Abstract
The lung disease of cystic fibrosis (CF) is characterized by a vicious cycle of airway obstruction, chronic bacterial infection, and vigorous inflammation, which ultimately results in bronchiectasis. Recognition that excessive and persistent inflammation is a key factor in lung destruction has prompted investigation into anti-inflammatory therapies. Although effective, the use of systemic corticosteroids has been limited by the unacceptable adverse effect profile. Inhaled corticosteroids (ICS) are a widely prescribed anti-inflammatory agent in CF, likely as a result of clinicians’ familiarity with these agents and their excellent safety profile at low doses in asthmatic patients. However, while multiple studies are limited by small sample size and short duration, they consistently failed to demonstrate statistically or clinically significant benefits of ICS use in CF. This review provides an overview of the inflammatory response in CF, the mechanisms of action of corticosteroids, the safety of ICS, and the literature relevant to the use of ICS in CF.
1. Introduction
Although cystic fibrosis (CF) affects nearly every organ system, lung disease accounts for most of the morbidity and mortality.[1] Epithelial cells, particularly those of exocrine glands, are the principal sites of expression of the CF trans-membrane conductance regulator (CFTR) and, therefore, are most affected by its dysfunction. In the airway, abnormal CFTR leads to a vicious cycle of airway obstruction, chronic bacterial infection, and vigorous inflammation, which ultimately results in bronchiectasis.[2] Relieving the obstruction by improving airway clearance and treating infections have been the cornerstones of therapy for decades, but more recently, recognition that inflammation drives lung destruction has prompted investigation into anti-inflammatory therapies. Inhaled corticosteroids (ICS) have been widely prescribed as anti-inflammatory agents in CF,[3,4] likely due in part to their safety profile and clinicians’ familiarity with this class of drug for asthma. This review provides an overview of the inflammatory response in the CF airway and the evidence for the use of ICS as anti-inflammatory agents. Articles were identified using PubMed (keywords ‘cystic fibrosis,’ ‘corticosteroids,’ ‘inhaled corticosteroids,’ and ‘anti-inflammatory’) and by discussion among the authors.
1.1 Characteristics of the Inflammatory Response in the Cystic Fibrosis (CF) Airway
In order to understand the approach to anti-inflammatory therapy for patients with CF, it is first necessary to review the inflammatory response in the CF airway (figure 1). CF patients have a predilection for infection with a limited spectrum of distinctive bacteria, such as Staphylococcus aureus, Haemophilus influenzae, Pseudomonas aeruginosa, Burkholderia cepacia complex, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans. Once challenged by viral or bacterial infection, massive numbers of neutrophils are recruited to the airway. Although inflammation is meant to contain infection, this ultimately fails in the CF airway. An exaggerated inflammatory response, excessive relative to the burden of infection, is responsible for much of the pathology in the CF lung.[7,8]
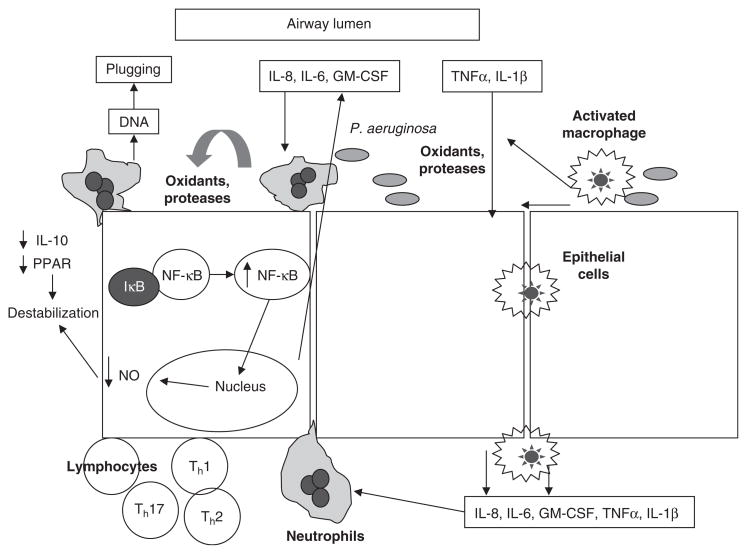
Fig. 1.
Schematic of inflammatory response in the cystic fibrosis airway. In response to a challenge by infectious agents, large numbers of neutrophils and other inflammatory cells are recruited to the airway. Upregulation of the transcription factor nuclear factor (NF)-κB plays a large role in this process by increasing transcription of multiple proinflammatory chemokines and cytokines. Decreased anti-inflammatory signaling leads to the destabilization of NF-κB and its inhibitory protein, IκB, resulting in a prolonged and excessive inflammatory response (modified from Konstan and Davis[5] and Chmiel and Davis,[6] with permission). GM-CSF= granulocyte-macrophage colony-stimulating factor; IL = interleukin; NO= nitric oxide; P. aeruginosa= Pseudomonas aeruginosa; PPAR= peroxisome proliferator activating receptor; Thx = T-helper cell x; TNFα = tumor necrosis factor-α.
Bronchoalveolar lavage (BAL) fluid from CF patients contains large concentrations of inflammatory mediators and massive amounts of neutrophils and neutrophil products.[9–13] This may not be surprising in adolescents and young adults, even those with mild disease, for most of these patients are chronically infected.[12] However, there is an impressive inflammatory burden even in CF infants.[2,10,11,14–16] BAL fluid from infected CF infants contains greatly elevated concentrations of neutrophil chemoattractants, neutrophils, and neutrophil products compared with BAL fluid from non-CF infants.[10,13–15] Moreover, these inflammatory markers are present, although at somewhat lower concentrations, in BAL fluid from apparently uninfected CF infants.[10] The presence of inflammation in the absence of detectable pathogens suggests that the inflammatory response in the CF lung may operate independently of an infectious stimulus. However, these findings may also be explained by failure to terminate the inflammatory response once the inciting stimulus has been eradicated and may actually represent an abnormal persistence of inflammation after clearance of early, transient infection.[7]
BAL fluid from infants with CF infected only with H. influenzae contains more neutrophils and interleukin (IL)-8, a potent neutrophil chemoattractant, for any given burden of H. influenzae than does BAL fluid from infants with underlying conditions other than CF who are infected with H. influenzae.[8,17] While it might seem effective to mount a more vigorous response to invading pathogens, eventually this excessive and prolonged inflammatory response becomes harmful to the CF lung. Neutrophil DNA and actin increase the viscosity of CF sputum and impair mucociliary clearance. Neutrophils also release oxidants and proteases, including elastase. Excessive amounts of oxidants overwhelm the antioxidant defenses and contribute to lung injury. The huge excess of elastase similarly overwhelms the antiprotease system in the airways and results in uninhibited proteolytic enzymatic activity. Elastase directly promotes structural damage by digesting elastin and other airway wall proteins, and worsens airway obstruction by impairing ciliary beating and by increasing mucus secretion. Elastase also promotes bacterial persistence by cleaving critical opsonins and receptors that are necessary for phagocytosis, and promotes the generation of neutrophil chemoattractants, particularly IL-8, leukotriene B4, and the complement component, C5a-like peptide. Bacteria and their products also promote the generation of chemoattractants, which recruit more neutrophils into the airways, fueling the vicious cycle of inflammation that leads to lung destruction.[7]
BAL fluid from patients with CF contains large concentrations of tumor necrosis factor (TNF)-α, IL-1β, IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF).[9] The synthesis of all of these proinflammatory cytokines is promoted by transcription factor nuclear factor (NF)-κB, which is activated by cellular interaction with bacteria, bacterial products, and proinflammatory cytokines. Interestingly, some studies found that CF airways are relatively deficient in IL-10 and nitric oxide (NO),[18–24] both of which preserve the function of the inhibitory protein-κB (IκB), the inhibitor of NF-κB. Decreased availability of IL-10 and NO would lead to less IκB-mediated inhibition of NF-κB; consequently, proinflammatory mediator production would increase. Therefore, an imbalance between IκB and NF-κB would result in the prolonged and excessive production of the mediators responsible for the damaging inflammation. In addition, CF tissues appear to be deficient in peroxisome proliferator activating receptor-γ (PPARγ).[25] When activated, PPAR forms a heterodimer with activated retinoid X receptor, an immunomodulator.[26] PPAR exerts its attenuating effects typically by inhibiting NF-κB activity via upregulation of IκB[27] or by competition with NF-κB for helicases. CF airway epithelial cell lines appear to have less PPARγ activity than do non-CF airway epithelial cell lines.[28] Decreased PPAR expression leads to an imbalance between IκB and NF-κB that favors increased inflammation in CF. The mechanisms proposed above are not mutually exclusive, and it is likely that a combination of the above processes fuels the aggressive and damaging inflammatory cascade.
