Abstract
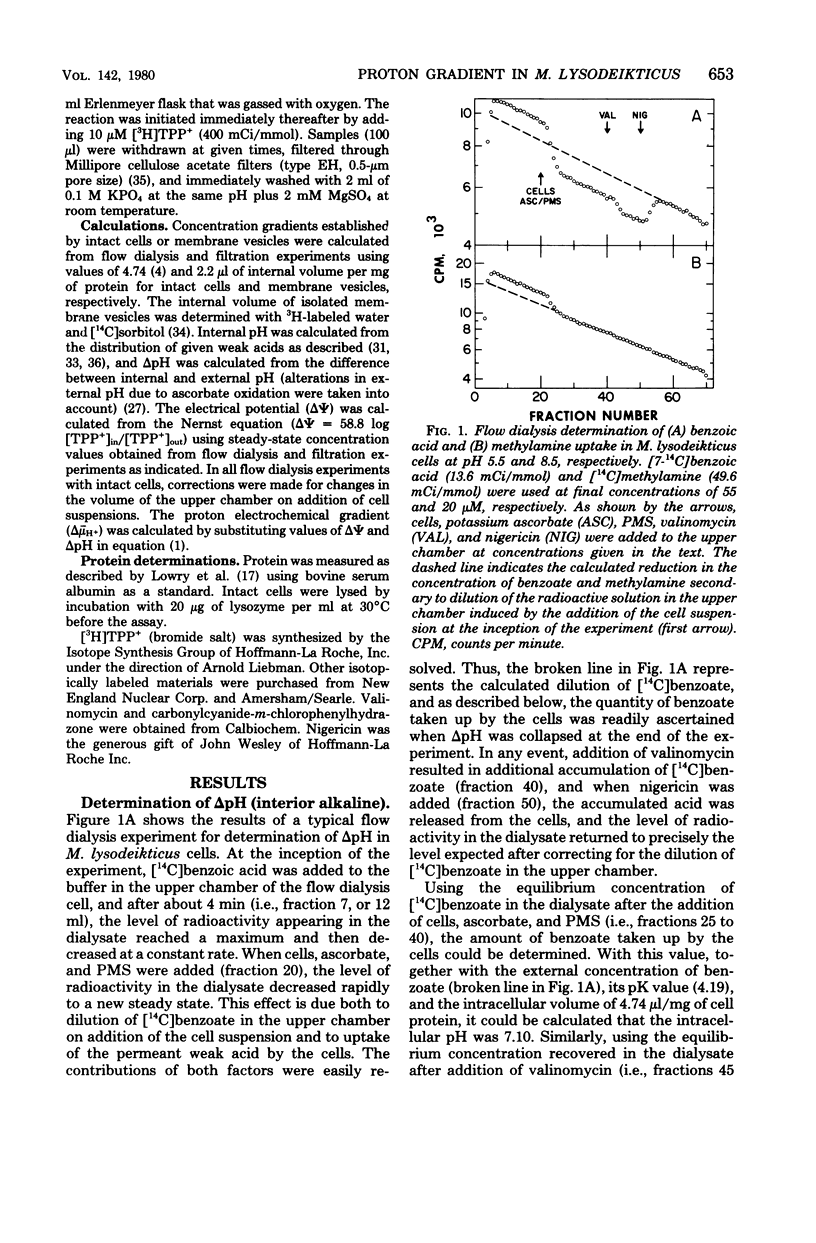
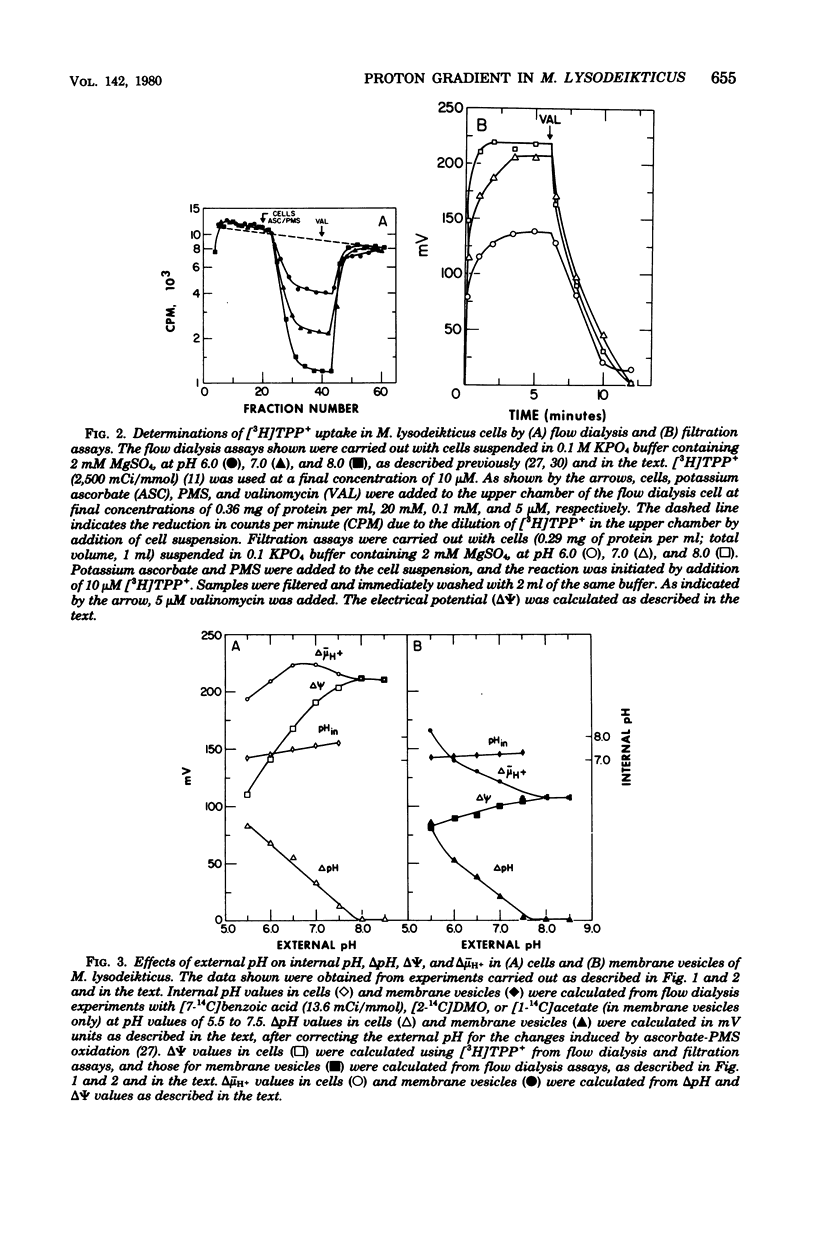
Using the distribution of weak acids to measure the pH gradient (delta pH; interior alkaline) and the distribution of the lipophilic cation [3H]tetraphenylphosphonium+ to monitor the membrane potential (delta psi; interior negative), we studied the electrochemical gradient or protons (delta mu- H+) across the membrane of Micrococcus lysodeikticus cells and plasma membrane vesicles. With reduced phenazine methosulfate as electron donor, intact cells exhibited a relatively constant delta mu- H+ (interior negative and alkaline) of -193 mV to -223 mV from pH 5.5 to pH 8.5. On the other hand, in membrane vesicles under the same conditions, delta mu- H+ decreased from a maximum value of -166 mV at pH 5.5 to -107 mV at pH 8.0 and above. This difference is related to a differential effect of external pH on the components of delta mu- H+. In intact cells, delta pH decreased from about -86 mV (i.e., 1.4 units) at pH 5.5 to zero at pH 7.8 and above, and the decreases in delta pH was accompanied by a reciprocal increase in delta psi from -110 mV at pH 5.5 to -211 mV at pH 8.0 and above. In membrane vesicles, the decrease in delta pH with increasing external pH was similar to that described for intact cells; however, delta psi increased from -82 mV at pH 5.5 to only -107 mV at pH 8.0 and above.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Rottenberg H., Caplan S. R. An estimation of the light-induced electrochemical potential difference of protons across the membrane of Halobacterium halobium. Biochim Biophys Acta. 1976 Sep 13;440(3):557–572. doi: 10.1016/0005-2728(76)90042-6. [DOI] [PubMed] [Google Scholar]
