Abstract
Whereas evidence for the role of psychosocial factors in cancer initiation has been equivocal, support continues to grow for links between psychological factors such as stress, depression, and social isolation and progression of cancer. In vitro, in vivo, and clinical studies show that stress- related processes can impact pathways implicated in cancer progression, including immuno-regulation, angiogenesis, and invasion. Contributions of systemic factors, such as stress hormones to the crosstalk between tumor and stromal cells, appear to be critical in modulating downstream signaling pathways with important implications for disease progression. Inflammatory pathways may also be implicated in fatigue and other factors related to quality of life. Although substantial evidence supports a positive effect of psychosocial interventions on quality of life in cancer, the clinical evidence for efficacy of stress-modulating psychosocial interventions in slowing cancer progression remains inconclusive, and the biobehavioral mechanisms that might explain such effects are still being established. This article reviews research findings to date and outlines future avenues of research in this area.
INTRODUCTION
Throughout history, scientists have pondered connections between psychosocial factors and diseases such as cancer.1 Epidemiologically established risk factors for carcinogenesis (eg, endocrine, environmental, socioeconomic, and genetic factors) only partially explain the risk for cancer initiation.2 Whereas evidence for the role of psychosocial factors in cancer initiation is limited and equivocal,3–6 evidence is stronger for links between psychological factors such as stress, depression, and social isolation and disease progression.7–9 Thus, this review focuses on literature related to disease progression.
STRESS RESPONSE
The physiological stress response is thought of as one of the probable mediators of the effects of psychosocial factors on cancer progression. The overall stress response involves activation of several body systems including the autonomic nervous system and the hypothalamic-pituitary-adrenal axis. The fight or flight response is elicited by the production of mediators, such as the catecholamines norepinephrine (NE) and epinephrine (E), from the sympathetic nervous system and the adrenal medulla. The hypothalamic-pituitary-adrenal response includes release of corticotropin-releasing hormone from the hypothalamus, inducing secretion of adrenocorticotrophic hormone from the anterior pituitary, resulting in downstream release of glucocorticoids such as cortisol from the adrenal cortex.10 Additional neuroendocrine factors are also modulated following stress, including dopamine, prolactin, nerve growth factor, substance P, and oxytocin.11,12 Stress can be acute (ie, short-lived) or chronic (ie, repetitive or occurring over an extended period of time).13 In chronic stress, the body remains in a constant state of overdrive, with deleterious downstream effects on regulation of stress response systems as well as many organ systems.14 A variety of stressors, including severe trauma, marital discord, bereavement, as well as depression and social isolation have been associated with dysregulation or alterations in various neuroendocrine hormones, particularly catecholamines and cortisol.15–19
STRESS-RELATED MECHANISMS RELEVANT TO CANCER PROGRESSION
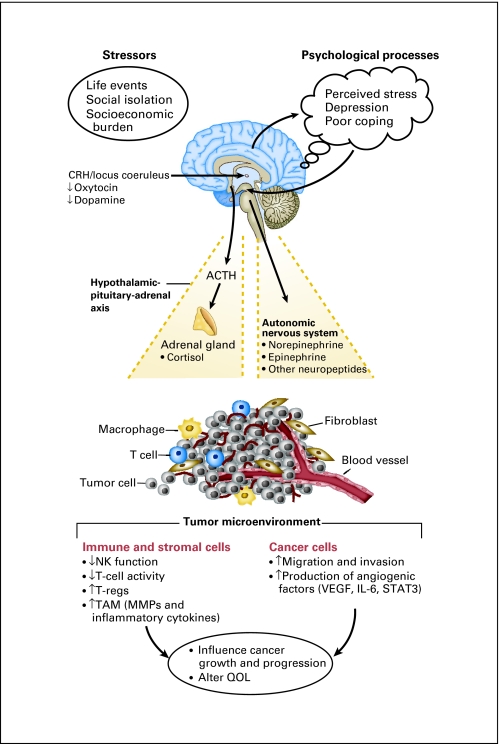
Cancer metastasis remains a difficult problem to manage and is responsible for most cancer-related mortality. Metastasis is a complex process that requires several steps to be successful, including angiogenesis, proliferation, invasion, embolization, and evasion of immune system surveillance.20 Increasing evidence shows that stress response pathways can affect many parts of this cascade (Fig 1). Here, we examine clinical, animal, cellular, and molecular findings relating psychosocial and behavioral factors (ie, stress, depression, social support/isolation) to processes implicated in cancer progression and metastasis.