It would appear that the most appropriate time to treat the excessive inflammatory response would be relatively early in life, prior to the establishment of the vicious cycle of obstruction, infection, and inflammation, and before the onset of structural damage to the airways. However, it is not known whether limiting inflammation in infancy would promote or retard the establishment of chronic infection. To some extent, the inflammatory response is likely protective early in the course of disease. Published clinical trials and clinical experience suggest that anti-inflammatory therapy needs to begin early in the course of disease.[29–33] It seems plausible that as lung disease progresses, therapies such as mechanical removal of secretions, antioxidants, and antiproteases, which limit the structural damage as a result of the inflammatory response, must accompany treatments aimed at reducing inflammation.
2. Anti-inflammatory Therapies in CF
At the present time, ibuprofen is the only anti-inflammatory drug recommended by the Cystic Fibrosis Foundation[34] and the Cochrane Database of Systematic Reviews[35] for use by CF patients for the purpose of slowing the loss of lung function. Although two long-term clinical trials support the efficacy and safety of twice-daily oral administration of high-dose ibu-profen[31,33] and a recent analysis of the Cystic Fibrosis Foundation Patient Registry revealed that real-world clinical use of ibuprofen is beneficial with an acceptable safety profile,[32] its use is quite limited[36] for reasons that are not entirely clear. Corticosteroids have received much more attention as an anti-inflammatory therapy for CF lung disease, and this review focuses on the use of ICS. A brief discussion of systemic corticosteroids is presented to provide the rationale for the use of (ICS). Other drugs used in CF with possible anti-inflammatory properties, such as azithromycin, are discussed elsewhere.[37]
2.1 Systemic Corticosteroids
Corticosteroids possess several potent anti-inflammatory effects (figure 2). They reduce the formation of mucus and edema, inhibit chemotaxis, adhesion, and activation of leukocytes, inhibit NF-κB activation, and interfere with the synthesis or actions of inflammatory mediators. Although corticosteroids have selectively been used to treat CF patients who also have asthma, allergic bronchopulmonary aspergillosis (ABPA), and corticosteroid responsive wheezing, a more general use to treat inflammation has been proposed in the past 2 decades. The rationale for evaluating systemic corticosteroids in the early 1980s was the observation that CF patients with hypogammaglobulinemia had better lung function than did age-matched CF patients with normal or elevated immunoglobulin concentrations.[38] If the immune and inflammatory responses were solely protective, one would have expected the opposite, so it was proposed that moderating these responses would be beneficial. This recommendation is based, in part, on the results of two double-blind, placebo-controlled clinical trials conducted to assess the effectiveness of long-term administration of oral prednisone.[29,30]
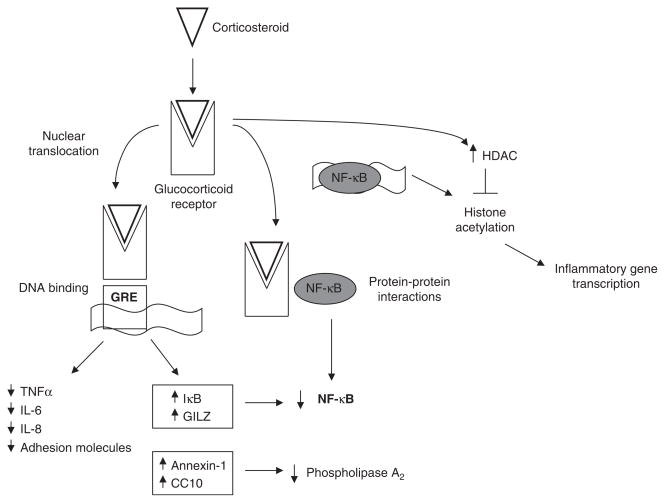
Fig. 2.
Mechanism of action of corticosteroids on inflammatory pathways relevant to cystic fibrosis (CF). Binding of corticosteroids to the cytoplasmic glucocorticoid receptor (cGR) results in the dissociation of the cGR from molecular chaperones that cover sites on the receptor that signal transport to the nucleus. Subsequent nuclear translocation allows the glucocorticoid-cGR complex to affect inflammatory signaling through multiple mechanisms. Binding to the glucocorticoid response-element (GRE) promoter region of corticosteroid-sensitive genes results in decreased transcription of proinflammatory gene products and increased transcription of anti-inflammatory gene products. The activity of nuclear factor (NF)-κB, central to the inflammatory response in the CF airway, is likely decreased by multiple mechanisms. These include upregulation of the inhibitory protein-κB (IκB), protein-protein interactions between the cGR complex, and increased histone deacetylase (HDAC) activity downstream of NF-κB binding to DNA. CC10 = Clara cell 10-kDa protein; IL = interleukin; GILZ = glucocorticoid-induced leucine zipper protein; TNFα = tumor necrosis factor-α.
