Abstract
This study was designed to examine the nature of object imitation performance in early autism. We hypothesized that imitation would be relatively preserved when behaviors on objects resulted in salient instrumental effects. We designed tasks in which, in one condition, the motor action resulted in a salient, meaningful effect on an object, whereas in the other condition, the same action resulted in a less salient effect because of differing object characteristics. The motor aspects of the tasks did not vary across conditions. Four participant groups of 2- to 5-year-olds were examined: 17 children with early-onset autism, 24 children with regressive onset autism, 22 children with developmental delays, and 22 children with typical development. Groups were matched on nonverbal skills, and differences in verbal development were examined as a moderator of imitative ability. Results revealed an interaction of group by condition, with the combined autism group failing more tasks than the combined comparison groups, and failing more tasks in the less salient condition than in the more salient condition, as hypothesized. Analyses of autism subgroups revealed these effects were primarily because of the regression onset group. Accuracy of motor performance was examined by analyzing errors. Among children passing imitative acts, there were no group differences and no condition effects in the number, type, or pattern of performance errors. Among children passing the tasks, the group with autism did not demonstrate more emulation errors (imitating the goal but not the means) than other groups. There was no evidence that either motor or attentional aspects of the tasks contributed to the poorer imitative performance of the children with autism.
Imitating another person’s behavior has long been seen as a powerful human learning mechanism (Baldwin, 1906; Bruner, 1972). Imitation of another person’s facial and vocal behavior, gestures, and actions begins early in development, with some imitative responses present at birth (Meltzoff & Moore, 1989) and a rapid expansion of intentional imitative responses seen in the second year of life (Kaye & Marcus, 1981; Masur & Ritz, 1984; McCabe & Uzgiris, 1983). Early childhood is an especially imitative period, and the ability to learn new behaviors through observation is considered to be a very important learning tool in the rapid development of young children’s behavioral repertoires.
A major issue in imitation studies concerns the multiple ways that imitation has been defined by different groups and the definition of imitation that is used in a particular study. Studies of human infants tend to define imitation as motor actions that match action patterns of the model. However, a matching action can occur via the process of emulation, in which the observer observes a means–end action on an object and then achieves the same goal state, but through whatever means is most readily available (Huang, Heyes, & Charman, 2002). This may look imitative, but the process has not involved copying the actions of the other; instead, only the goal has been copied. Tomasello, Savage-Rum-baugh, and Kruger (1993) have suggested a definition of imitation that involves copying both the means and the goal of the model, and he has examined the emulation–imitation distinction in animals and young children using paradigms that use a novel or unusual and unnecessary means to accomplish a specific goal. In the present paper, the term imitation is defined as intentional production of motor actions and postures that match both the means and the ends of the model.
Imitation appears to serve several functions (Uzgiris, 1981). One involves learning about goal-directed actions, the tools, and tasks of the world, referred to here as the apprenticeship function. This type of imitation serves instrumental learning and apprenticeship involving cultural transmission of skills and behaviors (Piaget, 1962). This function is consciously available and involves intentional actions aimed at copying the means–end relations observed in the model’s use of objects.
Imitation may also function to establish or maintain social relations. Imitation enhances positive feelings between social partners, fosters shared emotional states, and facilitates communication and empathy (Chartrand & Bargh, 1999; Iacoboni, 2006; Niedenthal, Barsalou, Winkielman, Krauth-Gruber, & Ric, 2005; Richardson, Marsh, & Schmidt, 2005). This social–communicative function of imitation is central to the theories suggesting that early imitation fosters the development of self-other understanding.
Of interest, the underlying function of the task appears to influence what matching process young children will use to reproduce the response. Young children appear to produce a more emulative response in an imitation task with a very meaningful means–end relation and a more imitative response with a task that is less easily mapped onto means–end schemas (the rational imitation effect; Gergely, Bekkering, & Kiraly, 2002). Similarly, when the intention of the model’s action is clear, children tend to reproduce the goal via emulation, whereas when they do not grasp the purpose of the action they are more likely to imitate the precise manner in which the model produced the target action (Williamson & Markman, 2006). In the present study we examine this distinction between emulation and imitation in two ways: by using contrasting conditions involving functional versus nonfunctional objects in the same means–end action patterns, and by rating errors involving the imitation of modeled actions unnecessary for creating the goal.
Imitation in Autism
In autism research, difficulty with imitation of other people’s movements and actions has been well documented in a variety of studies across the past 30 years (see Rogers & Williams, 2006, for the most recent review of this literature). Problems with imitation discriminate children with autism from those with other developmental disorders as early as age 2 (Charman et al., 1997; Rogers, Hepburn, Stackhouse, & Wehner, 2003; Stone, Ousley, & Littleford, 1997), and continue into adulthood (Bernier, Dawson, Murias, &Webb, 2007; Rogers, Bennetto, McEvoy, & Pennington, 1996). Studies have tended to use three kinds of tasks: actions on objects, manual and postural movements, and oral–facial movements. Persons with autism typically demonstrate impaired performance compared to controls on all three types of tasks (for some examples, see Charman et al., 1997; DeMyer et al., 1972; Ohta, 1987; Rogers et al., 1996, 2003; Stone et al., 1997), although imitation of actions on objects is generally considered to be less affected than gestural imitation (DeMyer et al., 1972; Hobson & Lee, 1999; Meyer & Hobson, 2004).
Imitation is considered by some to be one of the core neuropsychological deficits in autism, present very early in development, with possible cascading effects on social relations, interactions, and learning throughout the life span (Meltzoff & Gopnik, 1993; Rogers & Pennington, 1991;Williams, Whiten, Suddendorf, & Perrett, 2001). If imitation deficits contribute to the overall development of the behavioral phenotype of the disorder, then our understanding of the phenotype and of brain–behavior relations will be enhanced by understanding which of the many neuropsychological processes involved in imitation are affected in autism.
Several groups have suggested that imitation problems in autism reflect problems involving the social versus instrumental nature of the modeled action. Hobson and Lee (1999) reported no autism-specific differences in adolescents imitating very simple functional actions on objects. However, the group with autism was impaired at imitating the dynamic style of the experimenter’s movement. The authors interpreted this finding as indicating that imitation problems in autism reflect a difficulty in processing a social–affective stimulus (and the lack of innate processes of identification in autism); however, the two types of actions were not matched for difficulty level.
Ingersoll, Schreibman, and Tran (2003)published a similar finding in which initial autism differences in frequency of imitation of simple acts was eliminated when the actions resulted in strong sensory effects. Their interpretation focused on a different mechanism: involving a decreased motivation to imitate others.
The social versus instrumental distinction in autism involves differences at the level of stimulus input. However, other explanations of the imitation problems in autism discuss difficulties at the output, or motor execution, level: the dyspraxia explanation of imitation problems in autism (Mostofsky et al., 2006; Rogers, 1999). As reviewed byMostofsky et al. (2006), many studies have reported that children with autism have impaired motor performance. Several studies of older, high-functioning children and adolescents have documented a relation between motor impairments on standard measures and imitative abilities (Bennetto, 1999b; Smith & Bryson, 1998). Thus, the possible role of motor impairment needs to be considered in studies of imitation in autism, and this was addressed in the design of the current study in two ways, by using the same motor actions across conditions, and by examining execution errors.
Purpose of the Present Study
The results from imitation studies of actions on objects, gestural, affective, and facial imitation in autism (Rogers & Williams, 2006) suggest that the nature of the task has considerable effect on the imitative performance of people with autism. The present study was designed to explore how the nature of the task affects imitative performance in autism. We sought to test the hypothesis that accuracy of imitative performance in autism depends on the function of the task. We hypothesized that the apprenticeship, or instrumental learning function of imitation, indexed by functional actions with objects that stress means–end relations, would be relatively spared, whereas the social function of imitation, indexed by imitative acts that lack a salient means–end or instrumental function, would be more affected, exposing the social nature of the autism imitation deficit. Whether phrased as a lack of identification (Hobson, 1995; Hobson & Hobson, 2007), a deficit in “like me” understanding (Meltzoff, 2007), or a lack of self-other mapping (Rogers & Pennington, 1991), we expected that children with autism would be significantly less likely than others to imitate the posture and actions of the other in an object task that lacked instrumental salience.
To test the hypothesis, two sets of actions on objects were designed. The first set of items, characterizing the functional condition, was designed so that each target action resulted in a salient instrumental effect or meaningful goal. To discriminate between imitation (copying both the means and the goal of the modeled act) and emulation (copying the goal but not the means), each action had some novel and unnecessary feature that allowed us to examine the precision of the imitation of the means. Examples of these actions include using the teeth to tear the wrapper off a lollipop so that the candy could be licked, and using the mouth to hold a pen so that a line could be drawn on a piece of paper.
