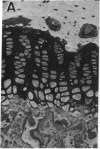
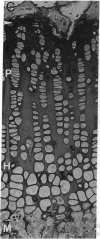
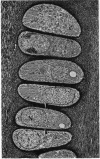
Abstract
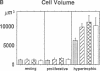
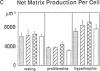
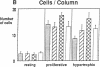
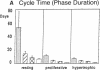
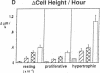
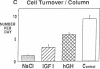
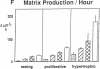
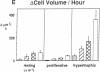
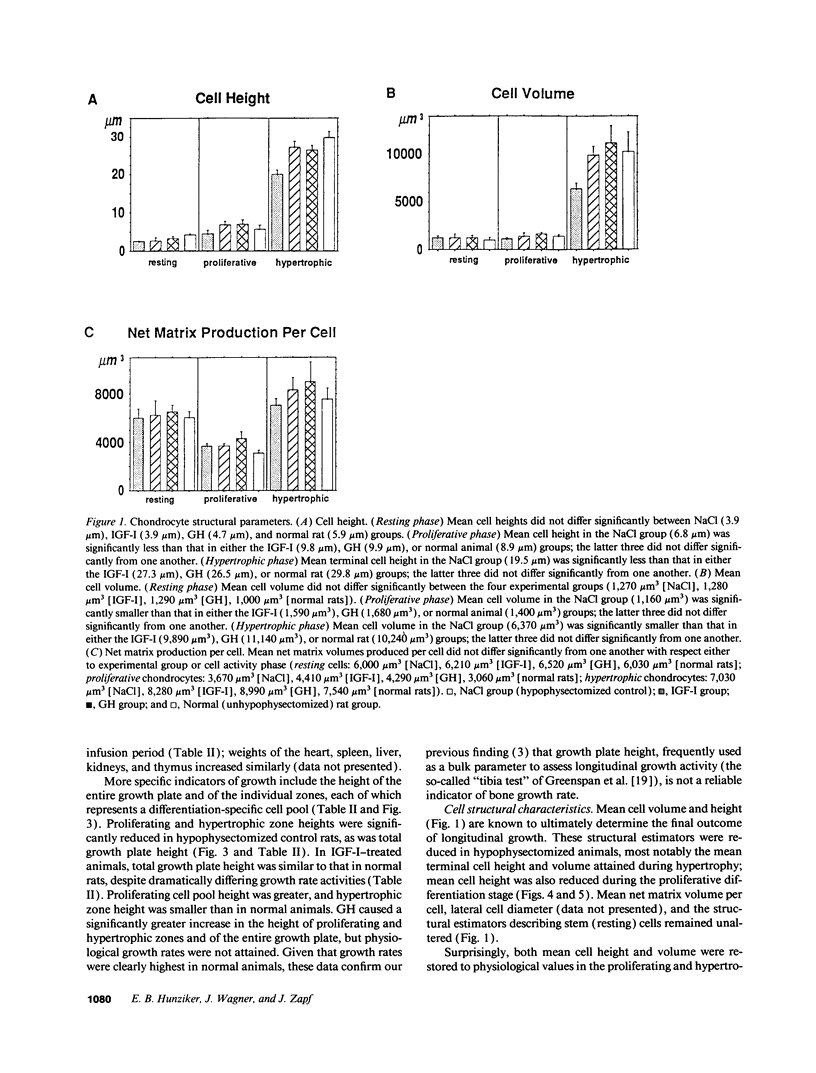
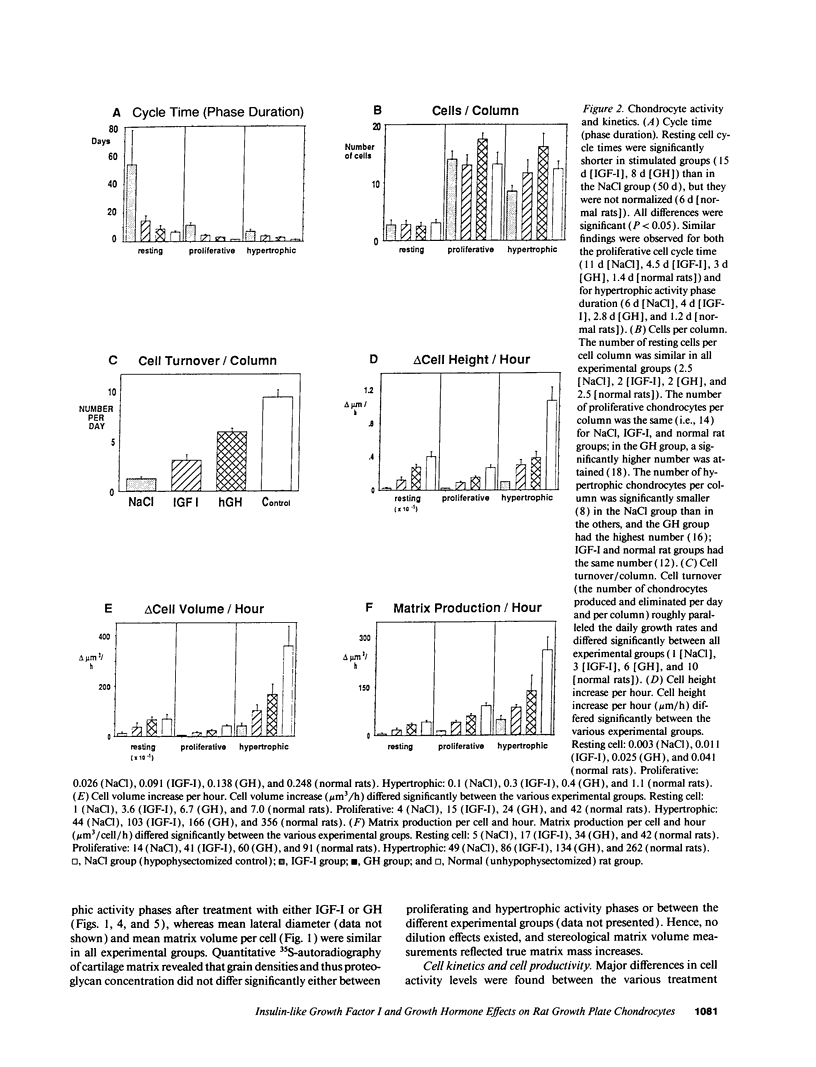
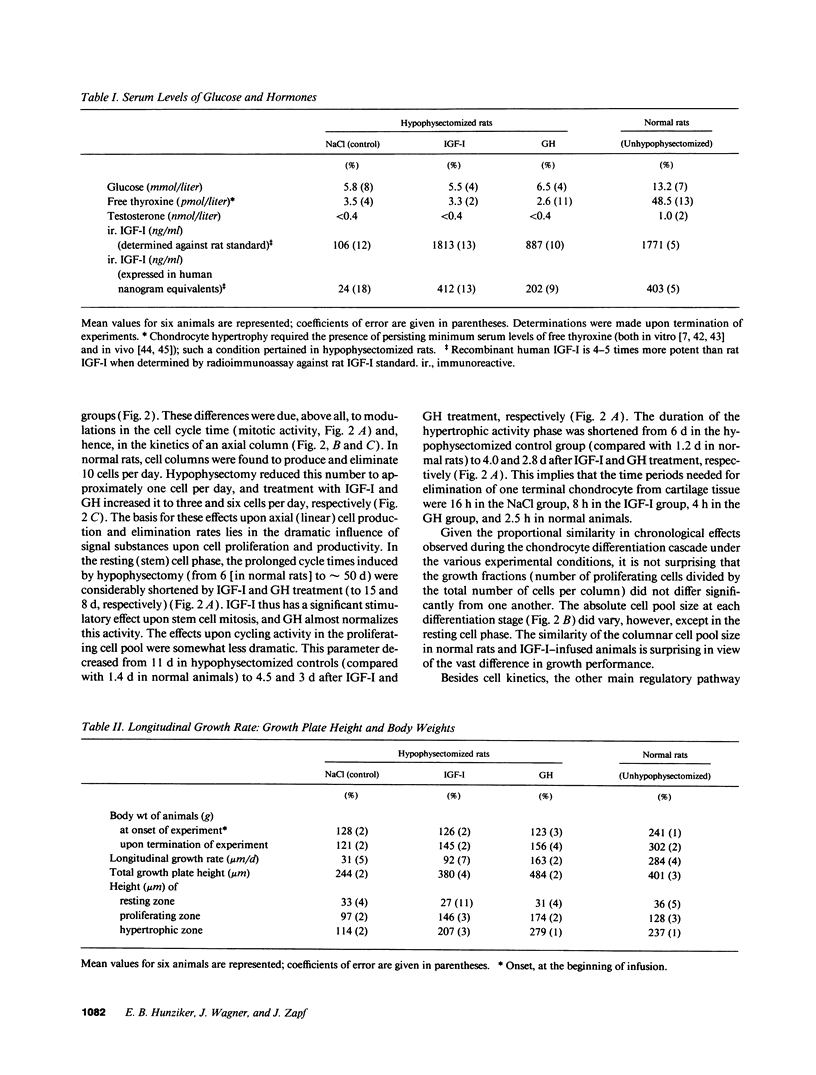
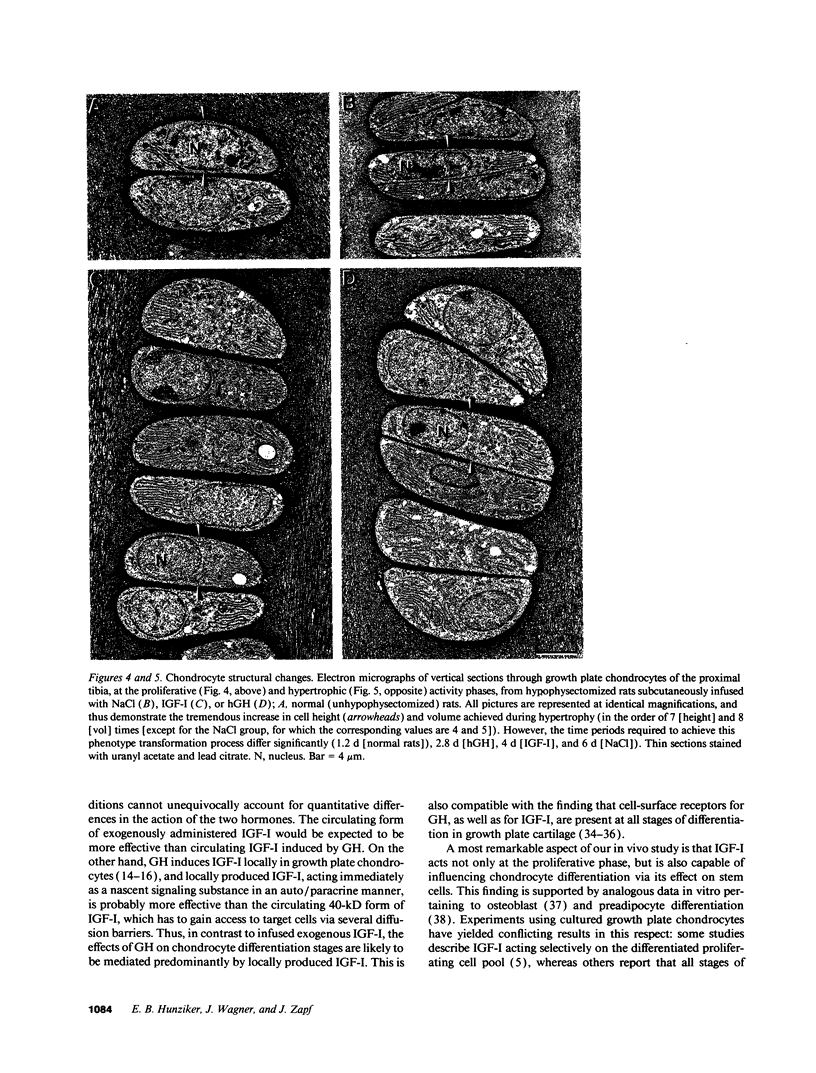
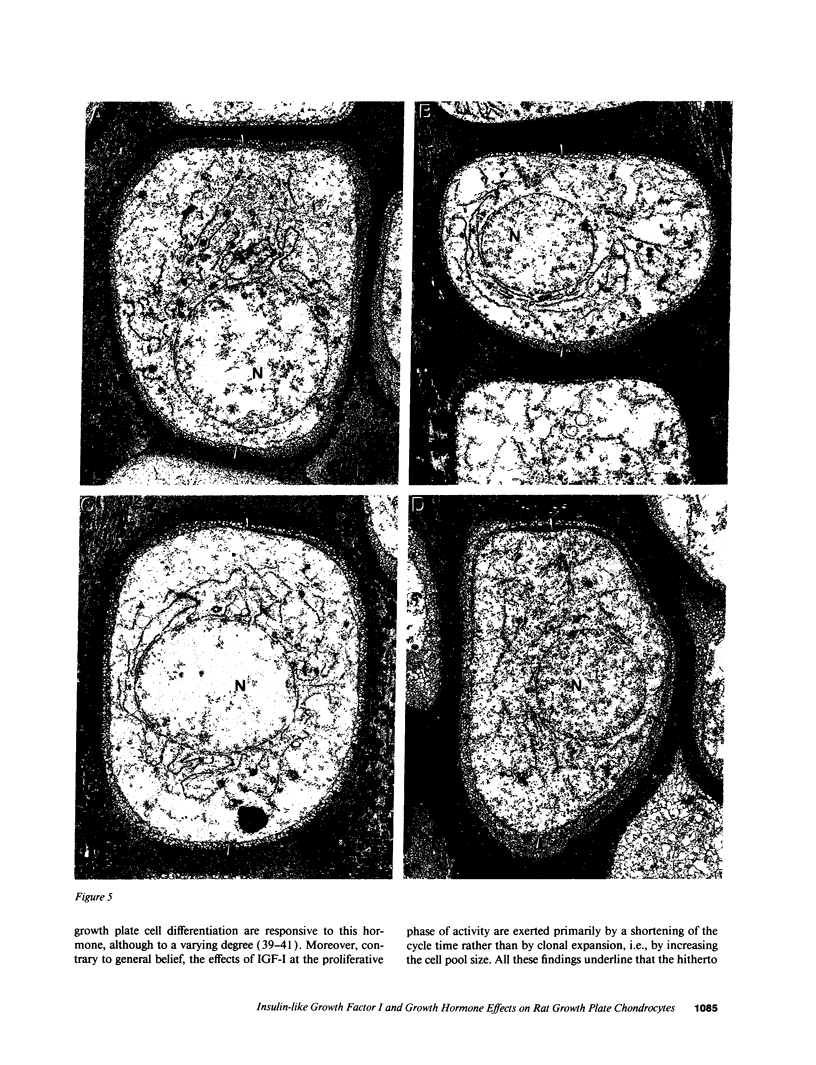
Skeletal growth depends upon enchondral ossification in growth plate cartilage, within which chondrocytes undergo well defined stages of maturation. We infused IGF-I or growth hormone (GH), two key regulators of skeletal growth, into hypophysectomized rats and compared their effects on growth plate chondrocyte differentiation using qualitative and quantitative autoradiography, stereology, and incident light fluorescence microscopy. Stem cell cycle time was shortened from 50 to 15 and 8 d after treatment with IGF-I and GH, respectively. Proliferating cell cycle time decreased from 11 to 4.5 and 3 d, and duration of the hypertrophic phase decreased from 6 to 4 and 2.8 d. Average matrix volume per cell at each differentiation stage was similar for normal, hormone-treated, and untreated hypophysectomized groups. Mean cell volume and cell height were significantly reduced by hypophysectomy at the proliferative and hypertrophic stages, but were restored to physiological values by IGF-I and GH. In contrast, cell productivity, i.e., increases in cell volume, height, and matrix production per unit of time, did not reach normal values with either IGF-I or GH, and this parameter was inversely proportional to cell cycle time or phase duration. IGF-I