In the first clinical trial of oral corticosteroids,[29] CF patients 1–12 years of age with mild-to-moderate lung disease were treated with alternate-day prednisone (2 mg/kg, maximum dose 60 mg/day) or placebo for 4 years; the group who received prednisone had better lung function, improved weight gain, fewer hospital admissions, and maintained constant IgG concentrations with no apparent adverse effects during the study period. This lack of adverse effects was puzzling, but most likely relates to the small number of patients enrolled in the study (21 in the treatment group and 24 in the placebo group). In fact, a follow-up analysis 5 years after completion of the first trial reported that 14 of 17 patients assigned to the corticosteroid group who completed the trial developed growth retardation, two developed cataracts, and two developed glucose abnormalities.[39]
In 1995, the results of a much larger multicenter trial were reported.[30] Two alternate-day dosing regimens (2 mg/kg and 1mg/kg, maximum dose 60mg/day)were compared with placebo in 285 CF patients 6–14 years of age with mild-to-moderate lung disease. Although beneficial effects on lung function were observed, particularly in patients infected with P. aeruginosa, so were serious adverse effects, including glucose intolerance, growth impairment, and cataracts, even at the 1 mg/kg dosage.[30] Follow-up analysis 6 years after the completion of this trial demonstrated that while catch-up growth occurred in most children, persistent deficits in growth after the therapy had been discontinued were seen in prepubertal boys.[40] Participants in the prednisone 1 mg/kg group also seemed to have an accelerated rate of decline in forced expiratory volume in 1 second (FEV1) after discontinuation of the drug. In the initial publication, investigators concluded that prednisone therapy (1 mg/kg administered on alternate days) should be individualized for each patient based upon their clinical course, response to standard therapy, and response to prednisone. If pulmonary function does not significantly improve within 6 months, then the drug should be discontinued. However, in view of the results from the follow-up analysis and results from recent studies that corticosteroids worsen osteopenia and osteoporosis,[41–44] and promote proximal skeletal muscle weakness[45] in CF patients who are already at high risk for such conditions, long-term use of systemic corticosteroids for slowing the progression of lung disease is no longer advocated.[46] Shorter courses of systemic corticosteroids have been proposed. To this end, the effect of a 12-week course of prednisolone on pulmonary function and serum cytokine and IgG concentrations was evaluated in a double-blind, placebo-controlled study in 24 children with CF.[47] The prednisolone-treated group received 2 mg/kg (maximum dose 40 mg) every day for 2 weeks tapered to 1mg/kg on alternate days for 10 weeks. Compared with placebo, the prednisolone group had an increase in forced vital capacity (FVC) and FEV1 that was associated with decreased serum IgG and cytokine concentrations. These findings suggest that short courses of corticosteroids may be a useful adjunct to the treatment of exacerbations, when inflammatory activity can be expected to be greatest. A recent pilot study demonstrated a trend towards improved pulmonary function in CF patients hospitalized for a pulmonary exacerbation who were treated with prednisone, compared with placebo, although the results failed to reach statistical significance likely as a result of the small population studied.[48]
3. Inhaled Corticosteroids (ICS)
The adverse effect profile of systemic corticosteroids has prompted the investigation of ICS as anti-inflammatory agents in CF. Due in part to their safety profile and clinicians’ familiarity with this class of drug for asthma, use of ICS as anti-inflammatory agents in CF has become widespread.[3,4] Despite the lack of data showing ICS to be effective anti-inflammatory agents in CF, they are frequently prescribed to preserve lung function by blocking inflammation.[3] In the US in 2006, 45.3% of CF patients were treated with ICS, although there is considerable variability among centers, with the range being from 0% to nearly 90%.[36] The first published study[49] evaluating the efficacy of ICS in CF was published 25 years ago, and multiple studies published since then have failed to demonstrate a consistent effect.[50–58] A recent update to the original Cochrane review published in 2000[59] concluded that evidence is “in-sufficient to establish if inhaled corticosteroids have a beneficial or harmful effect in people with CF.” Similarly, an expert committee assembled by the Cystic Fibrosis Foundation recently advised against the use of ICS as anti-inflammatory agents in adults and children 6 years of age and older who do not have asthma.[34] The published studies that have evaluated the use of ICS as anti-inflammatory agents in CF are summarized in table I and are discussed in more detail below.
Table I.
Clinical trials of inhaled corticosteroids (ICS) in cystic fibrosis (CF)
| Study (year) | Design | No. of patients | Mean or median age in years (range) | Drug/total daily dose | Treatment duration | Efficacy for primary outcome | Lung function (when reported) | Adverse effects |
|---|---|---|---|---|---|---|---|---|
| Schiotz et al.[49] (1983) | R, DB, PC, matched pairs | 26 | 14 (4–29) | BEC 400 μg | 16 wk | No effect on sputum inflammatory markers | FVC (mean, start of study → after treatment): PL 98% → 78%, BEC 89% → 91%, overall 90% → 85% No statistical tests reported | None reported |
| Van Haren et al.[50] (1995) | DB, PC, CO | 12 | 26 (16–45) | BUD 1600 μg | 6 wk | Decreased airway reactivity in 58% (≥1 doubling dose increase in PC20 histamine). Small improvement in cough and dyspnea scores | No effect FEV1 (after treatment): PL 2.2 L, BUD 2.3 L, FVC: PL 3.3 L, BUD 3.6 L | None reported |
| Nikolaizik and Schoni[51] (1996) | R | 49 | 19.8, SD 6.8 | BEC 1500 μg | 30 d | Positive effect on thoracic gas volume (p = 0.012). ICS had no effect on other measures | No effect FVC (mean, start of study → after treatment): no ICS 58% → 65%, ICS 55% → 65%, FEV1 (mean, start of study → after treatment): no ICS 40% → 48%, ICS 37% → 45% | No mention of AE reporting in paper |
| Balfour-Lynn et al.[52] (1997) | R, DB, PC, CO | 23 | 10.3 (7–17) | FLU 400 μg | 6 wk | No improvement in sputum inflammatory markers | No effect FVC: baseline 75%, PL 78%, FLU 78%, FEV1: baseline 64 %, PL 67%, FLU 65% | Reported AEs: PL 15 (65%), FLU 18 (78%). Most URI/CF pulmonary exacerbations. Two possibly drug related: transient cough, rash |
| Bisgaard et al.[53] (1997) | R, DB, parallel | 55 | 20 (not given) | BUD 1600 μg | 24 wk | Trend toward reduced decline in FEV1 | Intention to treat ΔFEV1: PL −0.187 L, BUD −0.032 L(p = 0.08) | PL 13, BUD 17 (overall incidences comparable) |
| Dauletbaev et al.[54] (1999) | R, open label, parallel | 26 | 26 (16–38) | FLU 1000 μg | 3 wk | No improvement in clinical symptom score, lung function or sputum inflammatory markers | FEV1 (mean, start of study → after treatment): PL 57 → 56% p > 0.2, FLU 56 → 53% p > 0.1 | No clinical AEs reported. Sputum superoxide anion release was higher in FLU group (34 vs 25 nmol/h/106 cells p = 0.02) |
| Wojtczak et al.[55] (2001) | Uncontrolled, prospective | 12 | 6 (1.5–13) | BEC 420 μg | 8 wk | Decreased BALF neutrophils (to 1/3 pretreatment levels, p = 0.03) | Not done as outcome measure | None reported. No increase in airway infection. No new Pseudomonas infections |
| Balfour-Lynn et al.[56] (2006) | R, DB, PC, MC, withdrawal trial | 171 | 14.6 (6–48) [FLU] 15.8 (6–53) [PL] | FLU varied doses | 6 mo | No difference in time to first exacerbation, HR 1.07 (0.68–1.7) | FVC (mean, start of study → after treatment): PL 90% → 90%, FLU 90% → 90%, FEV1 (mean, start of study → after treatment): PL 74% → 73%, FLU 76% → 76% | 24 withdrawals from each group. 136 AE in FLU group (3 SAE: head injury, gallstones, intra-abdominal sepsis). 161 AE in PL group (0 SAE) |
| De Boeck et al.[57] (2007) | R, DB, PC, MC | 27 | 8.2 (FLU) 9 (PL) | FLU 1000 μg | 12 mo | No difference in FEV1 | ΔFEV1 (prebronchodilator): PL −3.6%, FLU +3.9%, ΔFEV1 (post-bronchodilator): PL −3.9%, FLU +3.3% | Growth suppression: height gain PL 5.49 cm, height gain FLU 3.96 cm (p = 0.005). No increase in Pseudomonas infection or resp infections |
| Ren et al.[58] (2008) | Observational | 2565 | 6–17 | Varied | 2 y | Decreased rate of decline of FEV1 in patients regularly using ICS (p = 0.04) | Rate of change of FEV1 (% pred/y) after ICS: patients using ICS −0.44%/y, comparison group not using ICS −1.44%/y | Decreased WFA and HFA z-scores, increased use of insulin or oral hypoglycemics, and increased rates of Stenotrophomonas maltophilia, Burkholderia cepacia, and Aspergillus spp. in ICS users |
AE= adverse event; BALF = bronchoalveolar lavage fluid; BEC= beclomethasone; BUD= budesonide; CO= crossover; DB= double-blind; FEV1 = forced expiratory volume in 1 second; FLU = fluticasone propionate; FVC= forced vital capacity; HFA= height for age; HR= hazard ratio; MC= multicenter; NS= not significant; PC= placebo-controlled; PC20 = concentration provoking a 20% decline in FEV1; PL = placebo; pred = prednisone; R= randomized; resp = respiratory; SAE= serious AE; URI = upper respiratory tract infection; WFA=weight for age; Δ indicates change; → indicates after intervention/treatment.