Fig 1.
Effects of stress and psychological processes on the tumor microenvironment. The stress response results in activation of the autonomic nervous system and the hypothalamic-pituitary-adrenal axis. Factors released from these pathways can have direct effects on the tumor microenvironment, resulting in a favorable environment for tumor growth and progression. These dynamics can also adversely affect patient quality of life. CRH, corticotropin-releasing hormone; ACTH, adrenocorticotrophic hormone; NK, natural killer; T-regs, regulatory T cells; TAM, tumor-associated macrophages; MMP, matrix metalloprotinease; VEGF, vascular endothelial growth factor; IL, interleukin; STAT3, signal transducer and activator of transcription factor-3; QOL, quality of life.
Stress and Angiogenesis
Development of a blood supply is critical for tumor growth and metastasis. Many factors promote angiogenesis including vascular endothelial growth factor (VEGF), interleukin-6 (IL-6), transforming growth factor α and β, and tumor necrosis factor α.21,22 Social support has been shown to be related to lower levels of VEGF among patients with ovarian cancer perisurgically, both in serum23 and in tumor tissue.24 In vitro studies have found that NE and the β-agonist isoproterenol were both capable of inducing VEGF expression in ovarian and other cancer cell lines.25,26 Moreover, using orthotopic animal models of ovarian cancer, chronic restraint stress resulted in increased tumor burden and invasiveness, which was mediated by NE-driven increases in VEGF and angiogenesis.27 Similar effects were noted with the β-agonist isoproterenol and eliminated by using a β-blocker, thus, verifying the importance of adrenergic receptor signaling in mediating these effects.
Angiogenesis can also be stimulated by a disruption in the balance between pro- and antiangiogenic factors. IL-6 is a prominent angiogenic factor produced by tumor cells that disrupts this equilibrium.28,29 Clinically, patients with ovarian cancer with poorer social support had higher levels of IL-6 both in plasma and in ascites.30 Furthermore, NE was responsible for inducing IL-6 gene transcription through a Src-dependent mechanism, further demonstrating the role of tumor cells in activating stress pathways critical to their growth.31
Recent studies have also shown the involvement of signal transducer and activator of transcription factor-3 (STAT3) in promoting stress-mediated tumor-associated angiogenesis. STAT3 is involved in many protumorigenic pathways by activating downstream targets to promote proliferation and inhibit apoptosis. Although STAT3 can be activated by growth factors and cytokines, such as VEGF and IL-6, stress hormones, such as NE and E, can activate STAT3 independent of IL-6, leading to its translocation to the nucleus and subsequent binding to DNA to promote transcription of genes associated with cell survival, angiogenesis, and proliferation.32
Effects on Tumor Cell Migration and Invasion
Another key step in the metastatic cascade is the ability of a tumor cell to separate from the main tumor, invade through the basement membrane, and enter the blood supply. Stress hormones can affect these processes by increasing matrix metalloprotinease (MMP) production by tumor cells as well as by acting as chemoattractants to induce cell migration. Stress levels of NE increased the in vitro invasive potential of ovarian cancer cells by 89% to 198%, which was completely blocked by the β antagonist propranolol.33 Additional in vivo and in vitro studies demonstrated that NE and E significantly increased production of MMP-2 and MMP-9 by ovarian cancer cells through activation of the β-adrenergic pathway.33 Other studies have reported similar findings in several other tumor types including colon and head and neck cancers.26,34–36 Clinically, both depression and stress have been related to MMP-9 secretion by tumor-associated macrophages (TAM) in patients with ovarian cancer. As TAM are now known to promote a proinflammatory tumor microenvironment, downregulate cellular immunity, and enhance tumor growth and progression,37,38 effects of stress on TAM have important implications for tumor progression.24
Social support is thought to have direct links to health outcomes, as well as to moderate the effects of stress.39 For example, individuals with poor social support were shown to have impaired transcription of glucocorticoid response genes and increased activity of proinflammatory transcription control pathways.40 Social isolation has been related to upregulated mammary gland expression of murine orthologues of several key metabolic genes implicated in human tumorigenesis and to increased tumor growth in a murine breast cancer model.41 Among ovarian cancers from individuals with high levels of depression and low levels of social support, more than 200 upregulated gene transcripts were found that were consistent with activation of signaling pathways involved in tumor growth and progression (eg, CREB, NFKB, STAT, and ELK1) as compared to histologically and age-matched counterparts with high social support and low levels of depression.42 Collectively, emerging evidence has shown stress and psychosocial factors to be associated with key elements of the metastatic cascade, in both animal and human models.