and GH are thus capable of stimulating growth plate chondrocytes at all stages of differentiation, albeit to variable degrees with respect to individual cell activities. Although it is generally accepted that GH acts at both the stem and proliferating phases of chondrocyte differentiation, our data represent the first evidence in vivo that IGF-I is also capable of stimulating stem cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard R., Haynes K. M., Werther G. A., Waters M. J. The ontogeny of growth hormone receptors in the rabbit tibia. Endocrinology. 1988 Jun;122(6):2562–2569. doi: 10.1210/endo-122-6-2562. [DOI] [PubMed] [Google Scholar]
- Baxter R. C., Martin J. L. Structure of the Mr 140,000 growth hormone-dependent insulin-like growth factor binding protein complex: determination by reconstitution and affinity-labeling. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6898–6902. doi: 10.1073/pnas.86.18.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binoux M., Hossenlopp P. Insulin-like growth factor (IGF) and IGF-binding proteins: comparison of human serum and lymph. J Clin Endocrinol Metab. 1988 Sep;67(3):509–514. doi: 10.1210/jcem-67-3-509. [DOI] [PubMed] [Google Scholar]
- Breur G. J., VanEnkevort B. A., Farnum C. E., Wilsman N. J. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res. 1991 May;9(3):348–359. doi: 10.1002/jor.1100090306. [DOI] [PubMed] [Google Scholar]
- Burch W. M., McCarty K. S., Jr Hormonal stimulation of avian embryonic cartilage growth in vitro: histologic and ultrastructural features. In Vitro. 1984 Apr;20(4):329–338. doi: 10.1007/BF02618596. [DOI] [PubMed] [Google Scholar]