3.1 Mechanisms of Action of ICS
ICS were first used as an alternative to oral corticosteroids for the treatment of asthma in the 1970s. In the seminal paper by Brown et al.,[60] inhaled beclometasone was shown to improve asthma control and reduce the need for oral corticosteroids. Asthmatic patients with large numbers of eosinophils in their sputum had the most impressive responses; presence of eosinophils is an important distinction in the inflammation due to asthma from the neutrophil-driven inflammatory response in CF. While the success of ICS in asthma is attributed mainly to their ability to decrease the influx of T lymphocytes, eosinophils, and mast cells, rather than neutrophils, glucocorticoids have broad anti-inflammatory properties, so there is reason to believe ICS might be effective in treating CF-related inflammation. Glucocorticoids inhibit NF-κB activity, chemotaxis, and the synthesis of multiple proinflammatory mediators, including eicosanoids, all of which play roles in the excessive inflammatory response in CF.[2]
Glucocorticoids exert their biologic actions by binding to the cytosolic glucocorticoid receptor-α, the active form of the glucocorticoid receptor. The glucocorticoid-receptor complex then translocates to the nucleus, where it can affect inflammation by directly altering gene transcription (increasing production of anti-inflammatory gene products and decreasing production of proinflammatory gene products) or by interfering with other transcription factors. This latter mechanism is probably responsible for glucocorticoid-mediated downregulation of NF-κB, likely a major means by which glucocorticoids are effective anti-inflammatory agents. As described in section 1.1, NF-κB is a transcription factor that regulates many of the proinflammatory cytokines that characterize the exuberant inflammatory response in CF, including TNFα, IL-1β, IL-6, and IL-8. The glucocorticoid-receptor complex may inhibit NF-κB activation by protein-protein interaction, or by acting downstream of NF-κB binding to DNA through histone deacetylation.[61] Activated glucocorticoid-receptor complexes also increase the transcription anti-inflammatory gene products, including I-κB[62] and glucocorticoid-induced leucine zipper protein (GILZ).[63] This may be particularly important in CF because overexpression of GILZ in epithelial cells prevents lipopolysaccharide-mediated activation of NF-κB.[64]
There are conflicting data with regard to the relevance of the mechanisms of glucocorticoid action described above to the concentrations achieved using inhaled formulations. Much of the literature in this area focuses on the effects of ICS on eosinophilic inflammation, but a few studies have examined the impact of ICS on NF-κB signaling. Hart et al.[65] failed to show a reduction in NF-κB binding to DNA in bronchial biopsies from asthmatic patients treated with fluticasone propionate 1000 μg/day and, in fact, found elevated expression of the p65 subunit of NF-κB by immunostaining in the corticosteroid-treated group. However, in a similar study[66] in which asthmatic patients were treated with budesonide 800 μg/day, NF-κB staining of mucosal biopsies was reduced in the corticosteroid-treated group. This study also demonstrated decreased mucosal staining for TNFα and IL-8 after treatment with budesonide. Differences between study design and methodology in the two studies include a longer treatment period (9 weeks vs 4 weeks) in the study showing an effect[66] compared with the other study.[65] There are more consistent data with respect to the impact of ICS on NF-κB signaling in myofibroblasts, with decreased translocation of NF-κB to the nucleus seen in in vitro studies in which primary myofibroblast cell lines were treated with fluticasone propionate.[67] The relevance of altering myofibroblast signaling in CF is unclear.
While directing anti-inflammatory therapies to airway neutrophils seems a reasonable way to reduce adverse effects associated with systemic anti-inflammatory agents, there is evidence that neutrophils in the CF airway are not as responsive to corticosteroids as those still in the circulation. Corvol et al.[68] compared peripheral blood neutrophils with sputum neutrophils from 15 children with CF. Peripheral blood neutrophils spontaneously secreted more IL-8 than neutrophils from healthy controls, but dexamethasone decreased both spontaneous and lipopolysaccharide-stimulated IL-secretion from the CF circulating neutrophils. Sputum neutrophils from the CF patients secreted more IL-8 than the peripheral blood neutrophils from the same patient, and did not respond to suppression by dexamethasone. Stimulation with lipopolysaccharide did not increase IL-8 secretion from the sputum neutrophils, suggesting that they were already maximally stimulated by the environment in the CF airway. This suggests that even if ICS can be delivered to the sites of neutrophil recruitment in the airways, they may be less effective than in other inflammatory diseases.
In order for ICS to be effective, they must be deposited in the lung at the site of inflammation. This depends on several factors, including the delivery device, the properties of the drug itself, the particle size, and the state of the airways. Drugs that have been optimized for delivery to the small airways for the treatment of asthma may not be effective in CF simply because they cannot penetrate viscous mucus to get to the site of the inflammation. Evidence from asthmatic patients suggests that even mild airway obstruction decreases absorption of ICS,[69–71] although there is some variability between specific drugs. CF inflammation and airway damage is not uniform, so using higher doses of ICS in an attempt to overcome the difficulty in reaching the site of inflammation may not only be ineffective, but potentially deleterious as the relatively intact airways may allow for substantial absorption of the corticosteroid.
3.2 Efficacy of ICS in CF
A recent Cochrane review[59] concluded that evidence is “insufficient to establish if inhaled corticosteroids have a beneficial or harmful effect in people with CF.” Similarly, an expert committee assembled by the Cystic Fibrosis Foundation recently advised against the use of ICS as anti-inflammatory agents in adults and children ≥6 years of age who do not have asthma.[34] Investigators first began studying the use of ICS in CF over 25 years ago. Many of these studies have been limited to small numbers of patients and short courses of treatment, which may have limited their ability to detect a treatment effect. The reader is refered to table I for a summary of the studies discussed in this section, including the study design, drug and duration of therapy, efficacy results, and lung function data when reported. Schiotz et al.[49] conducted the first published randomized study of ICS in CF patients. Forty-eight adult and pediatric CF patients with chronic P. aeruginosa infection were randomized to receive either inhaled beclometasone 400 μg/day divided four times daily or placebo for 16 weeks immediately following a 2-week course of intravenous antibacterials. Participants randomized to active drug were matched to those receiving placebo by a clinical score, which included FVC, weight, chest x-ray score, and duration of P. aeruginosa infection. Subjects were matched by age and sex when possible. Five subjects were withdrawn by the investigators because of a deterioration of their pulmonary status and six were withdrawn as a result of noncompliance. None of these withdrawn subjects was matched to each other, so out of the original 24 pairs, data from only 13 pairs were analyzed. The investigators did not state if there was a difference in the withdrawal rate among groups. The primary outcome measure was analysis of sputum sol-phase immune complex activity, proteolytic activity, and sputum-to-serum albumin ratios. No differences were observed in the primary outcome measure. The patients receiving beclometasone had stable lung function during the study, with the median FVC 89% predicted before treatment and 91% after treatment. The patients receiving placebo had a median FVC of 98% prior to treatment, and 78% after treatment. Because there was no effect of beclometasone on the primary outcome measure, all participants were regarded as one group when using statistical analysis to evaluate the change in lung function. When all 26 participants were considered together, the median FVC decreased from 90% predicted to 85% predicted (p < 0.05) during the course of the study. No adverse effects were observed in either group.