The second set of object imitation items made up the nonfunctional condition and involved identical actions, but with different objects. Each nonfunctional task was matched to a corresponding task in the functional condition on all object-directed aspects of the action such as grasp of the object, dynamics of the movement, and positioning of the object with respect to the body or to other objects involved in the action. In addition, the target objects in the two conditions were matched on such features as size, shape, and general appearance. However, the actions had a less salient effect on these objects than on the objects in the functional condition. Examples involve using the teeth to tear a square piece of paper as opposed to a lollipop wrapper; and using the mouth to move a tongue depressor as opposed to a marker across a piece of paper.
The functional condition was considered to highlight the apprenticeship function of imitation, in that a meaningful and interesting effect on an object resulted from carrying out the action. The nonfunctional condition was considered to highlight the social function of imitation, in that the main reason to imitate a person doing a rather meaningless act on an object is to join them in the activity, to share the experience, or to be like the other person. We predicted that children with autism would show a greater tendency to imitate the more meaningful, functional actions than the less functional acts, unlike children with delayed or typical development, who we hypothesized would demonstrate either no effect of condition or reduced accuracy in the meaningful condition, as has been described previously (Gergely et al., 2002; Williamson & Markman, 2006).
A Critical Subgroup Analysis
Autism is no longer considered to be a homogeneous disorder at the biological level. Instead, it is currently considered to consist of differing subgroups with differing underlying biologies (Miles et al., 2005) that result in a final common behavioral constellation of symptoms. One such set of subgroups may involve those with differing onset patterns. Regressive-onset autism may have a different etiology than early-onset autism (Miles et al., 2005). Although several studies have examined onset differences in relation to IQ and language use (Luyster et al., 2005; Richler et al., 2006; Rogers & DiLalla, 1990; Werner, Dawson, Munson, & Osterling, 2005), no study has yet examined imitation skills. Given that imitation may begin functioning in the first days of an infant’s life (Meltzoff & Moore, 1989), and that early difficulty with imitation may potentially lead to a cascade of developmental problems associated with the autism phenotype (Rogers & Pennington, 1991), it is important to examine the homogeneity of imitation impairment across potential subgroups. Because regression typically occurs at a developmental period well past the period in which intentional imitation is well established, any imitation deficit associated with regressive onset autism would be expected to be a nonspecific and general deficit. Thus, in addition to the primary objective of examining the effect of the nature of the task on imitation in autism, a second objective of the present paper is to examine imitation performance in two different autism subgroups (those with early onset, and those with regressive onset), with the hypothesis that intentional imitation skills will be more severely affected in the early-onset subgroup than the regression subgroup.
Method
Procedures
This study was conducted under the approval of the institutional research review board at the University of California, Davis. The study was explained to the parents orally and in writing, written consent obtained, and any questions addressed gathering any data.
Participants
Eighty-five participants were included comprising four groups: early-onset autistic disorder (EOAD; n = 17), regressive-onset autistic disorder (RAD; n = 24), developmental delay of mixed etiology (DD; n = 22), and typically developing children (n = 22). All participants were recruited from the M.I.N.D. Institute Research Participant Recruitment Core, surrounding area regional centers, and word of mouth.
Children with autism had been previously diagnosed with an autism spectrum disorder by clinicians independent of this project, met current DSM-IV criteria of autistic disorder, and met standard clinical cutoffs for autistic disorder on the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999) and Autism Diagnostic Interview—Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994). No child had fragile X syndrome, seizures, or any other biological condition related to autism. The group of 41 children with autism was subdivided into two groups based on onset status. The early-onset group was defined by parents’ responses of no loss (score of 0) to two items on the ADI-R: question 11 (loss of at least five words), and question 25 (probable or definite loss of social abilities, such as social interest, engagement with others, etc.). The regression group was similarly defined by a score of 1 on question 11 and a score greater than 1 on question 25. Test–retest reliability has previously been established on all items of the ADI-R (Lord et al., 1994) and long-term reliability of parental reports of skill loss was recently reported as being higher than 80% (Luyster et al., 2005).
Preliminary analysis of ADOS and ADI-R scores revealed no significant differences in symptom severity between onset subtypes. We specifically recruited children with regression in order to have relatively equal numbers in the onset groups. Children in the early-onset subgroup ranged in age from 26 to 61 months; children in the regression subgroup ranged in age from 31 to 59 months.
The children in the DD group were recruited to provide both a chronological and an overall developmental age match for the groups with autism. All had normal vision and normal hearing, unimpaired hand use, and were mobile. None were considered by any clinician on our team to have autism and none met criteria for autistic disorder on the ADOS. The DD group was heterogeneous, and included 11 children with an established language delay, 3 with Down syndrome, 5 with global developmental delay, 2 with sensory integration disorder, and 1 with an unknown genetic syndrome. Children with DD ranged in age from 30 months to 59 months.
The typically developing group (TD) was recruited to provide a developmental age comparison for the children with autism. Children in the TD group all had normal hearing and vision and did not present with any significant medical or developmental concerns. None of these children met criteria for an autism spectrum disorder on any of the diagnostic instruments used in this study. Children in the TD group ranged in age from 14 to 41 months.
The majority of participants were Caucasian, middle class, and male. Examination of group differences on demographic variables (gender, ethnicity, and socioeconomic status [SES]) revealed no significant differences for ethnicity, χ2 (3) = 1.45, p = .69, or for SES (Hollingshead, 1975) F (3, 83) = 0.81, p = .45. There was a significant effect for gender, χ2 (3) = 12.10, p < .007, with a significantly greater proportion of females in the typical group (45.5%) than in the autism groups (9.8%) or the DD group (31.8%). Further analyses of any gender differences or Gender × Group interactions did not suggest any effect or interaction on imitation, and we did not include gender in any subsequent analyses.
The means, standard deviations, and omnibus F tests for group comparisons on chronological age, and developmental age measures are presented in Table 1. Although the 7.8-month difference between the early-onset and the regression subtypes of autism was significant (t = 2.49, p < .05), comparisons between subtypes for mental age scores (verbal, nonverbal, and overall mental age) were not significant (indeed, all differences were less than 1.6 months). Comparisons between the two subtypes on developmental quotients (mental age divided by chronological age) also revealed no significant difference (t = 1.21, p = .23). The significant difference among the ASD, DD, and typical groups on verbal mental age (VMA) scores were addressed in the analyses by statistically controlling for VMA in all subsequent analyses of imitation performance.
Table 1.
Chronological and developmental ages by group
| Age | Early Onset (n = 17) |
Regression (n = 24) |
DD (n = 22) |
Typical (n = 22) |
F | Signif. |
|---|---|---|---|---|---|---|
| Chronological | ||||||
| Mean | 39.15 | 46.95 | 44.36 | 23.36 | 30.60 | p < .001 |
| SD | 10.29 | 9.34 | 8.86 | 7.80 | ||
| Range | 26–61 | 31–59 | 30–59 | 14–41 | ||
| Overall mental | ||||||
| Mean | 23.31 | 24.30 | 29.82 | 25.57 | 1.88 | p = .14 |
| SD | 9.94 | 8.86 | 10.39 | 9.12 | ||
| Range | 13–44 | 12–52 | 12–49 | 16–47 | ||
| Verbal mental | ||||||
| Mean | 19.82 | 20.19 | 27.55 | 25.73 | 3.12 | p < .05 |
| SD | 10.46 | 9.66 | 10.82 | 9.68 | ||
| Range | 5–44 | 8–47 | 9–50 | 15–49 | ||
| Nonverbal mental | ||||||
| Mean | 26.79 | 28.42 | 32.09 | 25.41 | 1.97 | p = .13 |
| SD | 10.40 | 8.50 | 10.61 | 8.80 | ||
| Range | 15–55 | 16–57 | 14–49 | 17–48 |
Note: DD, developmental delay.
Measures
Symptoms of autism: ADI-R
The ADI-R (Lord et al., 1994) is a structured, standardized parent interview developed to assess the presence and severity of symptoms of autism in early childhood across all three main symptom areas involved in autism: social relatedness, communication, and repetitive or restrictive behaviors. Interviewers were trained according to research criteria. Reliability was maintained at 85% on the truncated (0–2) scoring system for 20% of participants across the period of data collection.