Stress and the Immune Response
The cellular immune response has been a central focus of much biobehavioral oncology research because of its role in immuno-surveillance and lysis of tumor cells.43 Experimental studies with animal models have demonstrated that tumor incidence and progression may be aggravated by chronic stress, including surgical stress, by suppressing type 1 (TH1) cytokines and cytotoxic activities of T cells and natural killer (NK) cells, impairing antigen presentation, and increasing regulatory T cells.44–47 Psychological states such as chronic stress, loneliness, and depression are known to downregulate the cellular immune response,48–50 largely via adrenergic and glucocorticoid signaling pathways. Stress has been related to decrements in a broad range of markers of cellular immunity in patients with breast cancer after surgery, including lower T-cell production of TH1 versus TH2 cytokines,51 decreases in T-cell response to mitogen stimulation, and impaired NK cell cytotoxicity.52,53 Among patients with advanced breast cancer, depression has been related to a reduction in the cellular immune response to a variety of specific antigens.54 Distress among patients with ovarian cancer at the time of surgery has been associated with poorer NK cell activity in tumor infiltrating lymphocytes (TIL) and lower T-cell production of TH1 versus TH2 cytokines in peripheral blood and TIL, whereas social support was related to greater NK activity in both peripheral blood and TIL.55,56 It should also be noted that inflammatory cytokines have been implicated in cancer-related fatigue and depression.57–60
Stress, Neuroendocrine Circadian Dysregulation, and Cancer Progression
There is clinical evidence that stress may disrupt the diurnal secretion of neuroendocrine hormones, such as cortisol, and that such disruption is related to diminished quality of life and poorer outcomes in some patients with cancer. Profound alterations in diurnal serum cortisol rhythms have been reported in animals with tumors and in a variety of patients with cancer.61,62 It is not clear to what extent these diurnal cortisol dysregulations derive from factors such as stress and depression, or if they are secondary to tumor-produced inflammatory products, or both.18,59,63,64 Dysregulations in diurnal cortisol have been associated with greater functional disability,65 fatigue,66 and poorer survival in women with breast cancer.67 Direct relationships between glucocorticoids and neoplastic growth have been documented. For example, glucocorticoids directly enhance a survival pathway and inhibit apoptosis of a mammary tumor cell line,68 downregulate expression of DNA repair genes including BRCA1,69 and decrease paclitaxel-induced apoptosis in a mammary cancer cell line.70 Patients with advanced breast cancer with higher mean diurnal cortisol concentrations showed suppressed cellular immunity to a number of antigens.54 Thus, glucocorticoids may have direct effects on tumor growth and development as well as effects on immunosurveillance and on factors related to quality of life.
Summary
These findings highlight the relevance of translational research testing pharmacologic agents on intermediate outcome markers of cancer progression as delineated above (eg, markers of angiogenesis, invasion, and metastasis) as well as more distal outcomes such as recurrence and survival. Potential pharmacologic approaches could include beta blockers, antidepressants, and anti-inflammatory agents as well as molecules specifically targeting the downstream pathways induced by stress. Psychosocial interventions may also modulate stress-related pathways by teaching individuals to behaviorally manage their stress responses.
