Abstract
Pb (lead) exposure and stress are co-occurring risk factors (particularly in low socioeconomic communities) that also act on common biological substrates and produce common adverse outcomes, including cognitive impairments. This study sought to determine whether lifetime Pb exposure combined with prenatal stress would enhance the cognitive deficits independently associated with each of these risk factors and to explore associated mechanisms of any observed impairments. Learning was evaluated using a multiple schedule of repeated learning and performance in female rats subjected to lifetime Pb exposure (0 or 50 ppm Pb in drinking water beginning in dams 2 months prior to breeding; blood Pb levels ∼10 μg/dl), to prenatal restraint stress on gestational days 16 and 17, or to both. Blood Pb, corticosterone levels, brain monoamines, and hippocampal nerve growth factor levels were also measured. Sequence-specific learning deficits produced by Pb, particularly the number of responses to correctly learn response sequences, were further enhanced by stress, whereas performance measures were unimpaired. Statistical analyses indicated significant relationships among corticosterone levels, frontal cortex dopamine (DA), nucleus accumbens dopamine turnover, and total responses required to learn sequences. This study demonstrates that Pb and stress can act together to produce selective and highly condition-dependent deficits in learning in female rats that may be related to glucocorticoid-mediated interactions with mesocorticolimbic regions of brain. These findings also underscore the critical need to evaluate toxicants in the context of other risk factors pertinent to human diseases and disorders.
Keywords: lead, prenatal stress, learning, monoamines, corticosterone, nerve growth factor
Adverse impacts of elevated blood lead (PbB) status on children's IQ have been reported at increasingly lower exposure levels. Recently, reductions of 7.4 IQ points were observed as PbB levels increased from 1 to 10 μg/dl, a reduction actually greater than that observed at PbB levels > 10 μg/dl (Canfield et al., 2003). A study of this same cohort designed to elaborate the specific behavioral domains impacted by Pb revealed marked impairments in the intradimensional-extradimensional set shift task (Cambridge Cognition), a measure of discrimination learning, reversal learning, and set shifting (Owen et al., 1991). We reported reversal learning deficits in a rat model of Pb exposure using a multiple schedule of repeated learning (RL) and performance (P) (Cohn et al., 1993).
Importantly, however, exposure to lead (Pb) does not occur in isolation but concurrently with numerous other risk factors that can also influence cognitive outcomes, including genetics, host risk factors, and other environmental chemical exposures. One such risk factor is stress, which, like Pb exposure, can occur at any period throughout the life span. Indeed, Pb and stress are co-occurring risk factors, particularly for low socioeconomic status communities. These communities have a higher percentage of children with elevated PbB levels and also sustain disproportionately higher incidences of many diseases and disorders, a phenomenon that has been hypothesized to arise from higher stress levels and chronically elevated stress hormones (Jones et al., 2009; Lupien et al., 2001). The consequences of prenatal stress (PS) in particular appear to have enduring consequences, including, as with Pb, deficits in cognitive functions, as reported both in human studies and animal models (King and Laplante, 2005; Laplante et al., 2004; Lordi et al., 2000).
The commonality of the cognitive deficits associated with Pb and stress may relate to the fact that they also share common biological substrates. Both Pb and stress act on the hypothalamic-pituitary-adrenal (HPA) axis (de Kloet et al., 1998; Rossi-George et al., 2009). In fact, we have demonstrated that maternal Pb exposure, like PS, induces a permanent change in HPA axis function including sustained hypercortisolism and altered glucocorticoid negative feedback (Cory-Slechta et al., 2004; Rossi-George et al., 2009; Virgolini et al., 2008). Both Pb and stress also interact extensively with brain dopamine (DA) and glutamate systems in regions such as nucleus accumbens and hippocampus that are important in the mediation of cognitive behaviors (Barrot et al., 2000; Cory-Slechta et al., 1999; Diorio et al., 1993; Piazza et al., 1996).
Given their co-occurrence as risk factors, common biological substrates and corresponding adverse outcomes, the current study was premised on the hypothesis that combined Pb exposure and PS might enhance the cognitive deficits produced by either alone. To evaluate this assertion, RL and associated neurochemical and biochemical measures were evaluated in female rats that had been exposed to lifetime Pb exposure, a model analogous to human environmental Pb exposure, to PS, or to the combination. This report focuses on outcomes observed in females; findings from male littermates will be reported in conjunction with those from males in another study involving exposures to maternal Pb and stress.
MATERIALS AND METHODS
Dams and Pb Exposure
Three-week-old female Long Evans rats (Charles River, Germantown, NY) were randomly assigned to receive distilled deionized water drinking solutions containing 0 or 50 ppm Pb acetate and housed in same Pb treatment condition pairs. Pb exposure was initiated 2 months prior to breeding to ensure elevated bone Pb levels and Pb body burden at the time of conception, and was continued throughout lactation, consistent with human exposure. When females reached 3 months of age, they were paired with 3-months old male Long-Evans rats for breeding. Animals were housed in a vivarium room maintained at 22°C ± 5°C with a 12-h light-dark cycle (lights on at 0700 h). Standard rat chow diet was provided ad libitum. All experiments were carried out according to NIH Guidelines and were approved by the University of Rochester Medical School University Committee on Animal Resources.
Breeding and PS
At proestrus, as determined by vaginal smears, female rats were mated with males (2:1) across two estrous cycles. The presence of vaginal plugs or sperm was considered indicative of pregnancy and deemed gestational day (GD) 1. Pregnant females in the control and Pb-treated group were weighed and further randomly subdivided to a nonstress (NS) or PS condition. Beginning on GD1, all pregnant females were individually housed for the remainder of pregnancy and lactation.
On GD16 and GD17, timed to correspond to the development of key brain regions (hypothalamic nuclei, hippocampus, striatum, frontal cortex) associated with the end points under study (Diaz et al., 1997; Yi et al., 1994), dams assigned to PS groups were weighed and subjected to a widely employed restraint stress procedure consisting of three 45-min restraint sessions (0900, 1200, and 1500 h) in plastic cylindrical devices (Ward and Weisz, 1984), a protocol we verified to elevate corticosterone levels and alter catecholamine levels in frontal cortex and nucleus accumbens of dams (Cory-Slechta et al., 2004). NS dams were weighed and subsequently left undisturbed in their home cages. This resulted in four Pb-stress conditions with the following numbers of litters: 0–NS (n = 12), 0–PS (n = 9), 50–NS (n = 8), and 50–PS (n = 8).
Offspring Procedures
Prebehavioral testing.
At delivery (postnatal day 1 [PND1]), litter size was recorded and number of pups culled to eight per litter, maintaining equal numbers of males and females wherever possible. Pups were weighed and weaned at PND21, separated by gender, and littermates of the same gender were housed as pairs.