ADOS—WPS Edition
The ADOS (Lord et al., 1999) is a semistructured standardized interview using developmentally appropriate social and toy-based interactions in a 30–45 min interview that elicits symptoms of autism in four areas: social interaction, communication, play, and repetitive behaviors. The ADOS consists of four different modules, each directed at a particular level of language ability. In the present study, all children received either Module 1 for preverbal children or those just beginning to speak, or Module 2 for children with reliable phrase speech. All examiners were trained to reliability of 85% or better item agreement over three consecutive administrations with a psychologist who was research reliable with the team at University of Michigan. Reliability was maintained at 85% by double-scoring 20% of protocols across the period of data collection.
Developmental
Mullen Scales of Early Learning (MSEL; Mullen, 1989). The MSEL is a standardized developmental test for children ages 3 months to 64 months. The test consists of four subscales used in establishing verbal and nonverbal abilities: fine motor, visual reception, expressive language, and receptive language. The MSEL was administered according to standard instructions, and all testers were trained and supervised by experienced clinicians. Mental age scores for verbal (receptive and expressive) and for nonverbal (fine motor and visual reception) as well as for overall intellectual functioning were used as matching criteria dues to floor effects on standardized scores for some children.
Experimental imitation task
The imitation task involved two conditions in which the same motor movement was applied to two objects of similar size and shape. In the functional condition, the movement activated the object’s affordance and resulted in an interesting instrumental act. In the nonfunctional situation, the same movement was applied to an object that afforded no such interesting effect. Table 2 delineates each pair of functional and nonfunctional items and the specific performance criteria, object affordance, and movement features of each item. The task was administered as part of a larger battery of tasks not being reported here. Imitation items were presented after a variety of playful interactions, in a counterbalanced order by task and functionality.
Table 2.
Experimental tasks
| Items |
Performance Criteria |
Key Item Features |
||||||
|---|---|---|---|---|---|---|---|---|
| Functional | Nonfunctional | Bilateral/ Unilateral |
Position | Location | Movement Dynamic |
Repetition | Object Affordance |
Unique Movement |
| Shake maraca | Shake potato masher | Object hand moves, other hand still | Grasps radially | Object strikes opposite hand | Lateral movement | Repeated movement (×3) | Makes sound | Hit against palm |
| Scribble marker | Scribble straw | Holds object in only one hand | NA | Marker tip on paper surface | Moves marker back and forth | Repeated movement (×3) | Makes line on paper | Scribbled wavy line |
| Blow cotton | Blow stone | NA | NA | Object on table surface | Child leans and blows on object | NA | Moves object across table | Blows on object to move |
| Shake bells | Shake cloth | Holds object in only one hand | NA | Holds above eye level | Lateral movement | Repeated movement (×3) | Makes sound | Waves over head |
| Line with marker in mouth | Line with stick in mouth | NA | Object held in mouth | Object tip on paper surface | Child moves head to move object tip | NA | Makes line on paper | Marker held in mouth |
| Poke putty | Poke sponge | Uses only one hand | Uses thumb pointed downward | NA | Drill thumb into object with rotation | NA | Makes hole in putty | Twists thumb into putty |
| Tear lollipop wrapper | Tear paper | Holds object in only one hand | NA | Places object between teeth | Pulls object in teeth away from mouth | NA | Removes wrapper | Tears with teeth |
| Lick lollipop | Lick plastic disk | Holds object in only one hand | NA | Brings object to protruding tongue | Pulls object down across tongue | Repeated movement (×3) | Tastes lollipop | Pulls object across tongue |
The experimenters were blind to the onset status of each child and to the precise hypotheses of the study, but not to group membership. Video coders were blind to all hypotheses, onset status, and group membership. The adult sat across from the child at a bare table, captured the child’s eye contact and attention, modeled a task three times in rapid succession, then gave the instruction, “Now you do it” and handed the materials to the child. If the child did not respond with an imitative attempt on the first trial, the task was administered up to two more times. Each child’s first imitative attempt on a task was used in later coding and scoring. Rewards were used to motivate all the children to attempt the tasks. All children were rewarded with either a social or tangible reward after each cooperative attempt that they made. For each child, rewards were used that the child sought and responded to positively.
All imitation items were coded from video recordings using Noldus Observer 5.0. Split-screen coding was used so that child responses could be compared to the adult model. The child’s response to each act was first coded using a 3-point pass–fail coding scale: 0 (if the child showed no response), 1 (if the child showed a contingent response that involved acting on the object but in a way that was unrelated to the model), and 2 (if the child showed some degree of imitation but with any number of errors as defined in Table 2).
The accuracy of imitation was also measured by coding the number of errors on each task made by the child. Six error categories were coded: bilateral versus unilateral (BU), location of action on target object (LC), limb position (LP), lack of repetition (RP), hand position/face position (HP), movement dynamics (MD), and emulation errors. Each specific error except for emulation errors was defined for each task within the coding instructions for that task.
For example, one functional task, Shake Maraca, involved hitting a maraca against the palm of the other hand. As shown below, the task was scored from0 to 4 and three specific errors were coded, LC, MD, and RP, each defined in the scoring criteria.
Scores
4—Hits maraca against opposite hand (LC) in lateral movement (MD) repeatedly (RP)
3—Shakes maraca in the air (not against table) but does not hit hand (LC error), or no lateral movement (MD error), OR not repeated (RP error)
2—Shakes maraca in air but 2 or more of the above errors apply
1—Contingent movement in hand or arm, or manipulates maraca, but does not meet target criteria
0—No contingent movement
Below is an example of scoring criteria for a nonfunctional action, tearing a piece of paper with the teeth. Three errors are defined: BU, LC, and MD.
4—Holds paper with one hand (BU), places paper between teeth (LC), and pulls away from mouth (MD) tearing the paper or pulling it rapidly from mouth (in attempt to tear)
3—Puts paper to mouth but holds with two hands (BU error), fails to put between teeth (LC error), OR does not pull away rapidly (MD error) or tear
2—Puts paper to mouth but two or more of the above errors apply
1—Some contingent movement of hand, arm, head, or face, but does not put paper to mouth
0—No contingent movement
A detailed list of scoring criteria for all items is available from the first author.
Emulation errors were identified for functional tasks; these involved a failure to enact the unique movement involved in the item. These unique movements are listed in Table 2. As an example, for the shake maraca item, the unique movement consisted of hitting the maraca against the palm. This movement was not necessary for the functional aspect of the item: making a sound. Thus, if a child shook the maraca in the air, rather than hitting it against the palm, this would constitute an emulation error indicating that the child was emulating the functional result of the action as opposed to imitating the means of the action.
Approximately 30% (26 out of 85) of the videos were coded by a second coder and reliabilities were checked routinely. The mean kappa for pass–fail scoring across items was .87, ranging from .65 for line with tongue depressor to 1.00 for shake bell loop. The mean intraclass correlation coefficient for error codes across all items was .77, ranging from .63 for location errors to .86 for hand position errors.
Two primary variables were generated from video coding: (a) the number of imitation items that were passed on the two scales, and (b) the total number of errors made on each of the two scales. Scores for each child were calibrated to the same count metric by correcting for the number of opportunities and pro-rating using the respective proportion (e.g., number of errors divided by total possible errors, for the number of items passed multiplied by the number of possible errors for the test).
Reliability analysis conducted on pass/fail scores revealed fair internal consistency for the functional scale (α = .67), and good internal consistency for the nonfunctional scale (α = .82). Internal consistency analyses of error scores per scale revealed an average item-total rank-order correlation of .46 for both the functional and nonfunctional scales, with individual item-total correlations ranging from .31 to .73. The correlation between error scores for the two scales was high (r = .59, p < .001), and was similar across groups, as revealed by a regression analysis that indicated no interaction between group and functional item error scores as a predictor of nonfunctional item error scores (range r = .43 for the DD group to r = .74 for the typical group).
Examination of the summary scale scores revealed that for the number of items passed, the distribution was highly negatively skewed, so we represented the data as a count of the number of items failed that produced a positively skewed distribution typical of count data that might result from a poisson process. The use of total number of items failed then allowed us to employ generalized estimating equations as a way to model the asymmetric count data as a negative binomial distribution and also to examine the effect of the repeated measures factor of condition (functional vs. nonfunctional) by modeling the within subject correlations (see Ballinger, 2004; Liang & Zeger, 1986). Examination of the number of errors as a second dependent variable likewise revealed positive skew and was thus also modeled as a negative binomial distribution using generalized estimating equations.
