of Cincinnati) and were bred at the University of New Mexico (Dalton et al., 2000). Age-matched C57BL/6 mice served as WT controls. Animals were housed in a temperature-controlled environment with a 12-h:12-h light-dark cycle, receiving standard mouse chow and water ad libitum. This study was approved by the University of New Mexico Institutional Animal Care and Use Committee and conforms to the National Institutes of Health animal care guidelines.
Exposure to TCDD and analysis of hepatic TCDD concentration.
TCDD dissolved in 1,4-p-dioxane was added to transgenic dough (Bio-Serv, Frenchtown, NJ) along with 0.1% bromophenol blue, and the mixture was folded together until the dye was evenly distributed throughout the dough. Pregelatinized corn starch was added to absorb excess water and reduce stickiness. The dough was then formed into 100 mg pills using a pill mold (Gallipot, St Paul, MN) coated with 5% magnesium stearate. Control pills were made by adding an equivalent volume of 1,4-p-dioxane and bromophenol blue to transgenic dough. Pills were analyzed for their TCDD content as described below and stored at 4°C. CYP1A1 WT and KO mice were fed one control or TCDD-containing pill (180 ng TCDD/kg, 5 days/week) for 35 days. Different batches of pills were made containing the appropriate amount of TCDD to adjust for 0.001 kg differences in body weight so that all mice received 180 ng TCDD/kg body weight throughout the entire exposure period.
TCDD was measured in the liver of control and TCDD-exposed CYP1A1 WT and KO mice after 35 days of treatment by a method based on EPA Method 1613 (Tetra- through Octa-Chlorinated Dioxins and Furans by Isotope Dilution HRGC/HRMS, 1994) (Huwe and Smith, 2005). All chemical standards used for the analysis were purchased from Wellington Laboratories (Guelph, ON). Tissue samples (0.2 g) were homogenized in saline (200 μl) using disposable pellet pestles and microtubes. An aliquot equivalent to 5 mg was transferred to a Teflon bottle containing methylene chloride:hexane (50:50) (20 ml), spiked with 13C-labeled recovery standards, and vigorously shaken. The extract was filtered through anhydrous sodium sulfate (20 g), solvent exchanged into hexane (10 ml), and applied to an automated dioxin cleanup instrument (Fluid Management Systems, Waltham, MA) for chromatography on tri-phasic silica, basic alumina, and carbon cartridges. 13C-labeled internal standards were added prior to high-resolution gas chromatography/high-resolution mass spectrometry analysis on an Autospec Ultima mass spectrometer (Waters, Milford, MA) coupled to an Agilent 6890 gas chromatograph. The limit of detection was 160 pg/g given the small sample size used for analysis (0.005 g).
Blood pressure analysis.
Ten-week-old mice were surgically implanted with blood pressure/activity telemeters (PA-C10; Data Sciences International, St Paul, MN) as previously described (Kopf et al., 2008). Mice were anesthetized with isoflurane, telemeter catheter was inserted into the left carotid artery, and body of the transmitter was placed sc. Beginning 7 days after surgery, baseline blood pressure values, including mean, systolic, and diastolic, and heart rate were recorded for 10 s every 15 min for 24 h/day for a total of 6 days.
Immunohistochemistry and Western blot analysis.
Aortic and mesenteric arteries were fixed in 10% neutral buffered-formalin or snap frozen. Fixed tissues were embedded in paraffin and sectioned at 7 μm. Sections were heated at 95°C for 15 min in 10mM Tris and 1mM EDTA, pH 9, cooled to room temperature, and then blocked for 1 h in 10% heat-inactivated goat serum (Gibco BRL, Gaithersburg, MD) in tris-buffered saline (pH 7.4). Blocked sections were incubated for 2 h with mouse anti-CYP1A1 monoclonal antibody (1/100; Santa Cruz Biotechnology), washed with PBS containing 1.0% bovine serum albumin and 0.05% Tween 20, incubated for 1 h with goat anti-mouse IgG-biotin (1/200; Southern Biotech), washed and incubated for 1 h with streptavidin-β-galactosidase (Southern Biotech), and washed again. Color was developed 30 min at 37°C with 0.1% X-gal (Sigma) as described previously (Walker et al., 1997).
Single frozen mesenteric arterial beds were pulverized while frozen and homogenized in RIPA lysis buffer and protein analyzed for CYP1A1 expression by Western blot. The protein concentration was determined by the Bradford assay. Protein samples were denatured by heating at 95°C in SDS loading buffer for 5 min, resolved by electrophoresis on a 10% SDS-polyacrylamide gel, and transferred to a polyvinylidene fluoride membrane. Membranes were probed using rabbit polyclonal anti-CYP1A1 (1/2000; Chemicon) followed by goat anti-rabbit IgM + IgG-HP secondary antibody (1/2000; Southern Biotech). Detection was performed using ECL reagent (Western Lighting -ECL kit; Perkin Elmer) and imaged on a Kodak Image Station 4000MM (Carestream Health, Rochester, NY). Membranes were then stripped using a mild stripping buffer, probed using a goat polyclonal anti-actin primary antibody (1/500 dilution) and a donkey anti-goat IgG- horseradish peroxidase (HRP) secondary antibody (1/2000 dilution). Band intensity was quantified using Kodak Image Station digital imaging software.
Assessment of plasma indices of renin-angiotensin system.