From weaning, pups were provided with unrestricted access to the same standard rat chow and to the same Pb drinking solutions that their dam had received. At 2 months of age, behavioral testing was initiated in a subset of females from each treatment group, using a single offspring per litter. A subset of additional female pups from these litters were maintained as nonbehaviorally tested “extra” offspring.
With the initiation of behavioral testing at 2 months of age, offspring were provided with sufficient food to allow a 2- to 3-g weight gain per day until reaching approximately 220 g. At this point, caloric intake was regulated for the duration of the experiment to maintain this weight as required for food-motivated behavioral performance. Because animals were pair housed, individual feeding was accomplished by separating the residents through the introduction of a cage divider at the time of feeding which remained in place for approximately 90–120 min.
Behavioral testing.
Behavioral test sessions were carried out Mon-Fri between 1000 and 1600 h in rat operant chambers (ENV-008; Med Associates, St Albans, VT) housed in sound-attenuated enclosures ventilated by a fan, equipped with a grid floor, speaker, house light, and three standard (nonretractable) response levers (L, left; C, center; R, right) configured horizontally on the front panel. Reinforcement consisted of the delivery of a 45-mg food pellet (Bioserv, Frenchtown, NJ) and behavioral contingencies, and data were controlled by SoftCtrl Cumulative Record interface and Med-PC Version IV Research Control and Data Acquisition software.
Assessment of learning relied on performance under a multiple schedule comprised of RL and P components, a paradigm we have previously shown to be sensitive to postweaning Pb exposure in male rats (Cohn et al., 1993). In the RL components, the subject was required to learn a sequence of three lever press responses, with each correct sequence resulting in food reinforcement; the correct three response sequence changed with each successive experimental session. In the P components, the subject was required to complete a sequence of three responses, but the specific sequence was constant across sessions (see below).
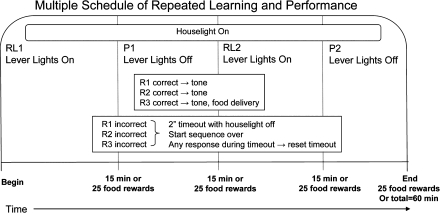
Assessment of behavior under the multiple schedule was preceded by lever press training (one session on fixed ratio 1 to 100 reinforcers carried out separately for each lever) followed by two- and then three-response sequence RL training, as previously detailed (Cohn et al., 1993). Advancement from two- to three-response sequence training occurred after 22 behavioral test sessions and from three-response sequence training to the multiple schedule after 23 behavioral test sessions. Subsequently, the multiple schedule was implemented (Fig. 1), where each behavioral test session was comprised of two presentations each of the RL and P components in the order RL1, P1, RL2, and P2, with each component lasting for 15 min or until 25 reinforcer deliveries, whichever occurred first. Different discriminative stimuli (lights above the three levers) were used to signal whether the RL or P component of the multiple schedule was currently in effect on the multiple schedule.
FIG. 1.
Schematic depicting the reinforcement contingencies of the multiple RL and P behavioral test paradigm.
Sequences used for the three-response RL training included CRL, RCL, CLR, RLC, LRC, and LCR; sequences with duplicative responses, for example, LLR, were excluded to minimize reinforcement of perseverative responding. Based on the high accuracy levels, it sustained during three-response sequence training (Fig. 3), the sequence LCR was assigned to be the correct sequence for the P components on the multiple schedule, and the remaining five sequences were used for the RL components, with each of these sequences occurring every fifth behavioral test session. Any error during the completion of the sequence in either the RL or P components initiated a 2-s time-out and turned off the houselight. Responses during the timeout extended the timeout until 2 s had elapsed without a response. Subjects were then required to begin the sequence anew. All correct responses produced a tone. Correct completion of a sequence resulted in food delivery, a tone stimulus, and the auditory click of the pellet delivery device.
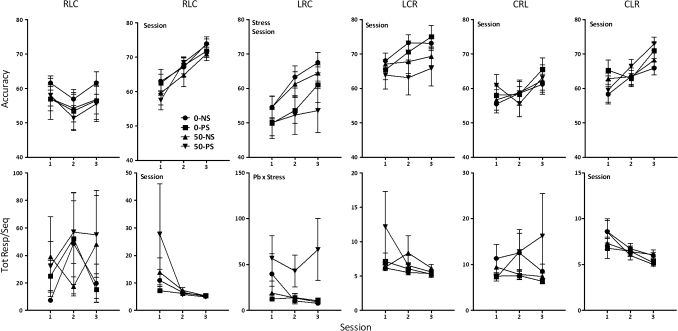
FIG. 3.
Group mean ± SE levels of accuracy (top row) and total responses required per completed sequence (bottom row) for groups (0–NS = 12, 0–PS = 9, 50–NS = 8, 50–PS = 8) as indicated for each of the six sequences used in the three-lever repeated learning (RL) training sessions. Pb = main effect of Pb, stress = main effect of stress, session = main effect of session, and Pb × stress = interaction of Pb by stress in statistical analysis.
Performance measures.
Measures included accuracy (correct responses divided by total responses), response rate (responses/time), total responses per completed sequence (total correct and incorrect responses/total completed sequences), session length (total session time), reinforcement density (total reinforcers/total responses), and total RL reinforcers (number of reinforcers earned in RL1 and RL2).
Blood Pb Determinations
Blood was collected from tail nicks in a subset of dams at three time points prior to the initiation of breeding (n = 10 at 3 weeks, n = 5 at 2 weeks, and n = 5 at 1 week prior to breeding) and at weaning (n = 2). Trunk blood was collected from offspring sacrificed at 5–6 days of age (n = 3 and 6, NS and PS, respectively) and from tail nicks in non-behaviorally tested female offspring at 2.5 months of age (n = 4 each, NS and PS offspring) and in behaviorally-tested offspring at the completion of behavioral testing, at approximately 10 months of age (0–NS = 12, 0–PS = 9, 50–NS = 8, and 50–PS = 8). Blood Pb was analyzed by anodic stripping voltammetry using the Lead Care II system with a detection limit of 3.3 μg/dl. All 0–NS and 0–PS values were below detection limits.
Corticosterone Determinations
For determination of corticosterone levels, trunk blood was collected from pups sacrificed at 5–6 days of age (0–NS = 7, 0–PS = 3, 50–NS = 7, 50–PS = 6) and by tail nicks from all behaviorally-tested offspring at approximately 4 months of age and again at 9 months of age (0–NS = 12, 0–PS = 9, 50–NS = 8, and 50–PS = 8) and from a subset of non-behaviorally tested female littermates at 10–11 months of age (0–NS = 5, 0–PS = 4, 50–NS = 3, and 50–PS = 2). Approximately 200 μl of blood was collected into pre-chilled tubes and centrifuged at 1685 × g for 10 min, after which serum corticosterone was measured using ImmuChem Double Antibody Corticosterone 125I kit according to the manufacturer's instructions (MP Biomedicals, Orangeburg, NY). All standards and samples were run in duplicate. The minimum detectable level in this assay was 7.7 ng/ml (Rossi-George et al., 2009).