Results
Participant cooperation with the tasks
Child visual attention was gained before the tasks were administered, and performance was only scored if the videos documented that the child was watching the examiner, so child visual attention as a variable was managed in the methodology. There was no significant effect of order on the imitation scores for the functional and nonfunctional scales.
We then examined whether the group with autism participated less than the other groups. Across the sixteen tasks, 21 children failed to perform a contingent act for at least one of the items (range = 0–5) within the first three trials. A Kruskal–Wallis analysis of group by number of no-response items revealed no systematic difference among groups for the number of no-response scores. In addition, an analysis of variance (ANOVA) of mean time-lag across all 16 items revealed no significant differences between groups. Finally, we examined the total number of demonstrations modeled by the experimenter across items and founda nonsymmetric distribution with positive skew and a standard deviation (2.52) much larger than the mean (1.59), with the majority of children exhibiting a score of zero. As such, generalized estimating equations were used as a way to model the asymmetric count data as a negative binomial distribution with a log link function. The use of generalized estimating equations also allowed us to examine the effect of the repeated measures factor of condition by modeling the within-subject correlations. This analysis revealed a significant main effect for group (Wald χ2 = 9.96, df = 3, p < .05) and a significant main effect for condition (Wald χ2 = 7.55, df = 1, p < .01). Post hoc testing of the group main effect revealed that the early-onset autism group required significantly more additional item demonstrations (M = 2.16, SD = 1.82) than the typical group (M = 0.92, SD = 0.92; t = 2.66, df = 38, p < .01) and the DD group (M = 1.09, SD = 1.35; t = 1.98, df = 38, p < .05). All other group comparisons were not significant. Examination of the main effect for condition revealed that a greater number of additional item demonstrations were given in the nonfunctional condition (M = 1.70, SD = 2.18) than in the functional condition (M = 1.05, SD = 1.41; t = 2.62, df = 84, p < .01). The main effect for VMA was also significant (Wald χ2 = 7.40, df = 1, p < .01) with an increase of roughly 0.4 demonstrations for each decrease of 1 SD in VMA. The number of item demonstrations in the functional imitation condition was unrelated to the number of items failed (Spearman r = .12, p = .26) but was significantly correlated with the error score (r = .32, p < .01). For the nonfunctional condition, the number of item demonstrations was significantly correlated with the number of items failed (r = .48, p < .001) and significantly correlated with the error score (r = .31, p < .001. Thus, the number of additional item demonstrations appeared to be a rough index of pass/fail and errors scores: the greater number of item demonstrations, the worse the overall performance.
Pass–fail performance
Pass–fail performance on each of the subscales was analyzed using the number of items failed modeled as a negative binomial distribution using generalized estimating equations, with VMA used as a covariate. The type of imitation condition (functional vs. nonfunctional) served as the two-level repeated measures factor and diagnosis (early-onset autism, regression, DD, and typical) served as the four-level between subjects factor. Planned comparisons for group main effects and for simple effects were conducted by looking at early-onset autism versus regression; DD versus typical; and autism (early-onset and regression collapsed) versus controls (DD and typical collapsed). Analysis revealed main effects for group (Wald χ2 = 9.42, df = 3, p < .05), condition (Wald χ2 = 4.94, df = 1, p < .05), and VMA (Wald χ2 = 32.87, df = 1, p < .001). There was also a marginally significant three-way interaction between group, condition, and VMA (Wald χ2 = 7.50, df = 3, p = .06).
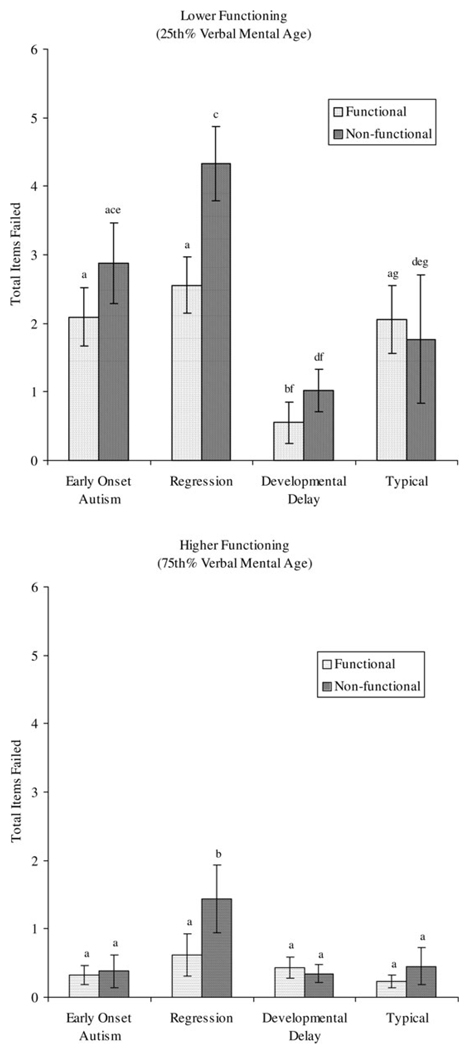
Follow-up tests of the three-way Condition × Group × Verbal Mental Age interaction effect were conducted by examining estimated marginal means for Group × Condition factors at the 25th and the 75th percentile scores of VMA separately (i.e., 14 months and 29.63 age equivalent scores, respectively). They were derived from the entire sample to represent the full range of scores associated with imitation performance regardless of diagnostic group, and then to provide idealized points of comparison between diagnostic groups of precisely the same VMA.
Estimated marginal means used for follow-up comparisons were calculated using these percentile scores in the model’s regression equation. Means and standard errors are depicted in Figure 1. Results of simple comparisons of group and imitation conditions at the 25th percentile of VMA (i.e., 14 months) revealed that the combined autism group failed significantly more items than the combined comparison group (DD and typical) in both the functional condition (t = 2.89, df = 84, p < .01) and the nonfunctional condition (t = 3.86, df = 84, p < .001). In individual group comparisons, the DD group failed significantly fewer items than the typical group in the functional condition (t = 2.61, df = 43, p < .01), but not in the nonfunctional condition. There were no differences between the early-onset and regression group in either condition. More items were failed in the nonfunctional condition than the functional condition for the combined autism group (t = 4.75, df = 40, p < .001), but not for the combined comparison group. Analysis of autism subgroups revealed that this condition effect was only significant for the regression group (t = 5.18, df = 23, p < .001).
Figure 1.
Group by imitation condition: items failed (at 25th and 75th percentiles for VMA). Means with the same subscripts are not significantly different.
Results of the same set of simple comparisons of group and imitation conditions at the 75th percentile ofVMA(i.e., 29.63 months) revealed only two significant differences: the regression group failed more items than the early-onset group in the nonfunctional condition (t = 2.12, df = 40, p < .05) and failed significantly more items in the nonfunctional condition than in the functional condition (t = 2.87, df = 23, p < .01).
Imitation accuracy
The next analysis examined the following question: given each child’s imitative responses, to what degree does imitative accuracy vary as a function of diagnosis and of condition? Accuracy scores were indexed by the total number of errors on passed items on a given scale. The total number of errors showed significant positive skew and was modeled as a count distribution (i.e., negative binomial) using generalized estimating equations. Pass–fail scores and accuracy scores were moderately correlated (Spearman ρ = .54, p < .001), so analyses of accuracy scores incorporated pass/fail scores as a covariate to account for this correlation.
Results revealed a marginally significant main effect for group (Wald χ2 = 7.24, df = 3, p = .07) and significant main effects for pass–fail scores (Wald χ2 = 9.65, df = 1, p < .01) and for VMA (Wald χ2 = 21.23, df = 1, p < .001). The main effect for condition was not significant. Examination of higher order interaction effects revealed no significant two-way or three-way interaction effects. Planned comparisons of group revealed no difference between the combined autism group (M = 7.67, SD = 3.21) and the combined comparison group (M = 7.14, SD = 2.81), and no difference between the DD group (M = 6.68, SD = 2.41) and the typical group (M = 7.65, SD = 2.83). There was, however, a significant difference between the early-onset group (M = 8.66, SD = 2.63) and the regression group (M = 6.92, SD = 2.76; t = 2.13, df = 40, p < .05).