Plasma renin activity (PRA) was determined using a commercial kit (GammaCoat Plasma Renin Activity 125I Kit; DiaSorin, Stillwater, MN). The PRA assay is a two-step process, where first angiotensin I is generated and second angiotensin I is detected by a radioimmunoassay. PRA is expressed as nanogram per milliliter per hour of generated angiotensin I. Plasma angiotensin converting enzyme (ACE) activity was determined using a commercial kit (Alpco Diagnostics, Salem, NH) (Senador et al., 2009). Plasma samples (10 μl) were incubated with 100 μl 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer containing the synthetic ACE substrate, 3H-hippuryl-glycyl-glycine, at 37°C for 1 h. Incubation was followed by acidification with 50 μl 1 N HCl to stop the reaction. Liberated 3H-hippuric acid was separated from unreacted substrate by extraction with 1.5 ml scintillation cocktail and measured in beta counter (Packard 18TR Liquid Scintillation Analyzer). ACE activity was expressed as units per liter. One unit of ACE activity was defined as the amount of enzyme required to release 1 μmol of hippuric acid per minute per liter of plasma at 37°C.
Assessment of cardiovascular ROS.
We used NADPH-dependent lucigenin luminescence to assess superoxide anion production as described previously (Kopf et al., 2008) and an amplex red assay to assess H2O2 production from aorta, heart, and kidneys. For lucigenin luminescence, a 15-mg cross section of frozen left ventricle, kidney, or 5-mm segment of aorta was thawed in ice-cold PBS and then incubated in ice-cold Krebs-Ringer buffer (20mM HEPES, 10mM dextrose, 127mM NaCl, 5.5mM KCl, 1mM CaCl2, and 2mM MgSO4, pH 7.4) for 10 min. Each tissue was then transferred to a well of a 96-well white opaque plate containing 200 μl of room temperature Krebs-Ringer buffer with 5μM lucigenin and 100μM NADPH. Luminescence was measured three times for 10 s at 2-min intervals using a multilabel counter (Wallac Victor2; Perkin Elmer), and the three measurements were averaged. Preincubation of tissue with 30μM tempol was used to confirm specificity for superoxide anion. For the amplex red assay, a 5-mm section of aorta or 20–25 mg of left ventricle tissue were incubated in 50 μl of Krebs/HEPES buffer (130mM NaCl, 4.7mM KCl, 1.5mM CaCl2, 1.2mM MgSO4, 1.2mM NaH2PO4, 25mM NaHCO3, 1mM HEPES, and 11.5mM glucose) and 50 μl of 0.2μM amplex red and 0.2 U/ml HRP in a well of a 96-well plate for 1 h at 37°C. Kidneys (half sections) were homogenized in Kreb/HEPES buffer, and 50 μl of the homogenate were added to 50 μl of the Amplex Red/HRP solution and incubated for 1 h at 37°C. Fluorescence was measure (560 nm excitation; 590 nm emission), and tissue H2O2 concentrations were derived from a H2O2 standard curve. Tissue H2O2 concentrations were standardized to tissue weight and total protein for heart and kidney, respectively. Total protein of kidney homogenates was determined by colorimetric assay (Bio-Rad Protein Assay).
Aortic reactivity analysis.
After 35 days of TCDD treatment, acetylcholine (ACh)-dependent vasorelaxation was assessed in the absence and presence of tempol as described previously (Kopf et al., 2008). Aortas were cleaned in ice-cold physiological saline (130mM NaCl, 4.7mM KCl, 1.18mM KH2PO4, 1.17mM MgSO4, 14.9mM NaHCO3, 5.5mM glucose, 26μM CaNa2EDTA, and 1.8mM CaCl2, pH 7.4), cut into 3-mm segments, and two rings per animal were mounted in a wire myograph (Radnoti Glass Technology Inc., Monrovia, CA) attached to a force transducer (Grass Technologies, West Warwick, RI). An ACh dose-response (10−5–10−9M) was conducted following preconstriction with phenylephrine (30μM). Following a 40-min washout, aortas were preincubated with tempol (30μM) for 20 min, and the ACh dose-response was repeated.
Analysis of gene expression.
Total RNA was isolated from the aorta, heart, or mesenteric arteries of CYP1A1 WT and KO mice treated for 35 days with control or TCDD, using RNeasy Fibrous Tissue Mini Kit (Qiagen, GmbH, Germany). Complementary DNA (cDNA) was synthesized using iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) with the supplied random primers and 250 ng RNA. PCR amplification was performed using an iCycler (Bio-Rad Laboratories) with a reaction mixture comprised of iQ SYBR Green Supermix (Bio-Rad Laboratories) with 500nM CYP1A1 or CYP1B1 primers (CYP1A1: sense, 5′ CAAAGAGCACTACAGGACA 3′; antisense, 5′ TTGGCATTCTCGTCCAGC 3′ and CYP1B1: sense, 5′ AATCAATGCGATTCTCCAGCTTTT 3′; antisense, 5′ CGACCGTATTCTTGGGGATGTAG 3′) and 250 pg cDNA/μl. We used RNA polymerase II (POL2: sense, 5′ TGACTCACAAACTGGCTGACATT 3′; antisense, 5′ TACATCTTCTGCTATGACATGG 3′) as a reference gene for CYP1B1 messenger RNA (mRNA) expression in aorta, heart, and kidney but found that glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was a better reference for the vascular tissues and thus used it (sense, 5′ CCAATGTGTCCGTCGTGGATC 3′; antisense, 5′ TGTAGCCCAAGATGCCCTTCA 3′) as the internal reference gene for CYP1A1 mRNA expression in aorta and mesenteric arteries. Cycle threshold data for both the target gene and the reference gene were used to calculate mean normalized expression as previously described (Lund et al., 2003).