- Böhme K., Conscience-Egli M., Tschan T., Winterhalter K. H., Bruckner P. Induction of proliferation or hypertrophy of chondrocytes in serum-free culture: the role of insulin-like growth factor-I, insulin, or thyroxine. J Cell Biol. 1992 Feb;116(4):1035–1042. doi: 10.1083/jcb.116.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orive L. M., Hunziker E. B. Stereology for anisotropic cells: application to growth cartilage. J Microsc. 1986 Jul;143(Pt 1):47–80. doi: 10.1111/j.1365-2818.1986.tb02765.x. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H. A personal history of the origin of the somatomedin hypothesis and recent challenges to its validity. Perspect Biol Med. 1989 Winter;32(2):194–211. doi: 10.1353/pbm.1989.0006. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- GREENSPAN F. S., LI C. H. Bioassay of hypophyseal growth hormone; the tibia test. Endocrinology. 1949 Nov;45(5):455-63, illust. doi: 10.1210/endo-45-5-455. [DOI] [PubMed] [Google Scholar]
- Green H., Morikawa M., Nixon T. A dual effector theory of growth-hormone action. Differentiation. 1985;29(3):195–198. doi: 10.1111/j.1432-0436.1985.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Scheiwiller E., Froesch E. R. Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4889–4893. doi: 10.1073/pnas.85.13.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Schmid C., Froesch E. R. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 1989 Dec;121(6):753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hill D. J. Stimulation of cartilage zones of the calf costochondral growth plate in vitro by growth hormone dependent rat plasma somatomedin activity. J Endocrinol. 1979 Nov;83(2):219–227. doi: 10.1677/joe.0.0830219. [DOI] [PubMed] [Google Scholar]
- Hunziker E. B., Schenk R. K. Cartilage ultrastructure after high pressure freezing, freeze substitution, and low temperature embedding. II. Intercellular matrix ultrastructure - preservation of proteoglycans in their native state. J Cell Biol. 1984 Jan;98(1):277–282. doi: 10.1083/jcb.98.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker E. B., Schenk R. K., Cruz-Orive L. M. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987 Feb;69(2):162–173. [PubMed] [Google Scholar]