PSYCHOSOCIAL INTERVENTION AND CANCER PROGRESSION
With evidence for associations between stress, social processes, and neuroendocrine changes that can impair quality of life and promote cancer progression, a logical extension is human research testing the effects of psychosocial interventions on quality of life, neuroendocrine parameters, and cancer progression. More than 300 trials of psychological interventions have been conducted in patients with cancer over the past 50 years.71–73 Most such intervention trials have been conducted in women with breast cancer.
In previous reviews,71–73 the consensus has been that different forms of psychosocial intervention that teach relaxation and stress management, help patients ventilate their feelings and anxiety, and provide social support are able to improve quality of life. Salient among these findings are the ability of psychosocial interventions to decrease pain and anxiety in patients with metastatic breast cancer with the most severe symptoms,74 a finding that has significant clinical implications. More recent studies published after the time of these reviews have generally supported a positive effect for psychosocial interventions on quality of life, depressed mood, distress, and social disruption in patients with cancer.75,76
Whether psychological interventions can affect cancer progression and survival has been more controversial. Reviews and commentaries conducted on this topic have produced varied conclusions.71–73,77,78 In the past 3 years, three trials of 12-month group interventions on cancer recurrence or survival in women with breast cancer have been completed. These trials recruited their cohorts during the 1990s and demonstrated good methodological strength, meeting nearly all of the revised CONSORT criteria.71 Each trial carefully planned and adequately powered their designs to detect recurrence or survival outcomes (at 80% to 90% power); used appropriate random assignment, follow-up periods, and statistical analyses (eg, survival analyses by intent to treat); delineated primary and secondary analyses from ancillary and exploratory analyses; clearly described stratification procedures, participant characteristics, and decision rules for including covariates; and reported outcome effects and precision (ie, 95% CI).
In one trial, patients with breast cancer with stage 2 to 3 disease were randomly assigned to standard care or 4 months of weekly and 8 months of monthly sessions of group-based cognitive behavioral intervention (eg, relaxation, coping skills training) in the weeks after surgery. Intervention participants showed a significant reduction in overall and breast cancer–specific mortality rates as well as reduced risk of breast cancer recurrence at a median of 11 years follow-up.79 Results were not attributable to site of accrual, sociodemographic factors, disease stage, prognostic markers, surgery type, or adjuvant therapies received during the trial nor extra-trial psychiatric medications or counseling received. In two other trials, one in the United States and one in Australia, women with metastatic breast cancer were assigned to a 12-month course of weekly group-based supportive expressive therapy, but neither showed an overall intervention-related survival advantage,80,81 essentially replicating an equally rigorous prior Canadian trial of supportive expressive therapy in women with metastatic disease published in 2001.74 Possible explanations for such divergent results include differences in patient populations (eg, metastatic v nonmetastatic disease) and covariates employed,78 and alterations in physiological effects due to variations in the interventions.82 It has also been suggested that optimizing neuroendocrine and immunologic status may require both psychological and pharmacologic interventions to fully mitigate the deleterious effects of stress biology on tumor growth and progression.83
Other recently completed trials reporting the effects of stress reduction techniques have mainly involved cognitive behavioral stress management—combining relaxation-based techniques with cognitive behavioral strategies to change negative thinking and build interpersonal coping skills—and mindfulness meditation-based stress reduction (MBSR) approaches. These two forms of intervention show similar effects on stress/distress and neuroendocrine and immunologic indicators in women with nonmetastatic breast cancer recruited during medical treatment.84 These effects have included decreases in afternoon and evening serum cortisol levels, increases in the T-cell lymphoproliferative response, and increased TH1 cytokine production and TH1/TH2 production ratio.85–89 Since the MBSR trials were not randomized clinical trials, caution is in order when interpreting these findings. Nevertheless, the magnitude of the changes in physiological indicators generally paralleled the size of the psychological effects of these interventions.84 In one randomized clinical trial of cognitive behavioral stress management, distress, social disruption, and cortisol decreases were paralleled by increased confidence in using relaxation as a coping strategy to manage stress,75,88 findings that mirror those of others conducting trials of cognitive behavioral interventions and MBSR.87,90
In addition to alterations in stress responses, it is also essential to consider whether the women who received psychological interventions in these trials successfully changed their health behaviors (eg, more exercise, better nutrition, less alcohol consumption, better adherence to hormonal medications and attendance at follow-up appointments) and actually got more effective medical treatment (eg, cointervention effects),71 and if these changes conferred greater protection against disease progression and facilitated general health. Nevertheless, such positive side effects of psychological interventions would contribute a net beneficial effect for the care of patients with cancer. Importantly, women assigned to psychological interventions in the Andersen et al82 trial were more likely to adhere to their chemotherapy regimen and received greater dose intensity than controls.