Statistical analysis.
Data were expressed as mean ± SE. Treatment- and genotype-related changes were analyzed by two-way ANOVA with post hoc Holm-Sidak comparisons. Aortic ACh relaxation and blood pressure were analyzed by repeated measures two-way ANOVA with post hoc Holm-Sidak comparisons. p < 0.05 was considered statistically significant.
RESULTS
Body and Organ Weights
We compared body and organs weights of CYP1A1 WT and KO mice and investigated whether 35 days TCDD exposure significantly altered these weights. Irrespective of TCDD exposure, CYP1A1 KO mice exhibited significantly smaller body weights compared with age-matched CYP1A1 WT mice (Table 1). In addition, all organs weighed from CYP1A1 KO mice were significantly smaller than WT, including liver, heart, and kidney. However, when organ weight was expressed as a percent of total body weight, only the liver/body weight ratio remained significantly smaller in CYP1A1 KO mice compared with WT mice. TCDD exposure significantly increased liver weight and liver/body weight ratio in both genotypes.
TABLE 1.
Comparison of Body and Organ Weights of 4-Month-Old CYP1A1 WT and KO Male Mice Exposed to TCDD for 35 Days
| CYP1A1 WT |
CYP1A1 KO |
|||
| Weight (g) | Control (n = 14) | TCDD (n = 12) | Control (n = 11) | TCDD (n = 10) |
| Body | 32.31 ± 0.92 | 32.97 ± 1.04 | 27.24 ± 0.41* | 27.27 ± 0.69* |
| Liver | 1.69 ± 0.06 (5.28 ± 0.12) | 2.00 ± 0.07† (5.94 ± 0.17)† | 1.32 ± 0.02* (4.83 ± 0.07)* | 1.52 ± 0.07*,† (5.54 ± 0.11)*,† |
| Heart | 0.129 ± 0.002 (0.40 ± 0.01) | 0.131 ± 0.002 (0.40 ± 0.01) | 0.109 ± 0.002* (0.40 ± 0.01) | 0.111 ± 0.003* (0.41 ± 0.012) |
| Kidney | 0.397 ± 0.006 (1.24 ± 0.03) | 0.449 ± 0.049 (1.37 ± 0.01) | 0.327 ± 0.006* (1.20 ± 0.02) | 0.334 ± 0.015* (1.22 ± 0.05) |
Note. Numbers in parentheses indicate organ weight/body weight × 100.
p < 0.05 versus corresponding WT.
p < 0.05 versus corresponding control group.
CYP1A1 Induction in Conduit and Resistance Arteries
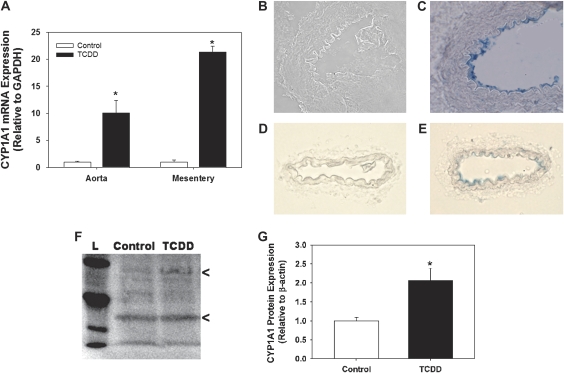
We sought to determine the spatial localization of TCDD-induced CYP1A1 expression in conduit and resistance arteries and the degree to which CYP1A1 was induced. We found that TCDD induced CYP1A1 mRNA expression by 10- and 21-fold in the aorta and mesenteric arteries, respectively (Fig. 1A). Furthermore, immunohistochemical analysis revealed that CYP1A1 expression localized to endothelial cells of both the aorta (Figs. 1B and 1C) and mesenteric arteries (Figs. 1D and 1E) with no detectable expression in vascular smooth muscle or adventitia. Although CYP1A1 expression was not detectable in control CYP1A1 WT or KO mice by immunohistochemistry (not shown), constitutive expression was detected at low levels in mesenteric resistance arteries of control CYP1A1 WT mice by Western blot (Fig. 1F) and was induced 2.1-fold by TCDD (Fig. 1G). The large difference between the degree of CYP1A1 mRNA and protein induction by TCDD in the mesentery may have resulted from differences in how the vessel beds were collected for each analysis. Analysis of pooled samples using a standardized collection method might provide a more accurate comparison of the level of CYP1A1 mRNA and protein induction by TCDD.
FIG. 1.
Comparison of CYP1A1 mRNA (A) and protein expression in aortic (B and C) and mesenteric arteries (D–G) of CYP1A1 WT mice treated with control or TCDD (180 ng/kg/day, 5 days/week) for 35 days. Representative sections of aorta and mesentery were stained without (B and D) or with primary CYP1A1 antibody (C and E). Positive β-galactosidase staining (blue) can be seen in the endothelium of both the aortic (C) and mesenteric artery (E). Representative Western blot (F) and quantification (G) of CYP1A1 expression from mesenteric arteries. L indicates molecular weight ladder and arrowheads indicate CYP1A1 (upper band) and β-actin (lower band). *p < 0.05 compared with control, n = 3 per treatment.