A randomized, placebo-controlled, crossover study of 12 subjects published in 1995[50] did demonstrate treatment with budesonide decreased airway reactivity in response to histamine; 7 of 12 subjects enrolled had an increase in PC20 (concentration provoking a 20% decline in FEV1) histamine by at least one doubling dose, and the geometric mean PC20 histamine increased from 1.6 to 3.2 mg/mL. No differences in FEV1 or FVC were seen. During budesonide treatment, dyspnea and cough scores also improved. On a scale of 0–3, baseline mean dyspnea at rest scores were 0.47; scores increased to 0.54 during placebo treatment, and decreased to 0.38 during budesonide treatment (p-value for placebo and budesonide scores <0.05). Similarly, cough scores were 1.19 at baseline, increased slightly during placebo treatment to 1.2, and decreased to 1.14 during budesonide treatment (p < 0.05). There were no significant differences in dyspnea on exertion, sputum production, morning or evening peak flow rates, or β2-agonist use. However, there was no washout period between the 6-week treatment arms, making these results difficult to interpret. In a study on 49 patients with CF who were hospitalized for a pulmonary exacerbation, Nikolaizik and Schoni[51] showed treatment with beclometasone for 30 days was associated with an improvement in thoracic gas volume and diffusing capacity of carbon monoxide. However, the study failed to demonstrate that beclometasone was associated with an improvement in the more clinically relevant measures of FEV1 or FVC.
In a longer outpatient study, 55 patients chronically infected with P. aeurginosa and with a mean FEV1 of 63% predicted were randomized to budesonide or placebo for a total of 6 months. The treatment courses were divided into two 12-week periods separated by a 2-week course of intravenous antibacterials.[53] The primary outcome measure was the rate of decline of FEV1 during the study period. In the intention-to-treat analysis, the budesonide group had a trend towards less decline compared with the placebo group (−0.032 vs −0.187 L; p = 0.08). When only those who completed the study were analyzed, the difference did reach statistical significance (−0.017 L with budesonide vs −0.198 L with placebo; p < 0.05). A small uncontrolled study published in 2001 demonstrated a decrease in neutrophils recovered in BAL fluid from 12 children treated with beclomethasone for 8 weeks; however, the lack of a control group makes this difficult to interpret.[55]
Balfour-Lynn et al.[52] failed to demonstrate an improvement in lung function, symptom scores, or their primary outcome of sputum inflammatory markers in 23 children treated with fluticasone propionate for 6 weeks in a randomized, double-blind, crossover study with an appropriate washout period. In fact, while sputum TNFα and free neturophil elastase levels did not change, IL-8 levels increased during treatment with fluticasone propionate. The investigators attributed this unexpected finding to the wide intra- and inter-subject variability found in sputum IL-8 levels in previous studies. Similarly, in a randomized, open-label study comparing fluticasone propionate treatment for 3 weeks with placebo, there was no difference in symptoms or lung function, but sputum cells isolated from the fluticasone propionate-treated subjects produced more of the free radical superoxide in response to stimuli.[54] The investigators postulated that this represents a normalization of the bactericidal activity of inflammatory cells, although they acknowledged that increased oxidative capacity may also be harmful.
Most of the dosing regimens used in these studies were extrapolated from asthma studies. While there is a flat dose-response curve in asthma, with the large majority of asthmatic patients gaining control with low-dose ICS and little additional benefit obtained from moderate or high doses, these dosages may be inadequate to penetrate the large amounts of mucus present in the CF lung. A recent randomized, placebo-controlled trial tested the hypothesis that high-dose ICS might show a benefit where low doses had not.[57] Twenty-seven children at multiple sites were randomized to fluticasone propionate at a total dosage of 1000 μg/day (divided twice daily) or placebo for 12 months. No difference in lung function was seen in the active treatment group. However, these children did exhibit slowed height velocity with no catch-up growth observed over the subsequent 12 months off the drug. This may be a particularly concerning adverse effect in children with CF given the association of decreased height and weight in early childhood with decreased pulmonary function 3 years later.[72]
Part of the difficulty in determining if there is a benefit to using ICS in CF is that many CF patients are currently treated or have been treated in the past with these drugs. A study from the UK[56] therefore tested if withdrawing this common therapy decreases the time to first exacerbation, or adversely effects lung function, antibacterial use, or bronchodilator use. In this randomized, placebo-controlled, multicenter trial, after a 2-month run-in period during which all subjects were transitioned to the same ICS (fluticasone propionate), 171 adult and pediatric patients were randomized to fluticasone propionate or placebo for 6 months. There was no difference in the primary outcome of time to first exacerbation with a hazard ratio of 1.07 (95% CI 0.68, 1.70), nor were there differences in oral and intravenous antibacterial use or bronchodilator use. There was also no change in lung function, although this study was not powered to detect this outcome. More subjects in the placebo group withdrew from the study (23% vs 14% in the fluticasone propionate group), but this difference also did not reach statistical significance.
Although not a randomized study, Ren et al.[58] published the results of the largest study examining the role of ICS as a means to slow the progression of lung disease in children with CF. Using data from the Epidemiologic Study of CF (ESCF), an observational, longitudinal, encounter-based database,[73] the authors analyzed data from 2978 children who were not receiving ICS for at least 2 years following enrollment in ESCF. Patients who had at least 3 visits in the 24-month period after ICS were prescribed and who had ICS use recorded on at least 80% of the visits were considered to be receiving long-term ICS treatment. A control group of patients enrolled in ESCF who never received ICS during the same 4-year period was selected for comparison. A significant decrease in the rate of decline in lung function was seen in the group that consistently reported using ICS (from −1.52%/year decline prior to use of ICS to −0.44% after initiation of ICS, p = 0.002). Children who had never received ICS had a rate of decline of −1.44%/year during the 4-year period. Patients who received ICS therapy had decreased weight for age and height for age Z scores after the index visit (p < 0.001), and increased use of insulin or oral hypoglycemic agents compared with those not treated with ICS (p = 0.024). While there was no increase in the rates of acquisition of P. aeruginosa, patients receiving long-term ICS therapy did have a small but significant increase in cultures positive for S. maltophilia, B. cepacia, and Aspergillus spp. compared with those never receiving ICS. While epidemiologic studies are inherently subject to bias, the cost of performing a prospective randomized study of this magnitude would be prohibitive.
3.3 Safety of ICS in CF
A recent Cochrane review of ICS in CF concluded that there is insufficient evidence to determine if ICS are beneficial or harmful.[59] While there is some evidence to suggest that ICS can be safely administered to children with CF without adrenal suppression,[53,55] a case report of profound adrenal suppression in a 28-year-old man with CF and ABPA treated with itraconzole and low-dose inhaled fluticasone propionate emphasizes the importance of designing studies of sufficient duration in CF to evaluate the effects of ICS on the adrenal glands.[74] Moreover, other long-term complications of corticosteroids, including growth failure, glucose intolerance, cataract formation, and decreased bone mineral density, have not yet been adequately evaluated in patients with CF. Growth failure is a particularly concerning adverse effect in children with CF given the association of decreased height and weight in early childhood with decreased pulmonary function 3 years later,[72] and the slowed height velocity seen in a study of high-dose ICS[57] with no catch-up growth observed over the subsequent 12 months off the drug. The association of ICS with earlier acquisition of P. aeruginosa and other organisms is also concerning.[58,75]
3.4 Potential use of ICS in CF Asthma
ICS are also commonly used in CF patients to treat airway reactivity, or ‘CF asthma.’ Diagnosing asthma in CF patients can be problematic; wheezing is a common physical finding in CF, but this may be the result of the underlying CF lung disease (i.e. mucosal edema from chronic infection, airway obstruction from thick secretions), rather than classic asthma. Diagnosing asthma in young children without CF can be difficult; it is a clinical diagnosis based on a pattern of recurrent or chronic cough and wheeze accompanied by bronchospasm and airway inflammation. Guidelines for the diagnosis and management of childhood asthma include recommendations to rule out other diseases that may cause similar symptoms, including CF.[76] In the ESCF, asthma is reported if “in the treating physician’s opinion, asthma contributes significantly to the patient’s lung disease,” and consideration for the diagnosis of asthma is recommended if the child has episodic airway obstruction that is relieved by bronchodilators, a strong family history of asthma, or evidence of atopy.[73] Using this diagnosis, asthma was reported in 19%[73] of the US and Canadian patients enrolled, higher than the 7.7% current prevalence of asthma reported in the general US population.[77] While the use of ICS has not been specifically studied for CF-associated asthma, it seems reasonable to initiate a trial for patients with recurrent episodes of bronchospasm and continue therapy if there is a clinical response. As with any therapy, this should be accompanied by monitoring for continued efficacy over time and adverse effects, particularly adrenal suppression and growth impairment.