- Hunziker E. B., Schenk R. K. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol. 1989 Jul;414:55–71. doi: 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson O. G., Jansson J. O., Gause I. A. Growth hormone stimulates longitudinal bone growth directly. Science. 1982 Jun 11;216(4551):1237–1239. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- Isaksson O. G., Nilsson A., Isgaard J., Lindahl A. Cartilage as a target tissue for growth hormone and insulin-like growth factor I. Acta Paediatr Scand Suppl. 1990;367:137–141. doi: 10.1111/j.1651-2227.1990.tb11650.x. [DOI] [PubMed] [Google Scholar]
- Isgaard J. Expression and regulation of IGF-I in cartilage and skeletal muscle. Growth Regul. 1992 Mar;2(1):16–22. [PubMed] [Google Scholar]
- Isgaard J., Möller C., Isaksson O. G., Nilsson A., Mathews L. S., Norstedt G. Regulation of insulin-like growth factor messenger ribonucleic acid in rat growth plate by growth hormone. Endocrinology. 1988 Apr;122(4):1515–1520. doi: 10.1210/endo-122-4-1515. [DOI] [PubMed] [Google Scholar]
- Kavumpurath S., Hall B. K. Lack of either chondrocyte hypertrophy or osteogenesis in Meckel's cartilage of the embryonic chick exposed to epithelia and to thyroxine in vitro. J Craniofac Genet Dev Biol. 1990;10(3):263–275. [PubMed] [Google Scholar]
- Kember N. F. Cell population kinetics of bone growth: the first ten years of autoradiographic studies with tritiated thymidine. Clin Orthop Relat Res. 1971 May;76:213–230. doi: 10.1097/00003086-197105000-00029. [DOI] [PubMed] [Google Scholar]
- Lewinson D., Harel Z., Shenzer P., Silbermann M., Hochberg Z. Effect of thyroid hormone and growth hormone on recovery from hypothyroidism of epiphyseal growth plate cartilage and its adjacent bone. Endocrinology. 1989 Feb;124(2):937–945. doi: 10.1210/endo-124-2-937. [DOI] [PubMed] [Google Scholar]