TCDD Liver Concentration and CYP1B1 Induction
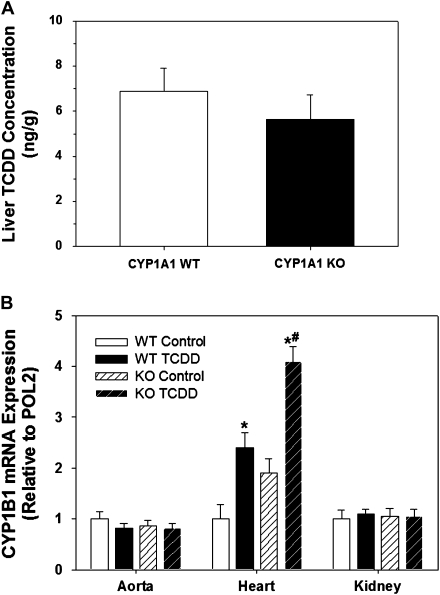
To determine if CYP1A1 WT and KO mice were exposed to similar levels of TCDD after 35 days, we measured the hepatic concentration of TCDD as an index of total body burden (Diliberto et al., 2001) and CYP1B1 mRNA induction as an index of AHR activation in extrahepatic tissues (Puga et al., 2004). TCDD accumulated to the same extent in the liver of CYP1A1 WT and KO mice after 35 days (Fig. 2A), whereas hepatic TCDD was not detected in any control mice. Although neither constitutive nor TCDD-inducible CYP1B1 mRNA expression differed between CYP1A1 WT and KO mice in aorta and kidney, the TCDD-induced expression of CYP1B1 mRNA was significantly greater in CYP1A1 KO hearts (Fig. 2B). Although CYP1B1 mRNA expression in the control KO hearts tended to be higher than in the WT hearts, it is notable that the degree of induction by TCDD was equivalent between genotypes (WT TCDD: 2.4 ± 0.5-fold induction; KO TCDD: 2.1 ± 0.1-fold, p > 0.6).
FIG. 2.
Comparison of liver TCDD concentration (A) and induction of CYP1B1 mRNA from cardiovascular tissues (B) of CYP1A1 WT and KO mice treated with control or TCDD (180 ng/kg/day, 5 days/week) for 35 days. *p < 0.05 compared with corresponding control group of the same genotype; #p < 0.05 compared with TCDD-treated CYP1A1 WT mice; n = 6 per genotype (A) and n = 4 per group (B).
TCDD Induction of Superoxide Anion and H2O2
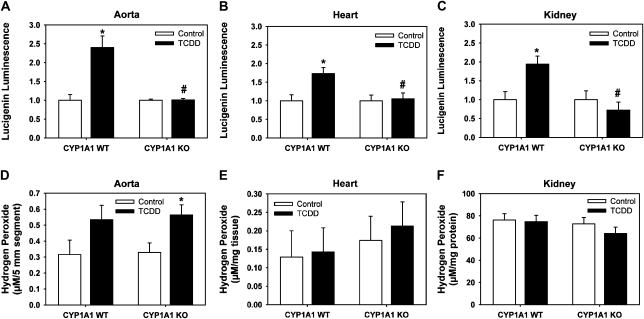
Because TCDD induction of superoxide anion and H2O2 in cultured endothelial cells requires CYP1A1 (Kopf and Walker, 2010), we determined whether TCDD induction of ROS in vivo also required CYP1A1. TCDD induced a significant increase in superoxide anion production in aorta, heart, and kidney in CYP1A1 WT mice, but these increases were absent from TCDD-exposed CYP1A1 KO mice (Figs. 3A–C). Additionally, TCDD induced an increase in H2O2 production from the aorta of both mouse genotypes, although the increase was significant only in CYP1A1 KO mice (Fig. 3D). TCDD did not increase H2O2 in the heart or kidney of either genotype (Figs. 3E and 3F).
FIG. 3.
Comparison of superoxide anion and H2O2 production from cardiovascular tissues of CYP1A1 WT and KO mice treated with control or TCDD (180 ng/kg/day, 5 days/week) for 35 days. Superoxide production, as measured by NADPH-dependent lucigenin luminescence, from aorta (A), heart (B), and kidney (C). H2O2 production, as measured using an amplex red assay, from aorta (D), heart (E), and kidney (F). Two-way ANOVA demonstrated significant differences based on treatment and genotype for lucigenin luminescence in all tissues and only based on treatment for amplex red in aorta. *p < 0.05 compared with corresponding control group of the same genotype; #p < 0.05 compared with TCDD-treated CYP1A1 WT; n = 3–7 per group.
TCDD Induction of Aortic Endothelial Dysfunction
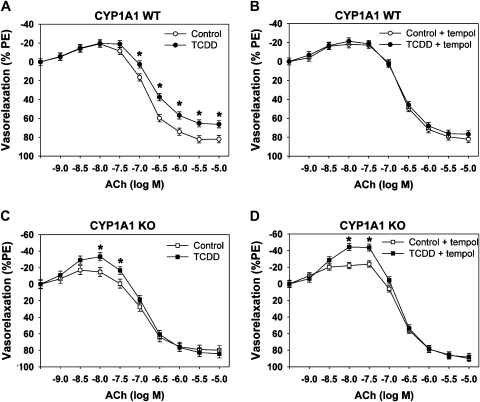
To determine if CYP1A1 mediated TCDD-induced endothelial dysfunction, we assessed ACh-dependent relaxation in aortic rings from control and TCDD-treated CYP1A1 WT and KO mice. As reported previously (Kopf et al., 2008), TCDD attenuated ACh-dependent relaxation, which was normalized by preincubation of aortic rings with the superoxide dismutase mimetic, tempol (Figs. 4A and 4B). In contrast, TCDD failed to alter ACh-mediated relaxation in aortic rings from CYP1A1 KO mice but rather enhanced ACh-dependent contraction at low doses (Figs. 4C and 4D).