When designing studies of psychological interventions in patients with cancer, it is also reasonable to consider other stress-related health outcomes beyond survival and disease recurrence, such as the incidence of opportunistic infections during and after the completion of surgical and adjuvant therapy. Stress-related changes in infectious disease processes are well-established.91–93 Stress reduction interventions showed improved neuroendocrine and immune parameters in persons with HIV,94 and also decreased the risk of developing persistent squamous intraepithelial lesions in women coinfected with HIV and human papilloma virus. The latter findings suggest that stress reduction may reduce the carcinogenic activity of opportunistic infections in some settings.95
FUTURE DIRECTIONS
There is growing evidence from in vitro, in vivo, and clinical studies that stress-related processes can impact pathways implicated in cancer progression, including immunoregulation, angiogenesis, and invasion. Contributions of systemic factors, such as stress hormones, to the crosstalk between tumor and stromal cells appears to be critical in modulating downstream signaling pathways with important implications for progression. Although effects of stress mediators and pharmacologic blockers of stress hormones on tumor progression have been demonstrated in animal models, effects of these pathways on progression of tumors in clinical models has not been well-characterized to date and provide an important avenue for future investigation. As stress mediators not only have effects on tumor growth but have effects on many related physiological processes, examination of how biobehavioral pathways contribute to effectiveness of chemotherapy and immunomodulatory therapies, fatigue, pain, and cognitive sequelae of chemotherapy will be important future lines of research. The role of stress-related immunosuppression in promoting tumor immune escape mechanisms and modulating the effectiveness of immunotherapy programs has been minimally studied and presents a fertile area for investigation.96,97 Understanding how the biobehavioral pathways outlined here are exacerbated by socioeconomic and cultural stressors and how all these factors interact with dynamics of tumor progression in diverse populations should be examined. The clinical evidence for efficacy of psychosocial interventions in slowing cancer progression remains inconclusive, and the biobehavioral mechanisms that might explain such effects are still being established. As cancer therapy moves toward greater personalization, it will be important to define those most likely to benefit from behavioral and/or pharmacologic interventions blocking the adverse effects of psychosocial factors on patient outcome.