Nerve Growth Factor Determinations
Hippocampal tissue were homogenized in ice-cold homogenate buffer (0.1M Tris-HCl, pH 7.0; 1M NaCl; 4mM EDTA; 2% Triton X-100) and protease inhibitors and centrifuged at 14,000 × g for 30 min. Nerve growth factor (NGF) levels were then determined using an enzyme-linked immunoabsorbent assay (ChemiKine Nerve growth Factor Sandwich ELISA Kit; Chemicon International, Billerica, MA) according to the specified protocols and optical density absorbance was read at 450 nm.
Neurochemical Determinations of Monoamines
Levels of DA, dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), norepinephrine (NE), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) from frontal cortex, nucleus accumbens, striatum, midbrain (ventral tegmental area and substantia nigra), and hypothalamus were analyzed using high performance liquid chromatography with electrochemical detection as previously detailed (Cory-Slechta et al., 2004; Virgolini et al., 2008). Concentrations of neurotransmitters were expressed as nanogram per milligram protein. Dopamine turnover (DA TO) was calculated as the DOPAC/DA ratio.
Statistical Analysis
PbB levels.
Because all 0–NS and 0–PS PbB levels were below detection limits, only values from 50–NS and 50–PS groups were analyzed. Blood Pb levels of dams were analyzed using ANOVA with time point (three time points pre-breeding and weaning) as a between-group factor. Blood Pb values of offspring were analyzed using ANOVA with stress (NS, PS) and time point as between-group factors. Post hoc tests were carried out using Fisher’s protected least significant difference (PLSD) tests as appropriate.
Corticosterone.
Corticosterone levels were analyzed using ANOVA with Pb, stress, and time point as between-group factors. When significant main effects or interactions involving time point were found, subsequent ANOVAs were undertaken separately for each time point followed by post hoc tests using Fisher’s PLSD tests as appropriate.
NGF.
Relative intensity values were analyzed using ANOVA with Pb and stress as between-group factors, and subsequent post hoc assessments were carried out using Fisher’s PLSD tests as appropriate.
Neurotransmitter levels.
Changes in neurotransmitter levels were analyzed separately for each neurotransmitter in each region using ANOVA with Pb and stress as between-group factors, and subsequent post hoc tests were carried out using Fisher’s PLSD tests as appropriate.
RL and P behavior.
Changes in accuracy and total responses per completed sequence during the three-response RL training program were first analyzed for sequence differences using repeated-measures analysis of variance (RMANOVA) that included Pb, stress, and sequence as between-groups factors and session as a within-group factor. Based on the confirmed sequence effects, subsequent RMANOVAs including Pb, stress, and sessions were carried out separately for each sequence.
Assessment of behavior under the multiple schedule for sequence LRC utilized RMANOVAs with sessions as a within-group factor and Pb and stress as between-group factors. In the event of main effects or interactions, subsequent ANOVAs or Fisher’s PLSD post hoc analyses were carried out as appropriate.
Relationships among neurochemical, biochemical, and behavioral outcomes.
To explore potential mechanistic relationships among behavioral, neurochemical, and biochemical end points, a correlation Z-test matrix was first generated using nucleus accumbens DA TO (based on significant Pb × stress interactions), frontal cortex DA (that showed a similar albeit nonsignificant [p = 0.10] pattern of Pb-stress interactions), 9-month corticosterone values (final values of behaviorally tested offspring), and NGF levels as independent variables, with the maximal value for total responses per completed sequence seen across all RL lever press sequences used for each individual subject (based on the observations that different Pb ± stress treated subjects exhibited notable impairments on different sequences) as the dependent variable. Subsequently, a forward stepwise regression analyses that included these variables was carried out that required an F of 4.00 to enter an independent variable and F = 3.996 to remove an independent variable from the final model.
In all analyses, post hoc analyses were based on significant main effects or interactions, and significance was defined as a p value of ≤ 0.05.
RESULTS
PbB Levels
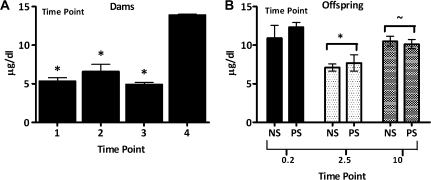
PbB levels of dams (Fig. 2A; time: F = 23.26, p < 0.0001) averaged 5–7 μg/dl prior to breeding but had increased to 13 μg/dl by the end of weaning. Offspring PbB levels (Fig. 2B; group: F = 9.26, p = 0.0006) were 10–13 μg/dl at 0.2 months, consistent with dam values at weaning, declined to 7 μg/dl (p < 0.0001) at 2.5 months but had increased again to approximately 10 μg/dl at 10 months, values higher than those at the 2.5-month time point but marginally lower than those at 0.2 months (2.5 vs. 10: p = 0.0001; 10 vs. 0.2: p = 0.0553). PbBs did not differ between NS and PS conditions.
FIG. 2.
(A) Mean ± SE PbB levels (μg/dl) of dams over three successive weeks prior to breeding (time points 1–3: n = 10 at 3 weeks, n = 5 at 2 weeks, and n = 5 at 1 week prior to breeding) and at weaning (time point 4: n = 2). “*” Significantly different from time point 4. (B) PbB levels (μg/dl) of NS and PS female offspring measured at PND5–6 (n = 3 and 6, respectively), at 2.5 months of age in nonbehaviorally tested offspring (EXTRA: n = 4 for both NS and PS) and in behaviorally tested offspring at the completion of testing (10–11 months of age; 0–NS = 12, 0–PS = 9, 50–NS = 8, 50–PS = 8). “*” Significantly different from time point 0.2 and 10 months; “∼,” marginally different from time point 0.2 months.
RL and Performance
Three-response training.
As expected (Cohn et al., 1993), sequence differences in accuracy levels, that is, in sequence difficulty (Fig. 3, top) were notable (F = 14.93, p < 0.0001); values of 0–NS controls on the first session ranged from 55–68% across the six sequences (from low to high on session 1: LRC = 54.5%, CRL = 55.3%, CLR = 58.3%, RLC = 61.6%, RCL = 63%, LCR = 68%). Subsequent analyses by sequence revealed stress-induced reductions in LRC accuracy (F = 5.59, p = 0.024), with 0–PS values 10% lower and 50–PS values 21% lower than 0–NS controls in the final training session. Additionally, a Pb × stress interaction occurred for total responses required for completed LRC sequences (Fig. 3, bottom, F = 5.97, p = 0.0197), with approximately eightfold higher values of the 50–PS group relative to all other groups (p = 0.011, 0.007, and 0.006 for 50–PS vs. 0–NS, 0–PS, and 50–NS, respectively).