FIG. 4.
Comparison of ACh-dependent relaxation of aortic rings between CYP1A1 WT and KO mice treated with control or TCDD (180 ng/kg/day, 5 days/week) for 35 days. ACh-dependent relaxation in vessels preconstricted with phenylephrine (PE) prior to (A, CYP1A1 WT; C, CYP1A1 KO) or after incubation with 10μM tempol (B, CYP1A1 WT; D, CYP1A1 KO). Two-way repeated measures ANOVA demonstrated significant differences based on treatment and ACh concentration. *p < 0.05 compared with corresponding control group; n = 6–7 per group.
TCDD Induction of Hypertension
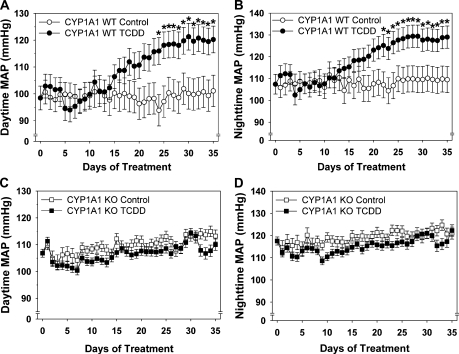
To determine if CYP1A1 also mediated TCDD-induced hypertension, we measured blood pressure by radiotelemetry from control and TCDD-treated CYP1A1 WT and KO mice. TCDD steadily increased both daytime and nighttime mean arterial blood pressure (MAP) in CYP1A1 WT mice beginning after 15 days, which plateaued at +20 mmHg after 25 days (Figs. 5A and 5B). Although CYP1A1 KO mice had a slightly higher baseline MAP (24 h MAP; KO: 109 ± 2.2 mmHg; WT: 101 ± 1.2; p < 0.05), TCDD failed to alter blood pressure in CYP1A1 KO mice over the 35-day exposure period (Figs. 5C and 5D).
FIG. 5.
Comparison of daytime and nighttime MAP between CYP1A1 WT and KO mice treated with control or TCDD (180 ng/kg/day, 5 days/week) for 35 days. Blood pressure radiotelemeters were implanted 14 days prior to treatment. MAP was measured for 7 days prior to treatment and continuously during 35-day treatment. Daytime and nighttime MAP from CYP1A1 WT mice (A and B) and CYP1A1 KO mice (C and D). Two-way repeated measures ANOVA demonstrated significant differences based on time and treatment. *p < 0.05 compared with CYP1A1 control group; n = 4–5 per group.
TCDD-Induced Activation of Renin-Angiotensin System
Because oxidative stress can stimulate renin expression (Itani et al., 2009) and an activated renin-angiotensin system (RAS) can induce additional cardiovascular oxidative stress, endothelial dysfunction, and hypertension, we further assessed whether TCDD exposure of CYP1A1 WT mice led to activation of the RAS. We found that plasma angiotensin II, ACE activity, and PRA were the same between control and TCDD-treated CYP1A1 WT mice (Table 2).
TABLE 2.
Plasma Angiotensin II Concentration, ACE Activity, and PRA in CYP1A1 WT Mice Treated with Control or TCDD for 35 Days
| CYP1A1 WT | ||
| Control (n = 8) | TCDD (n = 6–8) | |
| Ang II (pg/ml) | 30.9 ± 4.4 | 28.5 ± 4.4 |
| ACE (U/l) | 207.0 ± 4.8 | 205.0 ± 4.2 |
| PRA (ng Ang I/ml/h) | 3.29 ± 0.15 | 3.43 ± 0.16 |
DISCUSSION
CYP1A1 is one of the most highly induced genes in the liver and extrahepatic tissues of experimental animal models following AHR activation; however, its contribution to toxic end points other than cancer has been investigated in only a few instances. CYP1A1 KO mice are resistant to overt toxicity and mortality induced by a single high dose of TCDD, but no cardiovascular end points were investigated (Uno et al., 2004). Although CYP1A antisense morpholinos protected against TCDD-induced vascular dysfunction in zebrafish embryos in one study (Teraoka et al., 2003), they were not protective in another (Carney et al., 2004). Thus, our results establish for the first time that induction of CYP1A1 is an essential mediator of cardiovascular toxicity resulting from chronic dietary TCDD exposure.
The mechanism by which CYP1A1 mediates this cardiovascular toxicity remains to be elucidated; however, induction of CYP1A1 in the endothelium of both conduit and resistance vessels suggests that the vascular endothelium may be a target. Other studies have shown that CYP1A1 is highly induced in vascular endothelium (Garrick et al., 2005; Guiney et al., 1997; Schlezinger and Stegeman, 2000; Smolowitz et al., 1991; Stegeman et al., 1991) and that exposure to TCDD or other TCDD-like HAHs significantly impacts vascular structure and function (Jokinen et al., 2003; Toborek et al., 1995). The idea that the vascular endothelium is a target is further supported by our observations that TCDD induces endothelial dysfunction and that CYP1A1 induction is required to mediate this effect. It is notable that physiological levels of vascular shear stress induce endothelial expression of CYP1A1 in a manner consistent with an anti-atherogenic phenotype (Conway et al., 2009). Thus, although physiological mechanisms that induce endothelial CYP1A1 may be vasculoprotective, our data demonstrate that sustained xenobiotic-mediated induction is injurious.