3.5 Considerations Prior to Starting ICS
There are several practical considerations the treating physician must take into account before starting ICS in children with CF, starting with determining which drug to use, how to deliver it, and what dose to prescribe. The safety and efficacy profile of low dose ICS in children with asthma is excellent, but the optimal specific drug and dose for children with CF, even those who also have asthma, is not known. It is tempting to extrapolate from asthma studies, but the pathophysiology of recurrent wheezing in CF is probably different from classic childhood asthma, and the viscous mucous present in CF airways will change the absorption of any inhaled medication. CF airway disease may be localized, and when ventilation is directed to the healthiest areas of the lung, so are inhaled medications. When prescribed not for CF-related asthma, but as an anti-inflammatory therapy with the goal of decreasing the inexorable decline in lung function seen in CF, there are even fewer data available to determine the optimal delivery device, drug, and dose of ICS. Further studies to address these issues will be needed in order for CF healthcare providers to make evidence-based recommendations about the use of ICS. The optimal method of monitoring response to therapy also needs to be determined. This is a particular challenge in young children, who cannot expectorate sputum or perform pulmonary function testing. The routine therapies currently recommended for CF impose a considerable burden on patients and their families. This is reflected in the poor rates of adherence (<50%) to therapies that are proven to be effective.[78]Considerable thought should be given to adding therapies that may not be effective to the already complicated regimens asked of most CF patients.
4. Conclusion
CF lung disease begins early in life, is persistent, and is the leading cause of death in CF patients. An exuberant inflammatory response to chronic infection is a key contributor to the tissue destruction that characterizes CF lung disease. Anti-inflammatory therapies need to be started early in life and used regularly to prevent damage to the airway from inflammatory products. While ICS are commonly prescribed anti-inflammatory agents in CF, and despite over 25 years of studies summarized here, their benefits and risks have not been clearly established. There are many challenges both to studying the use of ICS and their mechanism of action in CF. Prospective studies with sufficient numbers of participants to determine the safety and efficacy of ICS will be difficult to conduct, given the large numbers of CF patients who already use these drugs. Although directing therapy to the airway neutrophil rather than circulating neutrophils is logical, it is unclear if activated neutrophils already recruited to the airway will be responsive to corticosteroids, and how the viscous mucus that lines the CF airway impacts access to airway neutrophils. It is tempting to start ICS early in life, before irreversible damage to the airways has been done, but there are real concerns about the effect ICS have on growth, and the consequences of impaired growth on lung function. The optimal specific drug, doses, delivery devices, and measures of response to therapy are not known in children with CF. The lack of proven benefit has recently led to a recommendation by a committee assembled by the Cystic Fibrosis Foundation against the long-term use of ICS in adults and children older than 6 years of age with CF who do not have asthma or ABPA.[34]
Advances in airway clearance, aggressive use of antibacterials, and advances in nutrition have drastically improved the life expectancy for CF patients born today compared with 50 years ago. However, survival has recently reached a plateau. While there is great hope that therapies directed at restoring CFTR function will soon result in a cure for CF lung disease, these therapies will not reverse the damage that has already occurred. Drugs that interfere with the cycle of infection and inflammation are the best hope for preventing the decline in lung function that is inevitable in CF. While ICS are widely available and widely used, the literature reviewed here does not support their routine use as an anti-inflammatory therapy in CF patients who do not have asthma.
Acknowledgments
Grant support from the National Institutes of Health Grant P30-DK27651, KL2RR024990, and the Cystic Fibrosis Foundation is gratefully acknowledged. The authors have no conflicts of interest that are directly relevant to the content of this review.
References
- 1.Davis P, Drumm ML, Konstan MW. Cystic fibrosis: state of the art. Am J Respir Crit Care Med. 1996;154 (5):1229–56. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 2.Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24 (2):137–42. doi: 10.1002/(sici)1099-0496(199708)24:2<137::aid-ppul13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Oermann CM, Sockrider MM, Konstan MW. The use of anti-inflammatory medications in cystic fibrosis: trends and physician attitudes. Chest. 1999;115 (4):1053–8. doi: 10.1378/chest.115.4.1053. [DOI] [PubMed] [Google Scholar]
- 4.Konstan MW, Butler SM, Schidlow DV, et al. Patterns of medical practice in cystic fibrosis: part II. Use of therapies. Pediatr Pulmonol. 1999;28 (4):248–54. doi: 10.1002/(sici)1099-0496(199910)28:4<248::aid-ppul3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Konstan MW, Davis PB. Pharmacological approach for the discovery and development of new anti-inflammatory agents for the treatment of cystic fibrosis. Adv Drug Deliv Rev. 2002;54:1409–23. doi: 10.1016/s0169-409x(02)00146-1. [DOI] [PubMed] [Google Scholar]
- 6.Chmiel JF, Davis PB. Inflammatory responses in the cystic fibrosis lung. In: Kirk KL, Dawson DC, editors. The CFTR chloride channel. Vol. 10. Georgetown (TX): Landes Biosci; 2003. pp. 160–80. [Google Scholar]
- 7.Chmiel JF, Konstan MW, Berger M. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol. 2002;23 (1):5–27. doi: 10.1385/CRIAI:23:1:005. [DOI] [PubMed] [Google Scholar]
- 8.Muhlebach MS, Stewart PW, Leigh MW, et al. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160 (1):186–91. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 9.Bonfield TL, Panuska JR, Konstan MW, et al. Inflammatory cytokines in cystic fibrosis lungs [published erratum appears in Am J Respir Crit Care Med 1996 Oct; 154 (4 Pt 1): 1217] Am J Respir Crit Care Med. 1995;152 (6):2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 10.Khan TZ, Wagener JS, Bost T, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151 (4):1075–82. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 11.Kirchner KK, Wagener JS, Khan TZ, et al. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am J Respir Crit Care Med. 1996;154 (5):1426–9. doi: 10.1164/ajrccm.154.5.8912759. [DOI] [PubMed] [Google Scholar]