Multiple schedule.
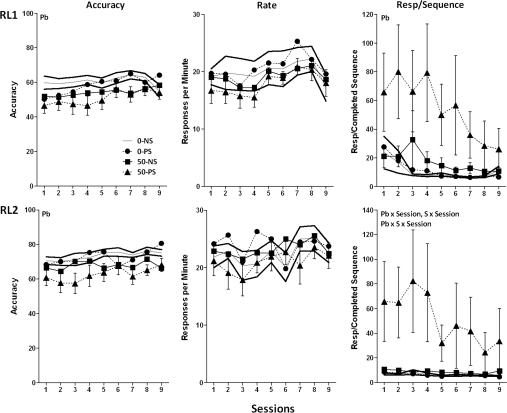
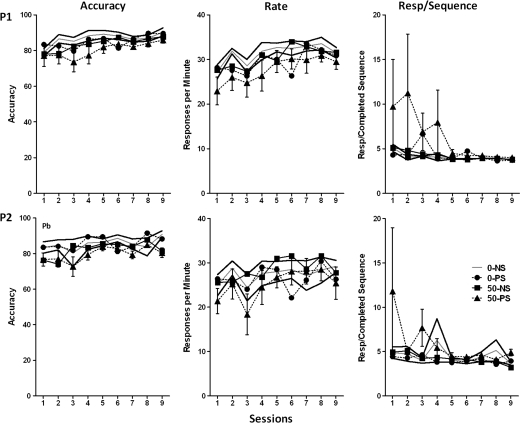
The five different sequences used in the RL component were completed at different accuracy levels across testing under the multiple schedule (main effect of sequence at final presentation [multiple schedule test sessions 42–46], F = 8.63, p < 0.0001; data not shown), with the same rank order in 0–NS controls as during training (LRC = 55.1%, CRL = 58.8%, CLR = 61.6%, RLC = 65.8%, RCL = 77.3%). Consequently, performance under the multiple schedule was analyzed separately by sequence. Again, performance under the sequence LRC exhibited statistically significant Pb ± stress-related differences (Fig. 4). Specifically, modest Pb-induced reductions in accuracy were observed in both RL1 and RL2 (F = 4.63, p = 0.038 and F = 8.89, p = 0.0052, respectively). A marked Pb-related increase in total responses per completed sequence was observed in RL1 (F = 5.06, p = 0.0031) with increases most evident in the 50–PS group. A corresponding effect in RL2 arose selectively from the markedly higher values of the 50–PS group relative to all other groups (F = 2.32, p = 0.02; post hoc 50–PS vs. 0–NS, 0–PS, and 50–NS = 0.0135, 0.0245, and 0.024, respectively). No response rates differences were seen in either RL1 or RL2.
FIG. 4.
Group mean ± SE levels of accuracy (left), response rate (middle), and total responses per completed sequence (right) in the RL1 (top) and RL2 (bottom) components of the multiple schedule of RL and performance across sessions using the sequence LRC (0–NS = 12, 0–PS = 9, 50–NS = 8, 50–PS = 8). Solid gray and black lines represent 0–NS control group mean and SE values, respectively. Pb = main effect of Pb, S = main effect of stress, session = main effect of session, Pb × S = interaction of Pb with stress; Pb × S × session = interaction of Pb by stress with session in the statistical analysis.
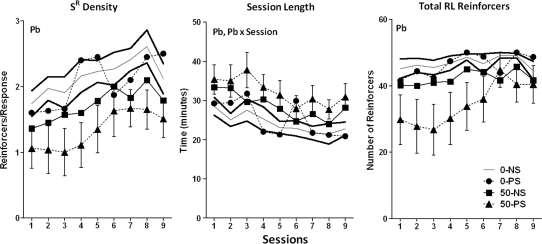
Consistent with the effects of Pb ± stress on the RL end points, Pb reduced reinforcement density (Fig. 5; F = 7.16, p = 00113) across sessions by 11–26% in the 50–NS group and by 28–48% in the 50–PS group. Similarly Pb reduced the total number of reinforcers earned during the RL components (F = 6.87, p = 0.0129) by 5–15% and 9–41%, respectively, again, effects that appear to predominate in the 50–PS group although not resulting in a Pb × stress interaction. In addition, Pb increased session length (F = 6.24, p = 0.0174; 108–133% and 122–140% for the 50–NS and 50–PS groups, respectively).
FIG. 5.
Group mean ± SE levels of reinforcement density, total session length, and total RL reinforcers on the multiple schedule of RL and performance across sessions requiring learning of the sequence LRC (0–NS = 12, 0–PS = 9, 50–NS = 8, 50–PS = 8). Solid gray and black lines represent 0–NS control group mean and SE values, respectively. Pb = main effect of Pb, Pb × session = interaction of Pb with session in the statistical analysis.
As expected, performance during the P1 and P2 was characterized by higher levels of accuracy, lower numbers of responses required for completed LRC sequences, and higher response rates than seen in the RL1 and RL2 components (Fig. 6). The only treatment effect consisted of a modest Pb-related reduction of 1–10% and 3–11% in P2 accuracy (F = 4.14, p = 0.0495) in the 50–NS and 50–PS groups, respectively; these effects had disappeared after six LRC behavioral test sessions.
FIG. 6.
Group mean ± SE levels of accuracy (left), response rate (middle), and total responses per completed sequence (right) during the P1 (top row) and P2 (bottom) components of the multiple schedule of RL and performance across sessions using LRC in the RL components of the session (0–NS = 12, 0–PS = 9, 50–NS = 8, 50–PS = 8). Solid gray and black lines represent 0–NS control group mean and SE values, respectively. Pb = main effect of Pb in statistical analyses.
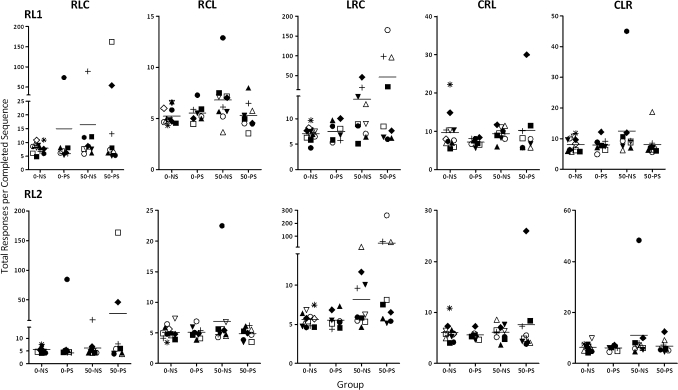
Although statistically significant Pb and stress effects were only detected for sequence LRC, there were notable outliers in the 0–PS, 50–NS, and 50–PS groups for other sequences as well relative to 0–NS distributions (Fig. 7). One 0–PS subject showed deviant performance for the sequence RLC. Among 50–NS subjects, four subjects showed performance well outside the control range for sequence LRC, but an additional subject showed marked impairments in the presence of sequences RCL and CLR. Among 50–PS subjects, four displayed notable deficits in the presence of the LRC sequence, but additional two subjects showed deficits when RLC (2) or CRL (1) served as the correct sequence. Thus, of the total subjects, 1 of 8 (13%) 0–PS subjects, 5 of 11 (45%; chi square = 17.6, p < 0.0001) 50–NS subjects, and 6 of 9 (67%; chi square = 28.13, p < 0.0001) 50–PS subjects exhibited behavioral deficits when considered across sequences.