One mechanism that could link sustained CYP1A1 induction to endothelial dysfunction is the production of superoxide anion. Superoxide anion can inactivate the vasodilator, nitric oxide, producing peroxynitrite and reducing endothelial-dependent vasodilation, and in our experiments, the endothelial dysfunction was normalized by the superoxide dismutase mimetic and antioxidant, tempol. Our results show that CYP1A1 induction is required to mediate TCDD-induced increases in superoxide anion in cardiovascular tissues, including aorta, which is consistent with our previous observations that CYP1A1 is required for TCDD-induced increases in superoxide anion in aortic endothelial cells in culture (Kopf and Walker, 2010). CYP1A1 itself could be the source of the superoxide anion via NADPH-dependent enzymatic uncoupling as has been shown in other models (Schlezinger et al., 2006; Shertzer et al., 2004; Zangar et al., 2004). It is also possible that CYP1A1 induction leads to the production of arachidonic acid hydroperoxides, which can lead to the subsequent release of superoxide anion or that the hypertension itself leads to increased vascular ROS. Thus, the specific cause-and-effect mechanism by which CYP1A1 mediates TCDD-induced superoxide anion remains to be determined.
It is interesting that although TCDD-induced superoxide anion production in the aorta, heart, and kidney was CYP1A1 dependent, TCDD induction of H2O2 in the aorta was not. Because this is not consistent with our observations in cultured endothelial cells (Kopf and Walker, 2010), it suggests that the source of H2O2 in the aorta is not CYP1A1 and may not be from the endothelium. It has been shown that AHR agonists can also induce gene targets within mouse aortic vascular smooth muscle cells (Karyala et al., 2004) and TCDD-induced changes in gene expression can differ significantly between intact aorta and cultured cells (Puga et al., 2004). Thus, although unexpected, it is not unreasonable that the source of TCDD-induced H2O2 in the aorta in vivo is not CYP1A1 and it is plausible that the source is not from the endothelium. It is possible that the enhanced CYP1B1 expression in the TCDD-exposed CYP1A1 KO mice could contribute to the increase in H2O2. The tissue in which we observed the increase in CYP1B1 mRNA expression (heart) does not correspond to tissue in which we measured an increase in H2O2 (aorta). Nonetheless, differences in CYP1B1 protein expression as well as the antioxidant capacity of the respective tissues could account for this apparent discrepancy. Future studies will be needed to delineate the specific sources of cardiovascular ROS and the mechanisms by which CYP1A1 mediates the increase in superoxide anion.
In addition to being required to mediate TCDD-induced superoxide anion and endothelial dysfunction, CYP1A1 was also required for TCDD-induced hypertension. Increased ROS and vascular dysfunction are causally associated with hypertension in experimental animal models, particularly those mediated by activation of the RAS (Didion et al., 2002). Although our data suggest that TCDD does not activate the RAS, we cannot completely rule out the possibility that there is an increased activation of angiotensin II receptors. Future studies using angiotensin II receptor blockers and antioxidant therapy and evaluating vascular reactivity of resistance vessels are needed to confirm the degree to which ROS, RAS, and vascular dysfunction contribute to TCDD-induced hypertension.
Lastly, although CYP1A1 KO mice were resistant to TCDD-induced cardiovascular toxicity, they exhibited phenotypic characteristics that were distinct from WT mice. CYP1A1 KO mice were significantly smaller than their age-matched WT controls, and MAP of CYP1A1 KO mice was slightly elevated (+8 mmHg). Although there was no evidence that this mild increase in blood pressure resulted in hypertension-related organ damage, such as cardiac hypertrophy, cardiovascular ROS, or vascular dysfunction, the increased MAP could contribute to an impairment of growth rate. The other notable difference between the CYP1A1 WT and KO mice was that TCDD-treated CYP1A1 KO mice exhibited an enhanced response to ACh-mediated vasocontraction at low doses. This suggests that TCDD may increase an endothelium-dependent contracting factor in CYP1A1 KO mice. One potential candidate would be cyclooxygenase 2, which is inducible by AHR activation (Kraemer et al., 1996) and which can increase the metabolism of arachidonic acid to prostanoids that mediate vasocontraction. Future studies are needed to determine whether cyclooxygenase 2 plays a role in TCDD-induced vascular dysfunction.
As noted earlier, epidemiology studies have linked exposure to TCDD and TCDD-like HAHs to hypertension in humans and these associations have been noted for both high exposure levels as well as to current background exposure levels (Everett et al., 2008a; Kang et al., 2006; Uemura et al., 2009). To compare our data with human exposures, we followed a similar dosing protocol (5 days/week for 35 days) as reported by Diliberto et al. (2001) and used the measured liver concentration (6–7 ng/g) as an index of total body burden. Thus, we estimated that the final body burden of these mice was 600–700 ng/kg body weight. Although this body burden is 15–100 times higher than current exposure levels to TCDD and TCDD-like HAHs in the United States (Ferriby et al., 2007), it is within the range estimated for Vietnam veterans and other individuals with known accidental or occupational dioxin exposure (100–8000 ng/kg body weight) (DeVito et al., 1995; Emond et al., 2005). Thus, our study provides biological plausibility for the link between human hypertension and exposure to TCDD-like chemicals. Nonetheless, future studies will be needed to establish the sensitivity of the hypertension response to TCDD and the degree to which even low level exposure may increase the susceptibility to hypertension in the presence of other common risk factors.