Footnotes
Supported in part by the Ovarian Cancer Research Fund, Program Project Development Grant, the University of Texas M.D. Anderson Cancer Center Ovarian Cancer SPORE (P50 CA083639), and Grants No. CA110793 and CA109298 (A.K.S.), R21CA88293, R01CA104825, CA102515, and R01CA140933 (S.L.), and 2R01CA064710-10A1 (M.H.A.) from the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Susan K. Lutgendorf, Anil K. Sood, Michael H. Antoni
Financial support: Susan K. Lutgendorf, Anil K. Sood, Michael H. Antoni
Data analysis and interpretation: Susan K. Lutgendorf, Anil K. Sood, Michael H. Antoni
Manuscript writing: Susan K. Lutgendorf, Anil K. Sood, Michael H. Antoni
Final approval of manuscript: Susan K. Lutgendorf, Anil K. Sood, Michael H. Antoni
REFERENCES
- 1.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncology. 2004;5:617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 2.Daly M, Obrams GI. Epidemiology and risk assessment for ovarian cancer. Semin Oncol. 1998;25:255–264. [PubMed] [Google Scholar]
- 3.Duijts SFA, Zeegers MPA, Borne BV. The association between stressful life events and breast cancer risk: A meta-analysis. Int J Cancer. 2003;107:1023–1029. doi: 10.1002/ijc.11504. [DOI] [PubMed] [Google Scholar]
- 4.Lillberg K, Verkasalo PK, Kaprio J, et al. Stressful life events and risk of breast cancer in 10,808 women: A cohort study. Am J Epidemiol. 2003;157:415–423. doi: 10.1093/aje/kwg002. [DOI] [PubMed] [Google Scholar]
- 5.Geyer S. Life events prior to manifestation of breast cancer: A limited prospective study covering eight years before diagnosis. J Psychosom Res. 1991;35:355–363. doi: 10.1016/0022-3999(91)90090-b. [DOI] [PubMed] [Google Scholar]
- 6.Michael YL, Carlson NE, Chlebowski RT, et al. Influence of stressors on breast cancer incidence in the Women's Health Initiative. Health Psychol. 2009;28:137–146. doi: 10.1037/a0012982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palesh O, Butler LD, Koopman C, et al. Stress history and breast cancer recurrence. J Psychosom Res. 2007;63:233–239. doi: 10.1016/j.jpsychores.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 9.Steel JL, Geller DA, Gamblin TC, et al. Depression, immunity, and survival in patients with hepatobiliary carcinoma. J Clin Oncol. 2007;25:2397–2405. doi: 10.1200/JCO.2006.06.4592. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 11.Ebner K, Rupniak NM, Saria A, et al. Substance P in the medial amygdala: Emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc Natl Acad Sci U S A. 2004;101:4280–4285. doi: 10.1073/pnas.0400794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakshmanan J. Nerve growth factor levels in mouse serum: Variations due to stress. Neurochem Res. 1987;12:393–397. doi: 10.1007/BF00993250. [DOI] [PubMed] [Google Scholar]
- 13.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 14.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 15.Seeman T, Berkman L, Blazer D, et al. Social ties and support and neuroendocrine function: The MacArthur studies of successful aging. Ann Behav Med. 1994;16:95–106. [Google Scholar]
- 16.Seeman T, McEwen B. Impact of social environment characteristics on neuroendocrine regulation. Psychosom Med. 1996;58:459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Seeman TE, Singer BH, Rowe JW, et al. Price of adaptation–allostatic load and its health consequences. Arch Intern Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 18.Kiecolt-Glaser JK, Bane C, Glaser R, et al. Love, marriage, and divorce: Newlyweds' stress hormones foreshadow relationship changes. J Consult Clin Psychol. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- 19.Tyrka AR, Wier L, Price LH, et al. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 21.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 22.Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194–204. doi: 10.1038/ncponc1051. [DOI] [PubMed] [Google Scholar]
- 23.Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- 24.Lutgendorf SK, Lamkin DM, Jennings NB, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–4521. [PubMed] [Google Scholar]
- 26.Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–10364. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 27.Thaker P, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and metastasis in ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–10800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen T, Nahari D, Cerem LW, et al. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 30.Costanzo ES, Lutgendorf SK, Sood AK, et al. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate IL-6 expression by human ovarian carcinoma cells through a SRC-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 32.Landen CN, Lin YG, Armaiz Pena GN, et al. Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res. 2007;67:10389–10396. doi: 10.1158/0008-5472.CAN-07-0858. [DOI] [PubMed] [Google Scholar]