FIG. 7.
Plots of median values for total responses per completed sequence calculated across sessions for individual subjects within each treatment group for each sequence used in the RL component of the multiple schedule. Each subject is identified by a unique symbol (0–NS = 12, 0–PS = 9, 50–NS = 8, 50–PS = 8). Dashed line designates the group mean value.
Brain Neurotransmitter Levels
Dopamine and metabolites.
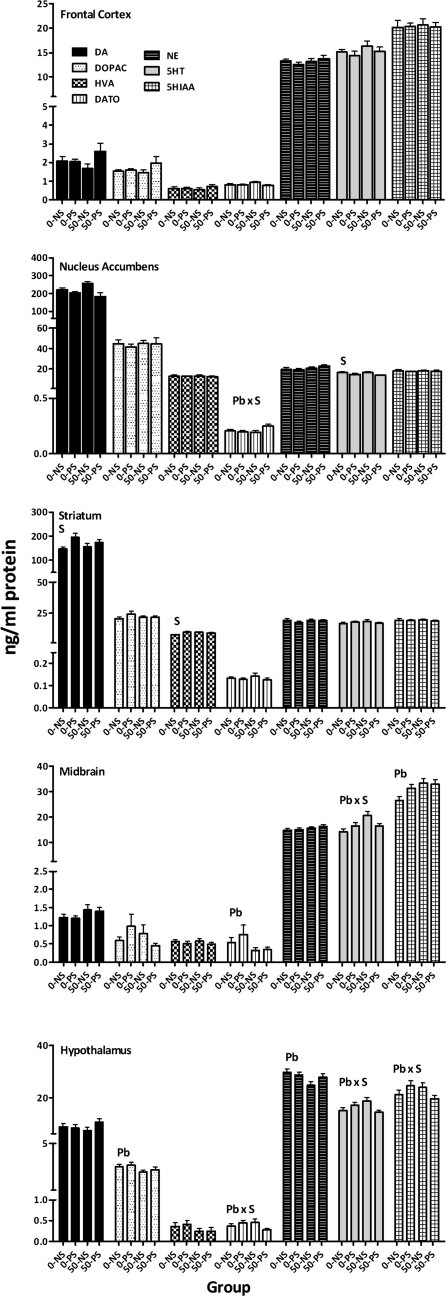
PS increased striatal DA by 19–34% (Fig. 8; F = 6.26, p = 0.017) and HVA by 18–28% (F = 8.86, p = 0.0048). Pb-induced changes included 36–42% reductions in DA TO in midbrain (F = 4.0, p = 0.052) and 10–16% reductions in hypothalamic DOPAC (F = 4.69, p = 0.036). Notably, nucleus accumbens DA TO was selectively increased by 21% in the 50–PS group (Pb × stress: F = 4.08, p = 0.0502, post hoc, p values for 50–PS vs. 0–NS, 0–PS, 50–NS = 0.0508, 0.026, and 0.018, respectively). 50–PS selective reductions in DA TO in hypothalamus (Pb × stress, F = 4.3, p = 0.044) also occurred, but only relative to the 0–PS (p = 0.04) and 50–NS groups (p = 0.0506).
FIG. 8.
Group mean ± SE levels of DA, DOPAC, HVA (ng/mg protein), DA TO (DOPAC/DA), NE, 5-HT, and HIAA in frontal cortex, nucleus accumbens, striatum, midbrain, and hypothalamus of behaviorally tested females at the completion of behavioral testing. Pb = main effect of Pb, S = main effect of stress, and Pb × stress = interaction of Pb with stress in statistical analysis.
Norepinephrine.
The only change observed was a Pb-related reduction in NE levels of 7–17% in hypothalamus (F = 5.33, p = 0.026).
Serotonin (5-HT) and metabolite (5-HIAA).
PS produced 11–17% reductions in nucleus accumbens 5-HT levels (F = 8.75, p = 0.005) and 24–25% increases in 5HIAA levels (F = 6.86, p = 0.012). In midbrain, a Pb × stress interaction for 5HT (F = 6.14, p = 0.017) resulted from a selective 44% increase in the 50–NS group (p = 0.0008, 0.026, and 0.026 for 50–NS vs. 0–NS, 0–PS, and 50–PS, respectively). A similar Pb × stress interaction for 5HT in hypothalamus (F = 6.18, p = 0.017) was based on a 25% increase in the 50–NS group relative to the 50–PS group (p = 0.028), whereas a marginal Pb × stress interaction for 5HIAA (F = 3.95, p = 0.0534) was based on higher levels in the 0–PS group relative to the 50–PS group (p = 0.049).
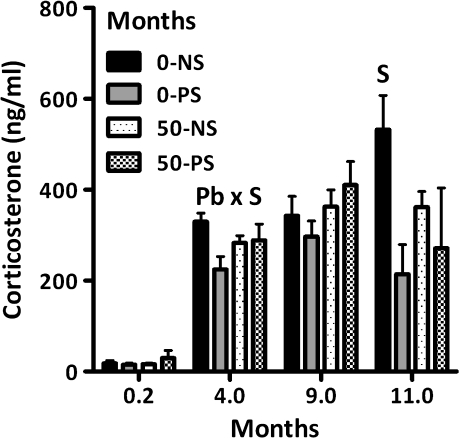
Corticosterone Levels
Corticosterone levels (Fig. 9), differed across age (F = 45.94, p < 0.0001). PND5–6 values (0.2 months) were lower than those at other times; corticosterone levels at 4 months of age were lower than those measured at 9 and at 11 months of age (all p values < 0.05). At PND5–6, no Pb or stress effects were detected. At 4 months, a Pb × stress interaction (F = 4.52, p = 0.039) reflected the significantly lower corticosterone levels of the 0–PS group relative to the 0–NS controls. At 11 months, 49–60% PS-associated reductions were observed (F = 6.30, p = 0.031).
FIG. 9.