In summary, our study provides experimental evidence that induction of CYP1A1 is a risk factor for vascular dysfunction and hypertension. Human exposure to AHR agonists that induce CYP1A1 is not limited to TCDD-like HAHs but also includes the polycyclic aromatic hydrocarbons found in tobacco smoke, particulate matter air pollution, and the diet. Thus, induction of CYP1A1 could represent an important risk factor for cardiovascular disease for a large number of individuals as a result of environmental pollutant exposure.
FUNDING
National Heart, Lung, and Blood Institute (HL078914) to M.K.W.
Acknowledgments
We thank Mary T. Walsh for her technical assistance and Dr Nancy Kanagy for her critical review of the manuscript.
References
- Air Force Health Study. An Epidemiological Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. 1997 Follow-up Examination and Results. Brooks AFB, TX: Armstrong Laboratory; 2005. [Google Scholar]
- Carney SA, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activation of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator pathway causes developmental toxicity through a CYP1A-independent mechanism in zebrafish. Mol. Pharmacol. 2004;66:512–521. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc. Res. 2009;81:669–677. doi: 10.1093/cvr/cvn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, Nebert DW. Targeted knockout of CYP1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem. Biophys. Res. Commun. 2000;267:184–189. doi: 10.1006/bbrc.1999.1913. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, Shertzer HG, Nebert DW, Puga A. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc. Toxicol. 2001;1:285–298. doi: 10.1385/ct:1:4:285. [DOI] [PubMed] [Google Scholar]
- DeVito MJ, Birnbaum LS, Farland WH, Gasiewicz TA. Comparisons of estimated human body burdens of dioxinlike chemicals and TCDD body burdens in experimentally exposed animals. Environ. Health Perspect. 1995;103:820–831. doi: 10.1289/ehp.95103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didion SP, Ryan MJ, Baumbach GL, Sigmund CD, Faraci FM. Superoxide contributes to vascular dysfunction in mice that express human renin and angiotensinogen. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1569–H76. doi: 10.1152/ajpheart.00079.2002. [DOI] [PubMed] [Google Scholar]
- Diliberto JJ, DeVito MJ, Ross DG, Birnbaum LS. Subchronic exposure of [3H]-2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female B6C3F1 mice: relationship of steady-state levels to disposition and metabolism. Toxicol. Sci. 2001;61:241–255. doi: 10.1093/toxsci/61.2.241. [DOI] [PubMed] [Google Scholar]
- Emond C, Michalek JE, Birnbaum LS, DeVito MJ. Comparison of the use of a physiologically based pharmacokinetic model and a classical pharmacokinetic model for dioxin exposure assessments. Environ. Health Perspect. 2005;113:1666–1668. doi: 10.1289/ehp.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett CJ, Mainous AG, 3rd, Frithsen IL, Player MS, Matheson EM. Association of polychlorinated biphenyls with hypertension in the 1999–2002 National Health and Nutrition Examination Survey. Environ. Res. 2008a;108:94–97. doi: 10.1016/j.envres.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Mainous AG, 3rd, Frithsen IL, Player MS, Matheson EM. Commentary on the association of polychlorinated biphenyls with hypertension. Environ. Res. 2008b;108:428–429. doi: 10.1016/j.envres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Ferriby LL, Knutsen JS, Harris M, Unice KM, Scott P, Nony P, Haws LC, Paustenbach D. Evaluation of PCDD/F and dioxin-like PCB serum concentration data from the 2001–2002 National Health and Nutrition Examination Survey of the United States population. J. Expo. Sci. Environ. Epidemiol. 2007;17:358–371. doi: 10.1038/sj.jes.7500498. [DOI] [PubMed] [Google Scholar]
- Gambier N, Marteau JB, Batt AM, Marie B, Thompson A, Siest G, Foernzler D, Visvikis-Siest S. Interaction between CYP1A1 T3801C and AHR G1661A polymorphisms according to smoking status on blood pressure in the Stanislas cohort. J. Hypertens. 2006;24:2199–2205. doi: 10.1097/01.hjh.0000249697.26983.aa. [DOI] [PubMed] [Google Scholar]
- Garrick RA, Woodin BR, Stegeman JJ. Cytochrome p4501a induced differentially in endothelial cells cultured from different organs of Anguilla rostrata. In Vitro Cell. Dev. Biol. Anim. 2005;41:57–63. doi: 10.1290/0409063.1. [DOI] [PubMed] [Google Scholar]
- Guiney PD, Smolowitz RM, Peterson RE, Stegeman JJ. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in vascular endothelium with toxicity in early life stages of lake trout. Toxicol. Appl. Pharmacol. 1997;143:256–273. doi: 10.1006/taap.1996.8051. [DOI] [PubMed] [Google Scholar]
- Huwe JK, Smith DJ. Laboratory and on-farm studies on the bioaccumulation and elimination of dioxins from a contaminated mineral supplement fed to dairy cows. J. Agric. Food Chem. 2005;53:2362–2370. doi: 10.1021/jf0480997. [DOI] [PubMed] [Google Scholar]
- Itani H, Liu X, Sarsour EH, Goswami PC, Born E, Keen HL, Sigmund CD. Regulation of renin gene expression by oxidative stress. Hypertension. 2009;53:1070–1076. doi: 10.1161/HYPERTENSIONAHA.109.130633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen MP, Walker NJ, Brix AE, Sells DM, Haseman JK, Nyska A. Increase in cardiovascular pathology in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin and 3,3',4,4',5-pentachlorobiphenyl. Cardiovasc. Toxicol. 2003;3:299–310. doi: 10.1385/CT:3:4:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HK, Dalager NA, Needham LL, Patterson DG, Jr, Lees PS, Yates K, Matanoski GM. Health status of Army Chemical Corps Vietnam veterans who sprayed defoliant in Vietnam. Am. J. Ind. Med. 2006;49:875–884. doi: 10.1002/ajim.20385. [DOI] [PubMed] [Google Scholar]