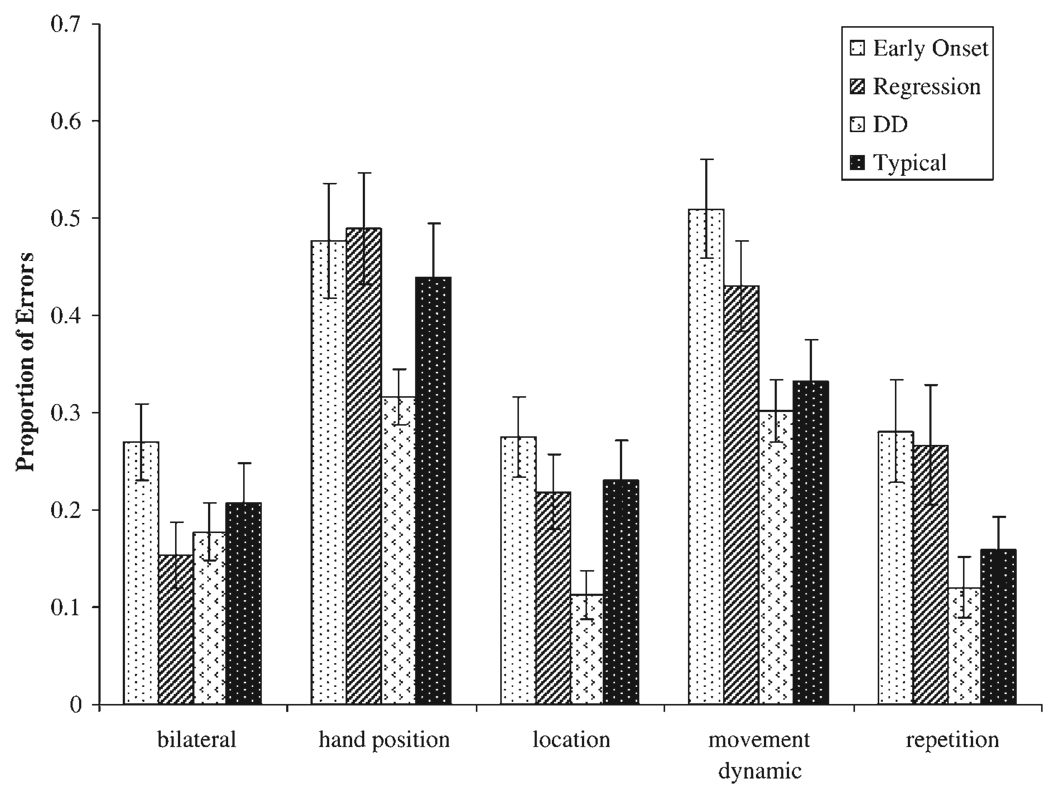
Error pattern analysis
To examine possible relations between types of errors and group differences, we examined the proportion of errors made by each group for five error types: bilateral errors, hand position errors, location errors, movement dynamic errors, and repetition errors. Proportion scores were calculated by dividing the total number of an error type by the total number of possible errors for that particular error type (e.g., total location errors made by each child divided by the total number of location errors that could have been made given the items passed by that child).1 Proportion scores were calculated across imitation condition and are shown in Figure 2. Analyses were conducted using a repeated measures analysis of covariance with VMA as a covariate, group as a four-level between-subjects factor, and error type as a five-level within-subjects factor. Results revealed a significant main effect for type of error, F (4, 316) = 15.23, p < .001, with hand position errors and movement errors being made proportionately more often than any other errors. We also found a significant main effect for group, F (3, 79) = 3.18, p < .05, due primarily to the early-onset autism group making significantly more overall errors than the DD group (t = 3.08, p < .05). There was no interaction effect between group and error type suggesting that all groups displayed roughly the same pattern of errors.
Figure 2.
Group by type of error: proportion of errors out of total possible (after controlling for overall mental age).
Emulation versus imitation
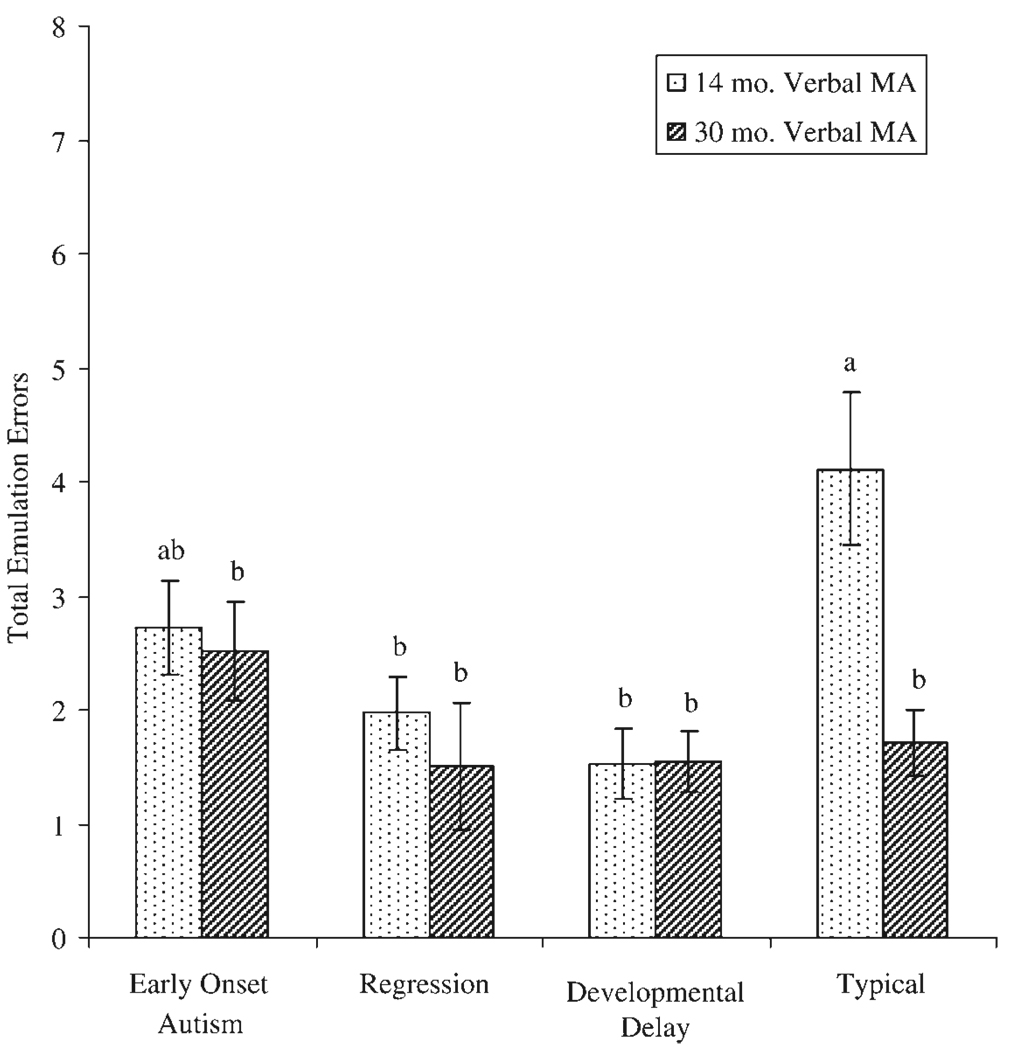
Finally, we examined the number of emulation errors made by each group. Data were modeled as a count distribution (negative binomial) using a generalized linear model with group, VMA, and pass–fail scores as independent variables. Mean emulation error scores by group and VMA (25th vs. 75th percentile score) are shown in Figure 3.
Figure 3.
Mean number of emulation errors by group (after controlling for overall mental age). Means with the same subscripts are not significantly different.
Analysis revealed a significant main effect for VMA (Wald χ2 = 5.92, df = 1, p < .05) and a significant main effect for group (Wald χ2 = 8.01, df = 3, p < .05). The interaction between group and VMA was also significant (Wald χ2 = 12.63, df = 3, p < .01). Simple effects of group were analyzed using estimated marginal means from the 25th percentile VMA (i.e., 14 months) and the 75th percentile VMA (i.e., 29.63 months). Results of simple effect comparisons revealed no differences between the combined autism group and comparison group at either VMA level. Individual planned group comparisons further revealed no differences between the early-onset and regression subgroups at either VMA level, but did reveal that the typical group exhibited significantly more emulation errors at the 25th percentile when compared to the DD group. Inspection of individual zero-order correlations between VMA and number of emulation errors likewise revealed a significant correlation only for the typical group, for whom VMA was significantly negatively related to the number of emulation errors (Spearman ρ = −.69, p < .001).
Discussion
The purpose of the study was to delve more deeply into the nature of imitative difficulties seen in autism. We designed a set of simple, novel object imitation tasks for preschoolers that held constant the motor demands of the tasks while varying the functionality of the means–end actions on the objects. There were several main findings.