- 12.Konstan MW, Hilliard KA, Norvell TM, et al. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation [published erratum appears in Am J Respir Crit Care Med 1995 Jan; 151 (1): 260] Am J Respir Crit Care Med. 1994;150 (2):448–54. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 13.McMorran BJ, Ouvry Patat SA, Carlin JB, et al. Novel neutrophil-derived proteins in bronchoalveolar lavage fluid indicate an exaggerated inflammatory response in pediatric cystic fibrosis patients. Clin Chem. 2007;53 (10):1782–91. doi: 10.1373/clinchem.2007.087650. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong DS, Grimwood K, Carlin JB, et al. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156 (4):1197–204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong DS, Grimwood K, Carzino R, et al. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ. 1995;310 (6994):1571–2. doi: 10.1136/bmj.310.6994.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birrer P, McElvaney NG, Rudeberg A, et al. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med. 1994;150 (1):207–13. doi: 10.1164/ajrccm.150.1.7912987. [DOI] [PubMed] [Google Scholar]
- 17.Noah TL, Black HR, Cheng P-W, et al. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis. 1997;175 (3):638–47. doi: 10.1093/infdis/175.3.638. [DOI] [PubMed] [Google Scholar]
- 18.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest. 1998;102 (6):1200–7. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balfour-Lynn IM, Laverty A, Dinwiddie R. Reduced upper airway nitric oxide in cystic fibrosis. Arch Dis Child. 1996;75 (4):319–22. doi: 10.1136/adc.75.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonfield TL, Konstan MW, Burfeind P, et al. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13 (3):257–61. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 21.Elphick HE, Demoncheaux EAG, Ritson S, et al. Exhaled nitric oxide is reduced in infants with cystic fibrosis. Thorax. 2001;56 (2):151–2. doi: 10.1136/thorax.56.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho LP, Innes JA, Greening AP. Exhaled nitric oxide is not elevated in the inflammatory airways diseases of cystic fibrosis and bronchiectasis. Eur Respir J. 1998;12 (6):1290–4. doi: 10.1183/09031936.98.12061290. [DOI] [PubMed] [Google Scholar]
- 23.Moeller A, Horak F, Jr, Lane C, et al. Inducible NO synthase expression is low in airway epithelium from young children with cystic fibrosis. Thorax. 2006;61 (6):514–20. doi: 10.1136/thx.2005.054643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas SR, Kharitonov SA, Scott SF, et al. Nasal and exhaled nitric oxide is reduced in adult patients with cystic fibrosis and does not correlate with cystic fibrosis genotype. Chest. 2000;117 (4):1085–9. doi: 10.1378/chest.117.4.1085. [DOI] [PubMed] [Google Scholar]
- 25.Ollero M, Junaidi O, Zaman MM, et al. Decreased expression of peroxisome proliferator activated receptor gamma in cftr−/− mice. J Cell Physiol. 2004;200 (2):235–44. doi: 10.1002/jcp.20020. [DOI] [PubMed] [Google Scholar]
- 26.Green S. PPAR: a mediator of peroxisome proliferator action. Mutat Res. 1995;333:101–9. doi: 10.1016/0027-5107(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 27.Vanden Berghe W, Vermeulen L, Delerive P, et al. A paradigm for gene regulation: inflammation, NF-kappaB and PPAR. Adv Exp Med Biol. 2003;544:181–96. doi: 10.1007/978-1-4419-9072-3_22. [DOI] [PubMed] [Google Scholar]
- 28.Davis PB, Gupta S, Eastman J, et al. Inhibition of proinflammatory cytokine production by PPAR gamma agonists in airway epithelial cells. Pediatr Pulmonol. 2003;36 (Suppl 25):268–9. [Google Scholar]
- 29.Auerbach H, Williams M, Kirkpatrick J, et al. Alternate-day prednisone reduces morbidity and improves pulmonary function in cystic fibrosis. Lancet. 1985;2 (8547):686–8. doi: 10.1016/s0140-6736(85)92929-0. [DOI] [PubMed] [Google Scholar]
- 30.Eigen H, Rosenstein B, FitzSimmons S, et al. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis: Cystic Fibrosis Foundation Prednisone Trial Group. J Pediatr. 1995;126 (4):515–23. doi: 10.1016/s0022-3476(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 31.Konstan MW, Byard PJ, Hoppel CL, et al. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332 (13):848–54. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 32.Konstan MW, Schluchter MD, Xue W, et al. Clinical use of ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2007;176 (11):1084–9. doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lands LC, Milner R, Cantin AM, et al. High-dose ibuprofen in cystic fibrosis: Canadian Safety and Effectiveness Trial. J Pediatrics. 2007;151 (3):249–54. doi: 10.1016/j.jpeds.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176 (10):957–69. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 35.Lands L, Stanojevic S. Oral non-steroidal anti-inflammatory drug therapy for cystic fibrosis. Cochrane Database Syst Rev. 2007;(4):CD001505. doi: 10.1002/14651858.CD001505.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Cystic Fibrosis Foundation Patient Registry. Annual Data Report to the Center Directors. Bethesda (MD): Cystic Fibrosis Foundation; 2006. 2007. [Google Scholar]
- 37.Chmiel JF, Konstan MW. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin Chest Med. 2007;28 (2):331–46. doi: 10.1016/j.ccm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler W, Williams M, Matthews WJ, et al. Progression of cystic fibrosis disease as a function of serum immunoglobulin G levels: a 5 year longitudal study. J Pediatr. 1984;104 (5):695–9. doi: 10.1016/s0022-3476(84)80946-4. [DOI] [PubMed] [Google Scholar]
- 39.Donati M, Haver K, Gerson W. Long-term alternate day prednisone therapy in cystic fibrosis [abstract] Pediatr Pulmonol. 1990;5:A322. [Google Scholar]
- 40.Lai H-C, FitzSimmons SC, Allen DB, et al. Risk of persistent growth impairment after alternate-day prednisone treatment in children with cystic fibrosis. N Engl J Med. 2000;342 (12):851–9. doi: 10.1056/NEJM200003233421204. [DOI] [PubMed] [Google Scholar]
- 41.Bhudhikanok GSB, Lim JB, Marcus RM, et al. Correlates of osteopenia in patients with cystic fibrosis. Pediatrics. 1996;97 (1):103–11. [PubMed] [Google Scholar]
- 42.Conway SP, Morton AM, Oldroyd B, et al. Osteoporosis and osteopenia in adults and adolescents with cystic fibrosis: prevalence and associated factors. Thorax. 2000;55 (9):798–804. doi: 10.1136/thorax.55.9.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flohr F, Lutz A, App EM, et al. Bone mineral density and quantitative ultra-sound in adults with cystic fibrosis. Eur J Endocrinol. 2002;146 (4):531–6. doi: 10.1530/eje.0.1460531. [DOI] [PubMed] [Google Scholar]
- 44.Fok J, Brown NE, Zuberbuhler P, et al. Low bone mineral density in cystic fibrosis patients. Can J Diet Pract Res. 2002;63 (4):192–7. doi: 10.3148/63.4.2002.192. [DOI] [PubMed] [Google Scholar]
- 45.Barry SC, Gallagher CG. Corticosteroids and skeletal muscle function in cystic fibrosis. J Appl Physiol. 2003;95 (4):1379–84. doi: 10.1152/japplphysiol.00506.2002. [DOI] [PubMed] [Google Scholar]
- 46.Cheng K, Ashby D, Smyth R. Oral steroids for cystic fibrosis. Cochrane Database Syst Rev. 2000;(2):CD000407. doi: 10.1002/14651858.CD000407. [DOI] [PubMed] [Google Scholar]
- 47.Greally P, Hussain M, Vergani D, et al. Interleukin-1 alpha, soluble interleukin-2 receptor, and IgG concentrations in cystic fibrosis treated with prednisolone. Arch Dis Child. 1994;71 (1):35–9. doi: 10.1136/adc.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dovey M, Aitken ML, Emerson J, et al. Oral corticosteroid therapy in cystic fibrosis patients hospitalized for pulmonary exacerbation: a pilot study. Chest. 2007;132 (4):1212–8. doi: 10.1378/chest.07-0843. [DOI] [PubMed] [Google Scholar]