- Lindahl A., Nilsson A., Isaksson O. G. Effects of growth hormone and insulin-like growth factor-I on colony formation of rabbit epiphyseal chondrocytes at different stages of maturation. J Endocrinol. 1987 Nov;115(2):263–271. doi: 10.1677/joe.0.1150263. [DOI] [PubMed] [Google Scholar]
- Makower A. M., Skottner A., Wroblewski J. Binding of insulin-like growth factor-I (IGF-I) to primary cultures of chondrocytes from rat rib growth cartilage. Cell Biol Int Rep. 1989 Aug;13(8):655–665. doi: 10.1016/0309-1651(89)90042-8. [DOI] [PubMed] [Google Scholar]
- Makower A. M., Wroblewski J., Pawlowski A. Effects of IGF-I, EGF, and FGF on proteoglycans synthesized by fractionated chondrocytes of rat rib growth plate. Exp Cell Res. 1988 Dec;179(2):498–506. doi: 10.1016/0014-4827(88)90287-x. [DOI] [PubMed] [Google Scholar]
- McConaghey P., Sledge C. B. Production of "sulphation factor" by the perfused liver. Nature. 1970 Mar 28;225(5239):1249–1250. doi: 10.1038/2251249b0. [DOI] [PubMed] [Google Scholar]
- Ohlsson C., Nilsson A., Isaksson O., Lindahl A. Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9826–9830. doi: 10.1073/pnas.89.20.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto R., Campanile G., Cancedda R., Dozin B. Thyroid hormone, insulin, and glucocorticoids are sufficient to support chondrocyte differentiation to hypertrophy: a serum-free analysis. J Cell Biol. 1992 Nov;119(4):989–995. doi: 10.1083/jcb.119.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALMON W. D., Jr, DAUGHADAY W. H. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957 Jun;49(6):825–836. [PubMed] [Google Scholar]
- Schlechter N. L., Russell S. M., Spencer E. M., Nicoll C. S. Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Steiner T., Froesch E. R. Insulin-like growth factor I supports differentiation of cultured osteoblast-like cells. FEBS Lett. 1984 Jul 23;173(1):48–52. doi: 10.1016/0014-5793(84)81015-7. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Humbel R. E., Froesch E. R. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982 Mar 18;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Wise L. S., Berkowitz R., Wan C., Rubin C. S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988 Jul 5;263(19):9402–9408. [PubMed] [Google Scholar]
- Trippel S. B., Corvol M. T., Dumontier M. F., Rappaport R., Hung H. H., Mankin H. J. Effect of somatomedin-C/insulin-like growth factor I and growth hormone on cultured growth plate and articular chondrocytes. Pediatr Res. 1989 Jan;25(1):76–82. doi: 10.1203/00006450-198901000-00017. [DOI] [PubMed] [Google Scholar]
- Trippel S. B., Van Wyk J. J., Foster M. B., Svoboda M. E. Characterization of a specific somatomedin-c receptor on isolated bovine growth plate chondrocytes. Endocrinology. 1983 Jun;112(6):2128–2136. doi: 10.1210/endo-112-6-2128. [DOI] [PubMed] [Google Scholar]
- Walker K. V., Kember N. F. Cell kinetics of growth cartilage in the rat tibia. I. Measurements in young male rats. Cell Tissue Kinet. 1972 Sep;5(5):401–408. doi: 10.1111/j.1365-2184.1972.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Werther G. A., Haynes K. M., Barnard R., Waters M. J. Visual demonstration of growth hormone receptors on human growth plate chondrocytes. J Clin Endocrinol Metab. 1990 Jun;70(6):1725–1731. doi: 10.1210/jcem-70-6-1725. [DOI] [PubMed] [Google Scholar]
- Zapf J., Hauri C., Waldvogel M., Futo E., Häsler H., Binz K., Guler H. P., Schmid C., Froesch E. R. Recombinant human insulin-like growth factor I induces its own specific carrier protein in hypophysectomized and diabetic rats. Proc Natl Acad Sci U S A. 1989 May;86(10):3813–3817. doi: 10.1073/pnas.86.10.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Walter H., Froesch E. R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest. 1981 Nov;68(5):1321–1330. doi: 10.1172/JCI110379. [DOI] [PMC free article] [PubMed] [Google Scholar]