We found that imitation performance was affected by both group and condition, and that these differences were most marked at younger developmental ages. At the lower developmental age range (i.e., 14 months), the group with autism as a whole had more failures across all imitation tasks than the comparison group, thus demonstrating a general imitation deficit. Further, as we hypothesized, only the combined autism group also showed a greater performance decrement in the nonfunctional condition than in the functional condition, although this effect was carried primarily by the regression group. In contrast, the combined comparison group showed equivalent performance in both conditions. At older developmental ages (i.e., 30 months), all groups demonstrated similar high levels of performance except for the regression group, who still evidenced more item failures in the nonfunctional condition compared to the functional condition. An interesting finding in this analysis was that the DD group performed significantly better than the typical group, although the difference involving passing only one more task on the average in both conditions. This is most likely because of the much greater age and thus greater range of experiences of the DD group (mean of 44 months, compared to 23 for the typical group). Children with this level of delay, by age 3.5 years, have generally been enrolled in early intervention programs and have had many more experiences with objects and with playing with adults than 1.5 year olds. It may also be related to their slightly, although not significantly, higher performance developmental age.
Now we will turn to the question of imitative accuracy. The combined group with autism did not differ significantly from the combined comparison group on total error scores, controlling for both VMA and number of passing scores, and there was no difference in the pattern of errors seen in the combined group with autism compared to the combined comparison group. Errors of hand position and movement trajectories were most frequent in all groups. There was no effect of the functionality of the tasks on accuracy of imitation.
We examined one error type, emulation, in greater detail, given the suggestion by some authors that children with autism rely on emulation (copying the goals of a task) rather than imitation (copying both the method and the goal) when copying actions on objects. We found no support for this hypothesis. Children with autism demonstrated no more emulation errors on tasks they passed than did children in the comparison group, and this was true of both lower VMA and higher VMA groups. In contrast, typically developing children with lower VMA scores made more emulation errors than those with higher VMAs.
Thus, the main group-related differences found in this study (other than an overall imitative deficit in autism) involved differential performance patterns on the functional and nonfunctional tasks, with the group with autism, particularly those with lower VMAs and with a regressive onset, demonstrating fewer passes on the nonfunctional than the functional task. Why would the relatively small differences between these two versions of the tasks affect the autism group so significantly? We believe that the imitative differences are because of nonintentional processes in both groups. The differences in the objects used across the two conditions are so subtle, and the actions being modeled are so emphasized, that it seems unreasonable to us to assume that volitional processes regarding imitation are involved in these children with an average mental age of around 24 months.
What nonintentional imitative processes would be affected by the task differences here? Attentional processes must be strongly considered. Of course, without eye-tracking methods, we cannot be sure that all children are attending to the same aspects of the stimulus for the same amount of time. However, several aspects of the experiment argue against an attentional interpretation. First, it included only children who were actively attending to the model in the analyses. Second, if children were attending only to the object manipulations and not the experimenter’s actions, then we should see increased emulation errors, which did not occur in the autism group. Third, the experimental design itself argues against attentional differences; the conditions are the same in every way except the functional nature of the object used.
Can motivational patterns account for the group differences? At one level, the children with autism appear to be exhibiting adequate motivation for the task. The children with autism are attending to the adult and providing as many contingent acts as the others. The tasks are essentially the same tasks across the two conditions, and this design characteristic provides additional motivational control. Thus, at the behavioral level of analysis, the children with autism appear motivated to engage in the task. However, in this experiment, children with autism respond as if the adult’s behavior in the less functional condition is somehow less salient to them than it is in the functional condition. This pattern cannot be explained by a general imitative deficit, but it fits well with Dawson’s and Mundy’s ideas about the social reward circuitry abnormalities in autism and reduced neural response to social stimuli (Dawson et al., 2004; Mundy & Neal, 2001).
How do our findings of autism-specific effects on imitation depending on the instrumental salience of the task fit with previous studies? The present paper builds on findings from two previous lines of work. Ingersoll et al. (2003) described the effects of sensory feedback on imitative frequencies. The findings from that paper also demonstrated that the nature of object characteristics affected the frequency of imitation for children with autism, but not for children with other diagnoses. However, in that study, the object differences involved strong sensory effects, and the interpretation had to do with the motivating effects on imitation of sensory feedback. In the present paper, the task differences generally involved subtle, functional object affordances as opposed to strong sensory experiences.
Also closely related is the work by Hobson and colleagues concerning imitation of actions versus style. The Hobson and Lee (1999) study, like the present one, identified greater difficulty for people with autism in what appears to be the more social aspect of imitation (what Hobson labels as identification) than in the more instrumental aspect of a means–end task (although the two conditions were not equated for difficulty). Hobson and Lee (1999) used tasks that varied widely across the instrumental and dynamic conditions and found group differences only in the dynamic, rather than the instrumental acts. The present study used more complex instrumental tasks, controlled for motor complexity across the instrumental and social conditions, and found autism-specific group differences in all imitative conditions and an autism-specific effect of condition involving greater imitation problems in the more social, less instrumental condition, building on and extending the findings from both Ingersoll et al. (2003) and Hobson and Lee (1999).
In this experiment, in the less meaningful condition, children are asked to imitate a playful adult doing an odd action with an object that creates no interesting effect. We suggest that the function of imitation in this task is largely social: to join, to share, to express the self-other social relation through a shared activity. A reciprocal frame has been set up in a call–response format, in which the adult’s behavior invites a child response. We believe that children without autism feel this invitation and respond accordingly, reciprocally and imitatively. (See Meltzoff, 2007, for similar ideas concerning children with typical development.) The oddness of the task may actually motivate the response, resulting in a more affectively infused and social reward-based experience than the more meaningful condition. Yet this capacity for sharing affect is a core deficit in autism, and we see significantly poorer imitative performance on this task in that group. As such, it may be that children with autism experience less social or affective meaning during these tasks, resulting in poorer imitation performance. In contrast, in the more meaningful condition, children with autism use their relatively intact means–end understanding to infer mechanical or instrumental meaning, resulting in improved performance.
An additional and potentially important finding involved the consistent pattern of greater difficulties for the autism regression onset group than the early-onset group. The regression subgroup in autism frustrates all developmental cascade theories of autism, which have at their core the assumption that seemingly small deficits or differences in early social processes in the first few months of infancy can cascade over time to cause the triad of impairments seen by age 18–24 months. Later onset cases, in which there is a greater period of time spent in more typical social engagement with parents, should result in less severe symptoms. However, they do not show milder symptoms in any domain than those with early infant onset (Luyster et al., 2005; Richler et al., 2006; Rogers, 2004;Werner et al., 2005). Furthermore, when differences have been revealed, the direction of the difference most often indicates poorer functioning in the regression group (Luyster et al., 2005; Richler et al., 2006; Rogers, 2004; Werner et al., 2005).
Our study is the first to compare imitative performance in the Regressed and Early-Onset Autism group, and our findings suggest that the regression group is more severely affected, both in terms of imitation performance and also in terms of their response to the functional–nonfunctional manipulation, showing an even greater divergence of performance in the two conditions than the early-onset group. Thus, having a longer period of nonautistic social development in infancy clearly did not provide protection from the severity of imitation problems of autism.
Thus, later onset of behavioral symptoms may yield more impairment than earlier onset, whether because of different biological events, reduced plasticity of neural development because of greater maturity at symptom onset, or other unknown reasons. It will be helpful for other imitation researchers to look for effects of onset status on imitative performance to determine whether our current finding is replicable, and if so, to examine more deeply the relations between onset status and imitative performance.
Another point to be discussed is the relationship between imitation and developmental maturity in this study. As in our own and others’ previous studies, we found imitative responses to be significantly related to developmental level. This was true for pass/fail scores and for accuracy of imitation, or number of errors, within those tasks that were passed. However, the differential response in autism to the functional/nonfunctional manipulation was more pronounced at a younger developmental age. At the higher developmental age the differential response in autism was only found for the regression group and was of a smaller magnitude. These findings may reflect the fact that imitation performance in autism follows a deviant pattern that is greatly magnified by developmental differences.