Group mean ± SE corticosterone levels (ng/ml) of female offspring at PND5–6 (time point 0.2; 0–NS = 7, 0–PS = 3, 50–NS = 7, 50–PS = 6) in behaviorally tested offspring at 4 and 9 months of age (0–NS = 12, 0–PS = 9, 50–NS = 8, and 50–PS = 8) and in nonbehaviorally tested offspring at 11 months of age (0–NS = 5, 0–PS = 4, 50–NS = 3, and 50–PS = 2). S = main effect of stress and Pb × stress = interaction of Pb with stress in statistical analysis.
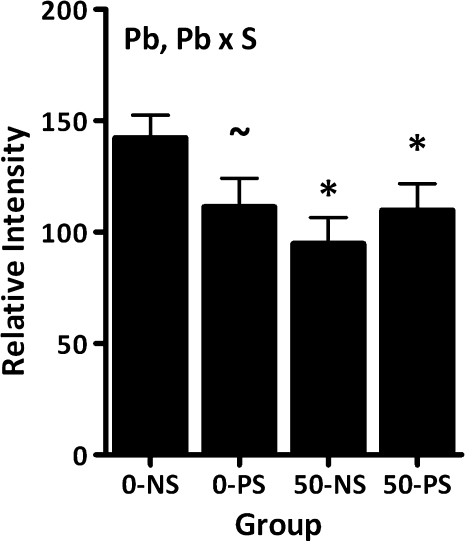
Hippocampal NGF
Reductions in hippocampal NGF levels were observed in all groups relative to 0–NS control (Fig. 10; main effect of Pb: F = 4.43, p = 0.0415; Pb × stress: F = 3.92, p = 0.054). Reductions of 22% in the 0–PS group were marginal (p = 0.062), whereas the 23–34% reductions in the 50–NS and 50–PS groups were significant relative to 0–NS control (p = 0.0068 and 0.0468, respectively).
FIG. 10.
Group mean ± SE levels of NGF in hippocampus of behaviorally tested females (0–NS = 12, 0–PS = 9, 50–NS = 8, 50–PS = 8). “*” Differs from 0–NS control; “∼,” marginally different from 0–NS control.
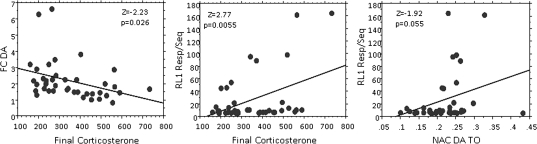
Relationships among Behavioral, Neurochemical, and Biochemical Outcomes
The Z score correlation matrix revealed a number of significant correlations (shown in Fig. 11) that included 9-month corticosterone levels with frontal cortical DA (Fig. 11, left: Z = −2.229, p = 0.0258, and with maximal total responses per completed sequence in RL1 (Fig. 11, center: Z = 2.774, p = 0.0055) and RL2 (data not shown; Z = −3.264, p = 0.0011) and of nucleus accumbens DA TO with maximal total responses per completed sequence in RL1 (Fig. 11, right: Z1.92, p = 0.0551). The critical role of 9-month corticosterone and of nucleus accumbens DA TO were further supported by a stepwise forward regression that included NGF, nucleus accumbens DA TO, frontal cortex DA, and 9-month corticosterone as independent variables and maximal RL1 total responses per completed sequence as the dependent variable. In a highly significant model (F = 8.023, p = 0.0014), the regression confirmed final corticosterone and nucleus accumbens DA TO as significant predictors of maximal RL1 total responses per completed sequence.
FIG. 11.
Simple linear regression analyses relating final corticosterone values of behaviorally tested females to frontal cortex DA values (left) and to the maximal total responses per completed sequence value to any sequence in RL1 (middle) and of nucleus accumbens DA TO to maximal total responses per completed sequence value to any sequence in RL1 (0–NS = 12, 0–PS = 9, 50–NS = 8, 50–PS = 8). Corresponding Z scores and p values are shown.
DISCUSSION
The current study presents evidence for enhanced impairments of learning in female offspring subjected to lifetime Pb exposure combined with PS in the form of marked increases in responses required to learn new sequences (Figs. 4 and 7). Statistically significant increases in total responses/completed sequence for a specific sequence, LRC, were observed in the 50–PS group relative to other groups during both three-response training and under multiple schedule conditions, with 8-fold increases observed in the latter. The performance under the LRC sequence was also characterized by lower accuracy levels during training (Fig. 3) than several other sequences, consistent with its greater difficulty to learn. As such, the findings with the LRC sequence are consistent with a literature demonstrating greater vulnerability to effects of drugs and other toxicants of behavior under conditions of lesser stimulus control, that is, greater difficulty (Winneke et al., 1977). That these deficits represented actual learning impairments was supported by the general absence of effects on behavior during the P components of the schedule, which involves the same motivational, motor, and sensory requirements as RL components but does not require learning per se.
Although statistically significant synergistic effects of Pb + stress were seen for only one of the five RL sequences, other individual PS, Pb, and Pb + PS subjects that did not exhibit deficits to LRC did show markedly impaired performance in the presence of other RL sequences (Fig. 7). That the deficits were so highly sequence dependent explains why Pb ± stress effects would be diluted in any statistical analysis that included all RL sequences, particularly in the face of the baseline differences in accuracy maintained by different sequences. Even still, a simple count of outliers for each sequence results in evidence that these deficits show Pb + stress additivity, that is, 13% of subjects affected for 0–PS, 45% for 50–PS, and 67% for 50–PS. For as yet undetermined reasons, individual variability in response to effects of Pb exposure has long been noted in experimental studies (Cory-Slechta et al., 1985) and has also characterized, for example, the PbB levels at which toxicity occurs in children (Davoli et al., 1996).
Performance of 50–PS females on the LRC sequence improved gradually across sessions, but not as much as controls (Fig. 4). Considered in the context of the human environment, however, where only a fixed or brief time might be allotted for learning specific concepts, thus precluding later improvement, such deficits might have residual escalating effects. For example, an insufficient grasp of basic mathematics concepts in early academic years is likely to increasingly impair subsequent grasp of more advanced mathematical concepts premised on the basics.
As a result of this impairment, Pb ± stress females also experienced a lower reinforcement density (Fig. 5). Intriguingly, these combined impairments parallel the effort-reward imbalance models of occupational stress studied in humans that posit a relationship between efforts spent and rewards received, with rewards based on money, esteem, promotion prospects, and security (Siegrist, 1996; Siegrist, 2001). Effort-reward imbalance has been associated with various adverse health outcomes, including coronary heart disease, psychiatric disorders, alcohol dependence, adverse well-being, and chronic illness. Measures of effort-reward imbalance have also been associated with markers of aging-related immune senescence including a reduction in the CD4:CD8 ratio and an increased number of late-differentiated CD27-CD28 CD8+ T lymphocytes (Bosch et al., 2009; Bosma et al., 1998; Head et al., 2004; Stansfeld and Candy, 2006). In the context of the effort-reward imbalance model, it could be posited that the behavioral consequences of Pb ± stress themselves contribute to such adverse health consequences.