- 33.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masur K, Niggemann B, Zanker KS, et al. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- 35.Drell TL, Joseph J, Lang K, et al. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res and Treatment. 2003;80:63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 36.Yang E, Bane CM, MacCallum RC, et al. Stress-related modulation of matrix metalloproteinase expression. J Neuroimmunol. 2002;133:144–150. doi: 10.1016/s0165-5728(02)00270-9. [DOI] [PubMed] [Google Scholar]
- 37.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews: Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen S. Social relationships and health. Am Psychol. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- 40.Cole SW, Hawkley LC, Arevalo JM, et al. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams JB, Pang D, Delgado B, et al. A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev Res. 2009;2:850–861. doi: 10.1158/1940-6207.CAPR-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutgendorf SK, DeGeest K, Sung CY, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: From immunosurveillance to tumor escape. Nature Immunology. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 44.Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst. 2005;97:1760–1767. doi: 10.1093/jnci/dji401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Eliyahu S, Page GG, Yimira R, et al. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Eliyahu S, Yirmiya R, Liebeskind JC, et al. Stress increases metastatic spread of a mammary tumor in rats: Evidence for mediation by the immune system. Brain Behav Immun. 1991;5:193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- 47.Greenfeld K, Avraham R, Benish M, et al. Immune suppression while awaiting surgery and following it: Dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21:503–513. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Kiecolt-Glaser J, Fisher L, Ogrocki P, et al. Marital quality, marital disruption, and immune function. Psychosom Med. 1987;49:13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Zorilla EP, Luborsky L, McKay JR. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 50.Irwin M. Psychoneuroimmunology of depression: Clinical implications. Brain Behav Immun. 2002;16:1–16. doi: 10.1006/brbi.2001.0654. [DOI] [PubMed] [Google Scholar]
- 51.Blomberg BB, Alvarez JP, Diaz A, et al. Psychosocial adaptation and cellular immunity in breast cancer patients in the weeks after surgery: An exploratory study. J Psychosom Res. 2009;67:369–376. doi: 10.1016/j.jpsychores.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andersen B, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thornton LM, Andersen BL, Crespin TR, et al. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav Immun. 2007;21:185–194. doi: 10.1016/j.bbi.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Lutgendorf S, Sood AK, Anderson B, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Lutgendorf SK, Lamkin D, Anderson B, et al. Depressed and anxious mood and T-cell cytokine producing populations in ovarian cancer patients. Brain Behav Immun. 2008;22:890–900. doi: 10.1016/j.bbi.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collado-Hidalgo A, Bower ME, Ganz PA, et al. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 58.Bower JE. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav Immun. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maier S, Watkins L. Cytokines for psychologists: Implications of bidirectional immune to brain communication for understanding behavior, mood, and cognition. Psychology Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 61.Sephton S, Spiegel D. Circadian disruption in cancer: A neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17:321–328. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 62.Touitou Y, Bogdan A, Levi F, et al. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: Relationships with tumour marker antigens. Br J Cancer. 1996;74:1248–1252. doi: 10.1038/bjc.1996.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Musselman DL, Miller AH, Porter MR, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. Am J Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 64.Jehn CF, Kuehnhardt D, Bartholomae A, et al. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- 65.Touitou Y, Levi F, Bogdan A, Benavides M, et al. Rhythm alteration in patients with metastatic breast cancer and poor prognostic factors. J Cancer Res Clin. 1995;121:181–188. doi: 10.1007/BF01198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bower JE, Ganz PA, Dickerson SS, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrino. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Sephton S, Sapolsky RM, Kraemer HC, et al. Early mortality in metastatic breast cancer patients with absent of abnormal diurnal cortisol rhythms. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 68.Moran TJ, Gray S, Mikosz CA, et al. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000;60:867–872. [PubMed] [Google Scholar]
- 69.Antonova L, Mueller CR. Hydrocortisone down-regulates the tumor suppressor gene BRCA1 in mammary cells: A possible molecular link between stress and breast cancer. Genes Chromosomes Cancer. 2008;47:341–352. doi: 10.1002/gcc.20538. [DOI] [PubMed] [Google Scholar]