- Karyala S, Guo J, Sartor M, Medvedovic M, Kann S, Puga A, Ryan P, Tomlinson CR. Different global gene expression profiles in benzo[a]pyrene- and dioxin-treated vascular smooth muscle cells of AHR-knockout and wild-type mice. Cardiovasc. Toxicol. 2004;4:47–73. doi: 10.1385/ct:4:1:47. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Huwe JK, Walker MK. Hypertension, cardiac hypertrophy, and impaired vascular relaxation induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are associated with increased superoxide. Cardiovasc. Toxicol. 2008;8:181–193. doi: 10.1007/s12012-008-9027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf PG, Walker MK. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol. Appl. Pharmacol. 2010;245:91–99. doi: 10.1016/j.taap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer SA, Arthur KA, Denison MS, Smith WL, DeWitt DL. Regulation of prostaglandin endoperoxide H synthase-2 expression by 2,3,7,8,-tetrachlorodibenzo-p-dioxin. Arch. Biochem. Biophys. 1996;330:319–328. doi: 10.1006/abbi.1996.0259. [DOI] [PubMed] [Google Scholar]
- Krushkal J, Ferrell R, Mockrin SC, Turner ST, Sing CF, Boerwinkle E. Genome-wide linkage analyses of systolic blood pressure using highly discordant siblings. Circulation. 1999;99:1407–1410. doi: 10.1161/01.cir.99.11.1407. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Porta M, Steffes M, Jacobs DR., Jr Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetologia. 2007;50:1841–1851. doi: 10.1007/s00125-007-0755-4. [DOI] [PubMed] [Google Scholar]
- Lind PM, Orberg J, Edlund UB, Sjoblom L, Lind L. The dioxin-like pollutant PCB 126 (3,3',4,4',5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol. Lett. 2004;150:293–299. doi: 10.1016/j.toxlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor (AhR) null mice is correlated with elevated angiotensin II, endothelin-1 and mean arterial blood pressure. Toxicol. Appl. Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- O'Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Rev. Environ. Health. 2008;23:167–202. doi: 10.1515/reveh.2008.23.3.167. [DOI] [PubMed] [Google Scholar]
- Puga A, Sartor MA, Huang MY, Kerzee JK, Wei YD, Tomlinson CR, Baxter CS, Medvedovic M. Gene expression profiles of mouse aorta and cultured vascular smooth muscle cells differ widely, yet show common responses to dioxin exposure. Cardiovasc. Toxicol. 2004;4:385–404. doi: 10.1385/ct:4:4:385. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Stegeman JJ. Dose and inducer-dependent induction of cytochrome P450 1A in endothelia of the eel, including in the swimbladder rete mirabile, a model microvascular structure. Drug Metab. Dispos. 2000;28:701–708. [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat. Toxicol. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp. Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shertzer HG, Clay CD, Genter MB, Chames MC, Schneider SN, Oakley GG, Nebert DW, Dalton TP. Uncoupling-mediated generation of reactive oxygen by halogenated aromatic hydrocarbons in mouse liver microsomes. Free Radic. Biol. Med. 2004;36:618–631. doi: 10.1016/j.freeradbiomed.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Smolowitz RM, Hahn ME, Stegeman JJ. Immunohistochemical localization of cytochrome P-450IA1 induced by 3,3',4,4'-tetrachlorobiphenyl and by 2,3,7,8-tetrachlorodibenzoafuran in liver and extrahepatic tissues of the teleost Stenotomus chrysops (scup) Drug Metab. Dispos. 1991;19:113–123. [PubMed] [Google Scholar]
- Stegeman JJ, Smolowitz RM, Hahn ME. Immunohistochemical localization of environmentally induced cytochrome P450IA1 in multiple organs of the marine teleost Stenotomus chrysops (Scup) Toxicol. Appl. Pharmacol. 1991;110:486–504. doi: 10.1016/0041-008x(91)90049-k. [DOI] [PubMed] [Google Scholar]
- Stoll M, Kwitek-Black AE, Cowley AW, Jr, Harris EL, Harrap SB, Krieger JE, Printz MP, Provoost AP, Sassard J, Jacob HJ. New target regions for human hypertension via comparative genomics. Genome Res. 2000;10:473–482. doi: 10.1101/gr.10.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem. Biophys. Res. Commun. 2003;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J. Biochem. Toxicol. 1995;10:219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sawachika F, Dakeshita S, Nii K, Satoh H, Sumiyoshi Y, et al. Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan's general population. Environ. Health. Perspect. 2009;117:568–573. doi: 10.1289/ehp.0800012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Sinclair PR, Gorman N, Wang B, Smith AG, Miller ML, Shertzer HG, Nebert DW. Cyp1a1(-/-) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol. Appl. Pharmacol. 2004;196:410–421. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Walker MK, Pollenz RS, Smith SM. Expression of the aryl hydrocarbon receptor (AhR) and AhR nuclear translocator during chick cardiogenesis is consistent with 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced heart defects. Toxicol. Appl. Pharmacol. 1997;143:407–419. doi: 10.1006/taap.1996.8068. [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol. Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 2004;199:316–331. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]