Neurochemical and biochemical outcomes from behaviorally tested females were used to explore potential mechanisms of impairments. Of particular interest in this regard were the correlations revealed between 9-month corticosterone levels with frontal cortical DA (which exhibited a marginal increase at 50–PS) and with maximal number of total responses required per completed sequence in both RL1 and RL2, as well as between nucleus accumbens DA TO, which exhibited a selective increase in the 50–PS group, with maximal value for total responses per completed sequence in RL1 (Fig. 11). The latter correlation is consistent with the known role for nucleus accumbens in learning/reversal learning (e.g., Floresco et al., 2009). We have demonstrated the significance of nucleus accumbens to the mediation of postweaning Pb effects on RL in rats, where blockade of N-methyl-D-aspartate glutamate receptors mimics the effects of Pb (Bauter et al., 2003; Cory-Slechta et al., 1999). Frontal cortex DA systems are also critical to mediation of reversal learning (McAlonan and Brown, 2003; Ragozzino, 2007).
Stepwise regression analysis also confirmed a critical role for 9-month corticosterone levels in mediation of Pb ± stress-induced increases in total responses/completed sequence in behaviorally tested females. The involvement of corticosterone is notable given the demonstrated interactions of glucocorticoids with frontal cortex and nucleus accumbens and corresponding monoaminergic and glutamatergic neurotransmitters. Glucocorticoids, for example, have been shown to be essential for maintaining working memory mediated by prefrontal cortex, with adrenalectomy impairing working memory, decreasing DA release, and upregulating prefrontal cortex D1 DA receptors, effects that were prevented via replacement of corticosterone (Mizoguchi et al., 2004). Nucleus accumbens DA is highly responsive to changes in glucocorticoids (Barrot et al., 2000; Cho and Little, 1999). The circadian cycle of mineralocorticoid versus glucocorticoid receptor activation maintains basal nucleus accumbens DA release; an overactivation of glucocorticoid receptors, as characterizes the stress response, can potentiate nucleus accumbens DA efflux (Tye et al., 2009).
Many studies also document the importance of DA-glutamate balance in the mesocorticolimbic system to learning and reversal behaviors (Takahata and Moghaddam, 1998; Takahata and Moghaddam, 2000). Glutamatergic receptors are known to be modified both by Pb exposure (Cory-Slechta et al., 1993; Guilarte, 1997; Guilarte and McGlothan, 1998) and by PS (Jia et al., 2010). Collectively, the findings suggest mesocorticolimbic DA-glutamate imbalance as a potential mechanism for these Pb ± stress-induced cognitive deficits. One potential scenario by which this might be incurred is the protracted HPA axis dysfunction produced by Pb ± stress leading to permanent alterations in both frontal cortex and nucleus accumbens monoamine and glutamate function. Such a scenario would also explain the patterns of correlations observed between 9-month corticosterone levels, frontal cortex DA, nucleus accumbens DA TO, and total responses per completed sequence. One complexity that will need to be considered in pursuit of such a hypothesis includes the inverted U-shaped curves that relate neurotransmitter function to such behaviors (Robbins and Arnsten, 2009) and that relate corticosteroid effects to behavior (Park et al., 2006).
Changes in NGF were also examined for potential mechanistic involvement. NGF reductions in the 0–PS group appear discrepant from prior reports of increased NGF in basal forebrain and hippocampus (males only) in offspring whose mothers had received corticosterone in drinking water from delivery (Scaccianoce et al., 2001). However, the impact of stress on NGF is highly dependent upon type of stressor (Scaccianoce et al., 2000), and experimental conditions here differed notably from those studied previously (Scaccianoce et al., 2001). Prior studies suggest an impact of postweaning Pb exposure on NGF, as demonstrated by reductions in NGF gene expression in dorsal hippocampus in adulthood (Schneider et al., 2001) and in p75 receptor number in the medial septum/vertical limb of the diagonal band of Broca (Kidd et al., 2008). The current study demonstrates reductions in hippocampal NGF in response to lifetime Pb exposure levels at considerably lower PbB levels than those in Schneider et al. (2001), with current values stabilizing at approximately 8–10 μg/dl (with slightly higher values likely occurring during gestation and weaning as would be expected in both humans and rodents [Dearth et al., 2002; Gulson et al., 2004]). The extent to which NGF reductions related to Pb ± stress-associated learning impairments in the current study is not yet clear, given that all Pb ± stress groups showed almost equivalent NGF reductions, an outcome not mirrored in behavioral changes, and by the more modest nature of NGF correlation with other outcomes, although these are based on small n's.
The current studies have particular significance for human health, particularly given reports that elevated blood Pb levels mediated inverse associations observed between socioeconomic status and cortisol levels in 9.5-year-old children (Gump et al., 2009), as well as the increases in systolic and diastolic blood pressure and the total peripheral vascular resistance response to an acute stress challenge (Gump et al., 2008), consistent with Pb-stress interactions in humans. Importantly, these findings also underscore the critical need to generate more realistic models of human environmental chemical exposure, for example, to establish effect levels of Pb and of other toxicants in the context of other extant risk factors with which they share either common biological substrates or adverse outcomes in cumulative risk or multiple hit models.
FUNDING
National Institute of Environmental Health Sciences (ES01212 to D.C.-S and ES01247 to T. Gasiewicz).