A final point has to do with a motor, or dyspraxia, hypothesis of imitation deficits in autism. Several findings work against a dyspraxia hypothesis concerning performance in the present experiment. First, there were no significant group differences on nonverbal performance, which is made up of Fine Motor and Visual Perception subtests of the MSEL. Second, we found no general autism-related differences in the error codes, which carefully examined motor performance on each task. For children who passed tasks, only those with regressive onset appeared to make more errors than others. Third, there was no autism-specific pattern of error production. Fourth, the experimental manipulation controlled for the motor demands of the tasks. Thus, for these particular tasks, we did not see evidence of differential motor performance affecting imitation. However, we chose tasks that would be relatively easy for the participants to do. Tasks that are more motorically complex to imitate may reveal a different pattern. However, examining this question requires use of fine-grained coding systems for motor errors as well as overall performance accuracy.
How do our findings fit with current hypotheses attributing autism-specific imitation deficits to a deficient mirror neuron network activation (Dapretto et al., 2006; Williams et al., 2006)? Rumiati et al. (2005) developed a neuropsychological model of imitation in which both the mirror neuron system (MNS) and long-term memory (LTM) and episodic or short-term memory processes are involved. This model attributes an important role for semantic memory processes in action imitations of meaningful stimuli, and a contrasting role for episodic memory in the imitation of meaningless actions. The theory suggests that although the LTM and semantic memory can support the MNS for familiar, meaningful actions through the use of prototypes of action patterns extracted from many previous experiences with the objects or actions involved a direct matching route involving the MNS is necessary for novel meaningless actions, which lack any representation in LTM memory. Thus, mirror neuron network impairments would be expected to have differential effects on imitation of novel meaningless actions. If, in the Rumiati model, the MNS functions supporting imitation were impaired, then performance on tasks like those used in this experiment would lead to three predictions: (a) greater overall imitation problems in the autism group, given that both types of performances involve MNS support: (b) greater autism deficits in the nonfunctional than the functional condition, given the greater dependence on MNS in this condition, and (c)more emulation errors in the autism group than the other group in the functional condition, given greater use of LTM prototypes from previous experiences with the objects. We found greater overall imitation deficits in the autism group, and differential effects on less meaningful tasks, as hypothesized by this model, but we did not find a greater number of emulation errors in the functional condition in the group with autism. Thus, our data are only partially congruent with the model.
A second explanation for the present findings involve brain mechanisms that work in conjunction with the mirror neuron system, rather than the mirror neuron system itself. Iacoboni (2005) has suggested that imitation of noninstrumental actions has a social component that involves limbic system activation facilitating MNS activation. Atypical amygdala functioning in autism, which has been suggested by a variety of researchers (e.g., Amaral, Bauman, & Mills Schumann, 2003; Bachevalier, 1994; Dawson et al., 2004), might differentially impair imitation of meaningless acts, an hypothesis that has some support from this study.
There are several limitations to the current study. Our groups were not matched by age. However, we controlled for developmental differences related to VMA in all analyses. Age differences might be expected to enhance imitation performance given greater experiences, all other things being equal, as may have been true for the DD group. However, given that the oldest group, the regression group, was also the most impaired in imitation, the age difference does not appear to be biasing our findings. The age issue also comes into play in choosing tasks. Certainly the younger children would have had many fewer experiences than the older ones with markers, in hands or mouths, and with lollipops, as well as with many other materials used here. However, the tasks were within the motor capabilities of all the children, even if not in the experiential repertoire. Task design with groups who are preverbal and very young is a challenge in this kind of research.
This experiment would have been strengthened by more rigorous monitoring of children’s attention. Future studies would benefit from using eye tracking methodology to examine the question of visual attention differences among groups as a source of variance in imitative responses. Third, we examined only one type of imitation, actions on objects, which prevents us from addressing the full range of imitative responses. It is particularly difficult to generalize from imitation of actions on objects to imitations of actions without objects. However, given that imitating actions on objects has often considered to be “spared” in autism, we would expect that examination of imitation of manual or facial gestures will demonstrate greater autism impairment than was found on these tasks. Fourth, examiners were not blind to child diagnoses, though they were unaware of onset status. The use of rewards may have inadvertently introduced a source of bias as well.
An additional limitation is in regard to the validity of the onset subclassifications. In our own study, onset status was not verified by any method other than parent report. Nevertheless, the validity of parent-reported regression and late onset of symptoms has been examined in a number of studies using home videos and behavioral coding of prospective video records during the first years of life (e.g., Goldberg, Thorsen, Osann, &Spence, 2007; Maestro et al., 2006; Osterling, Dawson, & Munson, 2002;Werner, Dawson, Osterling, &Dinno, 2000). Although such studies have found some subtle social deficits in children prior to parent reported age of regression, these studies have indeed documented significant differences between onset subtypes in a number of behavior domains prior to age of regression, with poorer performance in early- onset infants and toddlers, thereby providing a robust measure of construct validity for different onset patterns identified using parent report. Moreover, parent report on the ADI-R to identify onset subclassifications is currently the gold standard approach used by major autism network research projects (Luyster et al., 2005).
Another limitation of our research concerns the low internal consistencies of the error scores for both the functional and nonfunctional subscales. The low internal consistencies and relatively high correlation between the subscales suggests that our analyses regarding the accuracy of imitation, as measured by the number of errors, should be interpreted with some caution. Nevertheless, given that the analysis for accuracy did not reveal any main effect for condition nor any interaction terms involving condition, the analyses for accuracy scores essentially examined a combined accuracy score independent of subscale, thereby circumventing issues in a lack of subscale coherence and independence.
Finally, we interpret the current findings as suggesting that the social–reciprocal function of imitation is specifically and uniquely affected in autism, an interpretation also supported by the Hobson et al. studies (Hobson, 1995; Hobson & Hobson, 2007; Hobson & Lee, 1999) and the Ingersoll et al. (2003) study. This interpretation is best supported by the regression subgroup, as the early-onset autism subgroup demonstrated only a trend for the specificity of a social–reciprocal function deficit.
To conclude, we interpret our data as indicating that the social function of imitation, as a reciprocal response that marks a shared experience, is uniquely affected in autism and accounts for the group by condition interactions found in this experiment. Whether this social, reciprocal aspect of imitation could be the main source of all kinds of imitation impairment in autism is possible but not yet known.
Acknowledgments
This work was partially supported by Grant U19 HD35468-10 from the National Institute of Child Health and Human Development and by the MIND Institute. This site was one of the Collaborative Programs of Excellence in Autism. We acknowledge Sanja Sljivic and Diane Larzelere for support with manuscript preparation and the children and families who made this study possible.
Footnotes
For example: if a child passed all eight items for the functional scale, the total number of possible location errors would be 7. If the child made three location errors over these eight items, the proportional location error score would then be the number of location errors actually made by the child (3) divided by the total possible (7) yielding a proportion score of .43. For purposes of analysis, given that distributions of proportions have a non-constant variance across the range of possible values, we used an inverse sine transformation of the square root of proportion scores for purposes of analyses. For values of zero, scores were transformed using arcsine(sqrt(1/(4×n)), where n equals the number of trials for the given error type. For values of 1, scores were transformed using . Mean proportions shown in Figure 3, however, are untransformed, to facilitate interpretation.