- 70.Pang D, Kocherginsky M, Krausz T, et al. Dexamethasone decreases xenograft response to Paclitaxel through inhibition of tumor cell apoptosis. Cancer Biol Ther. 2006;5:933–940. doi: 10.4161/cbt.5.8.2875. [DOI] [PubMed] [Google Scholar]
- 71.Coyne J, Stefanek M, Palmer S. Psychotherapy and survival in cancer: The conflict between hope and evidence. Psychol Bull. 2007;133:367–394. doi: 10.1037/0033-2909.133.3.367. [DOI] [PubMed] [Google Scholar]
- 72.Newell S, Sanson-Fisher R, Savolainen N. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J NCL. 2002;94:558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 73.Spiegel D. Effects of psychotherapy on cancer survival. Nat Rev Cancer. 2002;2:383–389. doi: 10.1038/nrc800. [DOI] [PubMed] [Google Scholar]
- 74.Goodwin PJ, Leszcz M, Ennis M. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–1726. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- 75.Antoni MH, Kazi A, Lechner S, et al. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74:1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andersen BL, Farrar WB, Golden-Kreutz D, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;21:953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaufman P. Psychosocial interventions in breast cancer: To light a candle. Cancer. 2009;115:5617–5619. doi: 10.1002/cncr.24659. [DOI] [PubMed] [Google Scholar]
- 78.Stefanek M, Palmer S, Thombs B, et al. Finding what there is not: Unwarranted claims of an effects of psychosocial intervention on recurrence and survival. Cancer. 2009;115:5612–5616. doi: 10.1002/cncr.24671. [DOI] [PubMed] [Google Scholar]
- 79.Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kissane D, Grabsch B, Clarke D. Supportive-expressive group therapy for women with metastatic breast cancer: Survival and psychosocial outcomes from a randomized controlled trial. Psycho-Oncol. 2007;16:277–286. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- 81.Spiegel D, Butler LD, Giese-Davis J, et al. Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: A randomized prospective trial. Cancer. 2007;110:1130–1138. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 82.Andersen B, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: Could they reduce recurrence rates in oncological patients? Ann Surg Oncol. 2003;10:972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 84.McGregor B, Antoni MH. Psychological intervention and health outcomes among women treated for breast cancer: A review of stress pathways and biological mediators. Brain Behav Immun. 2009;23:159–166. doi: 10.1016/j.bbi.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGregor B, Antoni M, Boyers A, et al. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 86.Antoni MH, Lechner S, Diaz A, et al. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav Immun. 2009;23:580–591. doi: 10.1016/j.bbi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witek-Janusek L, Albuquerque K, Chroniak KR, et al. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phillips K, Antoni MH, Lechner S, et al. Stress management intervention reduces serum cortisol and increases relaxation during treatment for non-metastatic breast cancer. Psychosom Med. 2008;70:1044–1049. doi: 10.1097/PSY.0b013e318186fb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlson LE, Speca M, Faris P, et al. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Andersen B, Shelby R, Golden-Kreutz D. RCT of a psychological intervention for persons with cancer: I. Mechanisms of change. J Consult Clin Psychol. 2007;75:927–938. doi: 10.1037/0022-006X.75.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glaser R. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain Behav Immun. 1999;13:240–251. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- 92.Cohen S, Tyrrell DA, Smith AP. Negative life events, perceived stress, negative affect, and susceptibility to the common cold. J Personal Soc Psychol. 1993;64:131–140. doi: 10.1037//0022-3514.64.1.131. [DOI] [PubMed] [Google Scholar]
- 93.Pereira D, Antoni MH, Simon T, et al. Stress and squamous intraepithelial lesions in women with human papillomavirus and human immunodeficiency virus. Psychosom Med. 2003;65:427–434. doi: 10.1097/01.psy.0000041620.37866.89. [DOI] [PubMed] [Google Scholar]
- 94.Carrico A, Antoni MH. The effects of psychological interventions on neuroendocrine hormone regulation and immune status in HIV-positive persons: A review of randomized controlled trials. Psychosom Med. 2008;70:575–584. doi: 10.1097/PSY.0b013e31817a5d30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antoni MH, Pereira DB, Marion I, et al. Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. J Psychosom Res. 2008;65:389–401. doi: 10.1016/j.jpsychores.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nature Immunology. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benish M, Bartal I, Goldfarb Y, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15:2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]