References
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur. J. Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Bauter MR, Brockel BJ, Pankevich DE, Virgolini MB, Cory-Slechta D. Glutamate and dopamine in nucleus accumbens core and shell: sequence learning vs. performance. Neurotoxicology. 2003;24:227–243. doi: 10.1016/S0161-813X(02)00167-5. [DOI] [PubMed] [Google Scholar]
- Bosch JA, Fischer JE, Fischer JC. Psychologically adverse work conditions are associated with CD8+ T cell differentiation indicative of immunosenescence. Brain. Behav. Immunol. 2009;23:527–534. doi: 10.1016/j.bbi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Bosma H, Peter R, Siegrist J, Marmot M. Two alternative job stress models and the risk of coronary heart disease. Am. J. Public Health. 1998;88:68–74. doi: 10.2105/ajph.88.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N. Engl. J. Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Little HJ. Effects of corticosterone on excitatory amino acid responses in dopamine-sensitive neurons in the ventral tegmental area. Neuroscience. 1999;88:837–845. doi: 10.1016/s0306-4522(98)00264-4. [DOI] [PubMed] [Google Scholar]
- Cohn J, Cox C, Cory-Slechta DA. The effects of lead exposure on learning in a multiple repeated acquisition and performance schedule. Neurotoxicol. 1993;14:329–346. [PubMed] [Google Scholar]
- Cory-Slechta DA, O'Mara DJ, Brockel BJ. Learning versus performance impairments following regional administration of MK-801 into nucleus accumbens and dorsomedial striatum. Behav. Brain Res. 1999;102:181–194. doi: 10.1016/s0166-4328(99)00015-7. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ. Health Perspect. 2004;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B, Cox C. Performance and exposure indices of rats exposed to low concentrations of lead. Toxicol. Appl. Pharmacol. 1985;78:291–299. doi: 10.1016/0041-008x(85)90292-3. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Widzowski DV, Pokora MJ. Functional alterations in dopamine systems assessed using drug discrimination procedures. Neurotoxicology. 1993;14:105–114. [PubMed] [Google Scholar]
- Davoli CT, Serwint JR, Chisolm JJ., Jr Asymptomatic children with venous lead levels > 100 micrograms/dL. Pediatrics. 1996;98:965–968. [PubMed] [Google Scholar]
- Dearth RK, Hiney JK, Srivastava V, Burdick SB, Bratton GR, Dees WL. Effects of lead (Pb) exposure during gestation and lactation on female pubertal development in the rat. Reprod. Toxicol. 2002;16:343–352. doi: 10.1016/s0890-6238(02)00037-0. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Diaz R, Sokoloff P, Fuxe K. Codistribution of the dopamine D3 receptor and glucocorticoid receptor mRNAs during striatal prenatal development in the rat. Neurosci. Lett. 1997;227:119–122. doi: 10.1016/s0304-3940(97)00316-9. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav. Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Pb2+ inhibits NMDA receptor function at high and low affinity sites: developmental and regional brain expression. Neurotoxicol. 1997;18:43–51. [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL. Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res. 1998;790:98–107. doi: 10.1016/s0006-8993(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Gulson BL, Mizon KJ, Palmer JM, Korsch MJ, Taylor AJ, Mahaffey KR. Blood lead changes during pregnancy and postpartum with calcium supplementation. Environ. Health Perspect. 2004;112:1499–1507. doi: 10.1289/ehp.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Reihman J, Stewart P, Lonky E, Granger DA, Matthews KA. Blood lead (Pb) levels: further evidence for an environmental mechanism explaining the association between socioeconomic status and psychophysiological dysregulation in children. Health Psychol. 2009;28:614–620. doi: 10.1037/a0015611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Stewart P, Reihman J, Lonky E, Darvill T, Parsons PJ, Granger DA. Low-level prenatal and postnatal blood lead exposure and adrenocortical responses to acute stress in children. Environ. Health Perspect. 2008;116:249–255. doi: 10.1289/ehp.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J, Stansfeld SA, Siegrist J. The psychosocial work environment and alcohol dependence: a prospective study. Occup. Environ. Med. 2004;61:219–224. doi: 10.1136/oem.2002.005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N, Yang K, Sun Q, Cai Q, Li H, Cheng D, Fan X, Zhu Z. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev Neurobiol. 2010;70:114–125. doi: 10.1002/dneu.20766. [DOI] [PubMed] [Google Scholar]
- Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, Brown MJ. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123:e376–e385. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- Kidd SK, Anderson DW, Schneider JS. Postnatal lead exposure alters expression of forebrain p75 and TrkA nerve growth factor receptors. Brain Res. 2008;1195:113–119. doi: 10.1016/j.brainres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- King S, Laplante DP. The effects of prenatal maternal stress on children's cognitive development: Project Ice Storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier JF, Zelazo PR, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr. Res. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Lordi B, Patin V, Protais P, Mellier D, Caston J. Chronic stress in pregnant rats: effects on growth rate, anxiety and memory capabilities of the offspring. Int. J. Psychophysiol. 2000;37:195–205. doi: 10.1016/s0167-8760(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav. Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Takeda A, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J. Neurosci. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Woodson JC, Smith TP, Fleshner M, Diamond DM. Permissive influence of stress in the expression of a u-shaped relationship between serum corticosterone levels and spatial memory errors in rats. Dose Response. 2006;4:55–74. doi: 10.2203/dose-response.004.01.005.Park. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann. N. Y. Acad. Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, Virgolini MB, Weston D, Cory-Slechta DA. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: a potential biological unifying mechanism for their corresponding disease profiles. Toxicol. Appl. Pharmacol. 2009;234:117–127. doi: 10.1016/j.taap.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaccianoce S, Catalani A, Lombardo K, Consoli C, Angelucci L. Maternal glucocorticoid hormone influences nerve growth factor expression in the developing rat brain. Neuroreport. 2001;12:2881–2884. doi: 10.1097/00001756-200109170-00025. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Lombardo K, Angelucci L. Nerve growth factor brain concentration and stress: changes depend on type of stressor and age. Int. J. Dev. Neurosci. 2000;18:469–479. doi: 10.1016/s0736-5748(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Lee MH, Anderson DW, Zuck L, Lidsky TI. Enriched environment during development is protective against lead-induced neurotoxicity. Brain Res. 2001;896:48–55. doi: 10.1016/s0006-8993(00)03249-2. [DOI] [PubMed] [Google Scholar]
- Siegrist J. Adverse health effects of high-effort/low-reward conditions. J. Occup. Health Psychol. 1996;1:27–41. doi: 10.1037//1076-8998.1.1.27. [DOI] [PubMed] [Google Scholar]
- Siegrist J. Long-term stress in daily life in a socioepidemiologic perspective. Adv. Psychosom. Med. 2001;22:91–103. doi: 10.1159/000059278. [DOI] [PubMed] [Google Scholar]
- Stansfeld S, Candy B. Psychosocial work environment and mental health–a meta-analytic review. Scand. J. Work. Environ. Health. 2006;32:443–462. doi: 10.5271/sjweh.1050. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Glutamatergic regulation of basal and stimulus-activated dopamine release in the prefrontal cortex. J. Neurochem. 1998;71:1443–1449. doi: 10.1046/j.1471-4159.1998.71041443.x. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Target-specific glutamatergic regulation of dopamine neurons in the ventral tegmental area. J. Neurochem. 2000;75:1775–1778. doi: 10.1046/j.1471-4159.2000.0751775.x. [DOI] [PubMed] [Google Scholar]
- Tye SJ, Miller AD, Blaha CD. Differential corticosteroid receptor regulation of mesoaccumbens dopamine efflux during the peak and nadir of the circadian rhythm: a molecular equilibrium in the midbrain? Synapse. 2009;63:982–990. doi: 10.1002/syn.20682. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, Rossi-George A, Lisek R, Weston DD, Thiruchelvam M, Cory-Slechta DA. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008;29:812–827. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- Winneke G, Brockhaus A, Batissen R. Neurobehavioral and systemic effects of longterm blood-lead elevation in rats. I. Discrimination learning and open field behavior. Arch. Toxicol. 1977;37:247–263. doi: 10.1007/BF00330817. [DOI] [PubMed] [Google Scholar]
- Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Mol. Cell. Neurosci. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]