References
- Amaral DG, Bauman MD, Mills Schumann C. The amygdala and autism: Implications from non-human primate studies. Genes, Brain and Behavior. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Bachevalier J. Medial temporal lobe structures and autism: A review of clinical and experimental findings. Neuropsychologica. 1994;6:627–648. doi: 10.1016/0028-3932(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Baldwin JM. Social and ethical interpretations in mental development. New York: Macmillan; 1906. [Google Scholar]
- Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organizational Research Methods. 2004;7:127–150. [Google Scholar]
- Bennetto L. A componential approach to imitation and movement deficits in autism. Dissertation Abstracts International. 1999;60:8–19. [Google Scholar]
- Bernier R, Dawson G, Murias M, Webb S. EEG mu rhythm and imitation impairments in individuals with Autism Spectrum Disorder. Brain and Cognition. 2007;64:228–237. doi: 10.1016/j.bandc.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner J. Nature and uses of immaturity. American Psychologist. 1972;27:687–708. [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants with autism: An investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology. 1997;33:781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. The chameleon effect: The perception–behavior link and social interaction. Journal of Personality and Social Psychology. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Defining the early social attention impairments in autism: Social orienting, joint attention, and responses to emotions. Developmental Psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- DeMyer MK, Alpern GD, Barton S, DeMyer WE, Churchill DW, Hingtgen JN, et al. Imitation in autistic, early schizophrenic, and nonpsychotic subnormal children. Journal of Autism and Childhood Schizophrenia. 1972;2:264–287. doi: 10.1007/BF01537618. [DOI] [PubMed] [Google Scholar]
- Gergely G, Bekkering H, Kiraly I. Rational imitation in preverbal infants. Nature. 2002;415:755. doi: 10.1038/415755a. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Thorsen KL, Osann K, Spence MA. Use of home videotapes to confirm parental reports of regression in autism. Journal of Autism and Developmental Disorders. 2007;38:1136–1146. doi: 10.1007/s10803-007-0498-6. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Hobson RP. Identification: The missing link between joint attention and imitation? Development and Psychopathology. 2007;19:411–431. doi: 10.1017/S0954579407070204. [DOI] [PubMed] [Google Scholar]
- Hobson RP. Apprehending attitudes and actions: Separable abilities in early development? Development and Psychopathology. 1995;7:171–182. [Google Scholar]
- Hobson RP, Lee A. Imitation and identification in autism. Journal of Child Psychology and Psychiatry. 1999;40:649–660. [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Huang CT, Heyes C, Charman T. Infants’ behavioral reenactment of “Failed Attempts”: Exploring the roles of emulation learning, stimulus enhancement, and understanding of intentions. Developmental Psychology. 2002;38:840–855. doi: 10.1037//0012-1649.38.5.840. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Current Opinion in Neurobiology. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Understanding others: Imitation, language, and empathy. In: Hurley S, Chater N, editors. Perspectives on imitation: From mirror neurons to memes—Mechanisms of imitation and imitation in animals. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- Ingersoll B, Schreibman L, Tran QH. Effect of sensory feedback on immediate object imitation in children with autism. Journal of Autism and Developmental Disorders. 2003;33:673–683. doi: 10.1023/b:jadd.0000006003.26667.f8. [DOI] [PubMed] [Google Scholar]
- Kaye K, Marcus J. Infant imitation: The sensory-motor agenda. Developmental Psychology. 1981;17:258–265. [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule—WPS Edition. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Luyster R, Richler J, Risi S, Hsu WL, Dawson G, Bernier R, et al. Early regression in social communication in autism spectrum disorders: A CPEA study. Developmental Neuropsychology. 2005;27:311–336. doi: 10.1207/s15326942dn2703_2. [DOI] [PubMed] [Google Scholar]
- Maestro S, Muratori F, Cesari A, Pecini C, Apicella F, Stern D. A view to regressive autism through home movies. Is early development really normal? Acta Psychiatrica Scandinavica. 2006;113:68–72. doi: 10.1111/j.1600-0447.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- Masur EF, Ritz EG. Patterns of gestural, vocal, and verbal imitation performance in infancy. Merrill–Palmer Quarterly. 1984;30:369–392. [Google Scholar]
- McCabe MA, Uzgiris IC. Effects of model and action on imitation in infancy. Merrill–Palmer Quarterly. 1983;29:69–82. [Google Scholar]
- Meltzoff A, Gopnik A. The role of imitation in understanding persons and developing a theory of mind. In: Baron-Cohen S, Tager-Flusberg H, Cohen DJ, editors. Understanding other minds. Oxford: Oxford University Press; 1993. pp. 335–366. [Google Scholar]
- Meltzoff AN. “Like me”: A foundation for social cognition. Developmental Science. 2007;10:126–134. doi: 10.1111/j.1467-7687.2007.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation in newborn infants: Exploring the range of gestures imitated and the underlying mechanisms. Developmental Psychology. 1989;25:954–962. doi: 10.1037/0012-1649.25.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Hobson RP. Orientation in relation to self and other: The case of autism. Interaction Studies. 2004;5:221–244. [Google Scholar]
- Miles JH, Takahashi TN, Bagby S, Sahota PK, Vaslow DF, Wang CH, et al. Essential versus complex autism: Definition of fundamental prognostic subtypes. American Journal of Medical Genetics. 2005;135:171–180. doi: 10.1002/ajmg.a.30590. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Janciewicz EM, Goldberg MC, Denckla MB. Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society. 2006;12:314–326. doi: 10.1017/s1355617706060437. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Cranston, RI: T.O.T.A.L. Child, Inc; 1989. [Google Scholar]
- Mundy P, Neal RA. Neural plasticity, joint attention, and a transactional social-orienting model of autism. In: Glidden LM, editor. International review of research in mental retardation. San Diego, CA: Academic Press; 2001. pp. 139–168. [Google Scholar]
- Niedenthal PM, Barsalou LW, Winkielman P, Krauth-Gruber S, Ric F. Embodiment in attitudes, social perception, and emotion. Personality and Social Psychology Review. 2005;9:184–211. doi: 10.1207/s15327957pspr0903_1. [DOI] [PubMed] [Google Scholar]
- Ohta M. Cognitive disorders of infantile autism: A study employing the WISC, spatial relationships, conceptualization, and gestural imitation. Journal of Autism and Developmental Disorders. 1987;17:45–62. doi: 10.1007/BF01487259. [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Piaget J. Play, dreams, and imitation in childhood. New York: Norton; 1962. [Google Scholar]
- Richardson MJ, Marsh KL, Schmidt RC. Effects of visual and verbal interaction on unintentional interpersonal coordination. Journal of Experimental Psychology. 2005;31:62–79. doi: 10.1037/0096-1523.31.1.62. [DOI] [PubMed] [Google Scholar]
- Richler J, Luyster R, Risi S, Hsu WL, Dawson G, Bernier R, et al. Is there a regressive “phenotype” of autism spectrum disorder associated with the measles–mumps–rubella vaccine? A CPEA study. Journal of Autism and Developmental Disorders. 2006;36:299–316. doi: 10.1007/s10803-005-0070-1. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. An examination of the imitation deficit in autism. In: Nadel J, Butterworth G, editors. Imitation in infancy. Cambridge: University of Cambridge Press; 1999. pp. 254–283. [Google Scholar]
- Rogers SJ. Developmental regression in autism spectrum disorders. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:139–143. doi: 10.1002/mrdd.20027. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Bennetto L, McEvoy R, Pennington BF. Imitation and pantomime in high functioning adolescents with autism spectrum disorders. Child Development. 1996;67:2060–2073. [PubMed] [Google Scholar]
- Rogers SJ, DiLalla D. Age of symptom onset in young children with pervasive developmental disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29:863–872. doi: 10.1097/00004583-199011000-00004. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Pennington BF. A theoretical approach to the deficits in infantile autism. Development and Psychopathology. 1991;3:137–162. [Google Scholar]
- Rogers SJ, Williams JHG. Imitation in autism: Findings and controversies. In: Rogers SJ, Williams JHG, editors. Imitation and the social mind: Autism and typical development. New York: Guilford Press; 2006. pp. 277–309. [Google Scholar]
- Rumiati RI, Weiss PH, Tessari A, Assmus A, Zilles K, Herzog H, et al. Common and differential neural mechanisms supporting imitation of meaningful and meaningless actions. Journal of Cognitive Neuroscience. 2005;17:1420–1431. doi: 10.1162/0898929054985374. [DOI] [PubMed] [Google Scholar]
- Smith IM, Bryson SE. Gesture imitation in autism I: Nonsymbolic postures and sequences. Cognitive Neuropsychology. 1998;15:747–770. doi: 10.1080/026432998381087. [DOI] [PubMed] [Google Scholar]
- Stone WL, Ousley OY, Littleford CD. Motor imitation in young children with autism: What’s the object? Journal of Abnormal Child Psychology. 1997;25:475–485. doi: 10.1023/a:1022685731726. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Savage-Rumbaugh S, Kruger AC. Imitative learning of actions on objects by children, chimpanzees, and enculturated chimpanzees. Child Development. 1993;64:1688–1705. [PubMed] [Google Scholar]
- Uzgiris IC. Two functions of imitation during infancy. International Journal of Behavioral Development. 1981;4:1–12. [Google Scholar]
- Werner E, Dawson G, Munson J, Osterling J. Variation in early developmental course in autism and its relation with behavioral outcome at 3–4 years of age. Journal of Autism and Developmental Disorders. 2005;35:337–350. doi: 10.1007/s10803-005-3301-6. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G, Osterling J, Dinno N. Brief report: Recognition of autism spectrum disorder before one year of age: A retrospective study based on home videotapes. Journal of Autism and Developmental Disorders. 2000;30:157–162. doi: 10.1023/a:1005463707029. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and “mirror neuron”functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Williamson RA, Markman EM. Precision of imitation as a function of preschoolers’ understanding of the goal of the demonstration. Developmental Psychology. 2006;42:723–731. doi: 10.1037/0012-1649.42.4.723. [DOI] [PubMed] [Google Scholar]