- 49.Schiotz P, Jorgensen M, Flensborg E, et al. Chronic Pseudomonas aeruginosa lung infection in cystic fibrosis: a longitudinal study of immune complex activity and inflammatory response in sputum sol-phase of cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infections: influence of local steroid treatment. Acta Paediatr Scand. 1983;72 (2):283–7. doi: 10.1111/j.1651-2227.1983.tb09712.x. [DOI] [PubMed] [Google Scholar]
- 50.Van Haren EHJ, Lammers JWJ, Festen J, et al. The effects of the inhaled corticosteroid budesonide on lung function and bronchial hyperresponsiveness in adult patients with cystic fibrosis. Respir Med. 1995;89 (3):209–14. doi: 10.1016/0954-6111(95)90249-x. [DOI] [PubMed] [Google Scholar]
- 51.Nikolaizik WH, Schoni MH. Pilot study to assess the effect of inhaled corticosteroids on lung function in patients with cystic fibrosis. J Pediatr. 1996;128 (2):271–4. doi: 10.1016/s0022-3476(96)70407-9. [DOI] [PubMed] [Google Scholar]
- 52.Balfour-Lynn IM, Klein NJ, Dinwiddie R. Randomised controlled trial of inhaled corticosteroids (fluticasone propionate) in cystic fibrosis. Arch Dis Child. 1997;77 (2):124–30. doi: 10.1136/adc.77.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bisgaard H, Pedersen SS, Nielsen KG, et al. Controlled trial of inhaled budesonide in patients with cystic fibrosis and chronic bronchopulmonary Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 1997;156 (4):1190–6. doi: 10.1164/ajrccm.156.4.9612044. [DOI] [PubMed] [Google Scholar]
- 54.Dauletbaev N, Viel K, Behr J, et al. Effects of short-term inhaled fluticasone on oxidative burst of sputum cells in cystic fibrosis patients. Eur Respir J. 1999;14 (5):1150–5. doi: 10.1183/09031936.99.14511509. [DOI] [PubMed] [Google Scholar]
- 55.Wojtczak HA, Kerby GS, Wagener JS, et al. Beclomethasone diproprionate reduced airway inflammation without adrenal suppression in young children with cystic fibrosis: a pilot study. Pediatr Pulmonol. 2001;32 (4):293–302. doi: 10.1002/ppul.1122. [DOI] [PubMed] [Google Scholar]
- 56.Balfour-Lynn IM, Lees B, Hall P, et al. Multicenter randomized controlled trial of withdrawal of inhaled corticosteroids in cystic fibrosis. Am J Respir Crit Care Med. 2006;173 (12):1356–62. doi: 10.1164/rccm.200511-1808OC. [DOI] [PubMed] [Google Scholar]
- 57.De Boeck K, De Baets F, Malfroot A, et al. Do inhaled corticosteroids impair long-term growth in prepubertal cystic fibrosis patients? Eur J Pediatr. 2007;166 (1):23–8. doi: 10.1007/s00431-006-0198-9. [DOI] [PubMed] [Google Scholar]
- 58.Ren CL, Pasta DJ, Rasouliyan L, et al. Relationship between inhaled corticosteroid therapy and rate of lung function decline in children with cystic fibrosis. J Pediatr. 2008;153 (6):746–51. doi: 10.1016/j.jpeds.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Balfour-Lynn I, Walters S, Dezateux C. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev. 2000;1:CD001915. doi: 10.1002/14651858.CD001915. [DOI] [PubMed] [Google Scholar]
- 60.Brown H, Storey G, George W. Beclomethasone diproprionate: a new steroid aerosol for the treatment of allergic asthma. Br Med J. 1972;1 (5800):585–90. doi: 10.1136/bmj.1.5800.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20 (18):6891–903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Auphan N, DiDonato J, Rosette C, et al. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–90. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 63.Mittelstadt PR, Ashwell JD. Inhibition of AP-1 by the glucocorticoid-inducible protein gilz. J Biol Chem. 2001;276 (31):29603–10. doi: 10.1074/jbc.M101522200. [DOI] [PubMed] [Google Scholar]
- 64.Eddleston J, Herschbach J, Wagelie-Steffen AL, et al. The anti-inflammatory effect of glucocorticoids is mediated by glucocorticoid-induced leucine zipper in epithelial cells. J Allergy Clin Immunol. 2007;119 (1):115–22. doi: 10.1016/j.jaci.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 65.Hart L, Lim SAM, Adcock IAN, et al. Effects of inhaled corticosteroid therapy on expression and DNA-binding activity of nuclear factor kappa B in asthma. Am J Respir Crit Care Med. 2000;161 (1):224–31. doi: 10.1164/ajrccm.161.1.9809019. [DOI] [PubMed] [Google Scholar]
- 66.Wilson SJ, Wallin A, Della-Cioppa G, et al. Effects of budesonide and formoterol on NF-kappa B, adhesion molecules, and cytokines in asthma. Am J Respir Crit Care Med. 2001;164 (6):1047–52. doi: 10.1164/ajrccm.164.6.2010045. [DOI] [PubMed] [Google Scholar]
- 67.Cazes E, Giron-Michel J, Baouz S, et al. Novel anti-inflammatory effects of the inhaled corticosteroid fluticasone propionate during lung myofibroblastic differentiation. J Immunol. 2001;167 (9):5329–37. doi: 10.4049/jimmunol.167.9.5329. [DOI] [PubMed] [Google Scholar]
- 68.Corvol H, Fitting C, Chadelat K, et al. Distinct cytokine production by lung and blood neutrophils from children with cystic fibrosis. AJP Lung Cell Mol Physiol. 2003;284 (6):L997–1003. doi: 10.1152/ajplung.00156.2002. [DOI] [PubMed] [Google Scholar]
- 69.Brutsche MH, Brutsche IC, Munawar M, et al. Comparison of pharmacokinetics and systemic effects of inhaled fluticasone propionate in patients with asthma and healthy volunteers: a randomised crossover study. Lancet. 2000;356 (9229):556–61. doi: 10.1016/S0140-6736(00)02581-2. [DOI] [PubMed] [Google Scholar]
- 70.Harrison TW, Tattersfield AE. Plasma concentrations of fluticasone propionate and budesonide following inhalation from dry powder inhalers by healthy and asthmatic subjects. Thorax. 2003;58 (3):258–60. doi: 10.1136/thorax.58.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison TW, Wisniewski A, Honour J, et al. Comparison of the systemic effects of fluticasone propionate and budesonide given by dry powder inhaler in healthy and asthmatic subjects. Thorax. 2001;56 (3):186–91. doi: 10.1136/thorax.56.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konstan MW, Butler SM, Wohl MEB, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142 (6):624–30. doi: 10.1067/mpd.2003.152. [DOI] [PubMed] [Google Scholar]
- 73.Morgan WJ, Butler SM, Johnson CA, et al. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S. and Canada. Pediatr Pulmonol. 1999;28 (4):231–41. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 74.Parmar JS, Howell T, Kelly J, et al. Profound adrenal suppression secondary to treatment with low dose inhaled steroids and itraconazole in allergic bronchopulmonary aspergillosis in cystic fibrosis. Thorax. 2002;57 (8):749–50. doi: 10.1136/thorax.57.8.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt J, Davidson A, Seear M. Is the acquistion of Pseudomonas in cystic fibrosis patients increased by the use of inhaled corticosterois? Unexpected results from a double blind placebo controlled study [abstract] Pediatr Pulmonol. 1997;124 (Suppl 14):A318. [Google Scholar]
- 76.EPR-3. Expert Panel Report 3: guidelines for the diagnosis and management of asthma. National Heart, Lung and Blood Institute National Asthma Education and Prevention Program; Bethesda (MD): 2007. Aug 28, [Google Scholar]
- 77.Akinbami L. Asthma prevalence, health care use and mortality: United States, 2003–05. Hyattsville (MD): Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 78.Modi AC, Lim CS, Yu N, et al. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006;5 (3):177–85. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]