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Friedberg I. Phosphate transport in Micrococcus lysodeikticus. Biochim Biophys Acta. 1977 May 2;466(3):451–460. doi: 10.1016/0005-2736(77)90338-8. [DOI] [PubMed] [Google Scholar]
- Friedberg I. The effect of ionophores on phosphate and arsenate transport in Micrococcus lysodeikticus. FEBS Lett. 1977 Sep 15;81(2):264–266. doi: 10.1016/0014-5793(77)80531-0. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Molecular biology and energetics of membrane transport. J Cell Physiol. 1976 Dec;89(4):575–593. doi: 10.1002/jcp.1040890414. [DOI] [PubMed] [Google Scholar]
- Konings W. N. Active transport of solutes in bacterial membrane vesicles. Adv Microb Physiol. 1977;15:175–251. doi: 10.1016/s0065-2911(08)60317-3. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A., Davidson L. F., Filip S. J., Jr, Zuckerman R. S., Guffanti A. A. The protonmotive force and beta-galactoside transport in Bacillus acidocaldarius. J Biol Chem. 1978 Jul 10;253(13):4599–4603. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lichtshtein D., Dunlop K., Kaback H. R., Blume A. J. Mechanism of monensin-induced hyperpolarization of neuroblastoma-glioma hybrid NG108-15. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2580–2584. doi: 10.1073/pnas.76.6.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G., Glynn P., Yamane T., Navon G. On the measurement of pH in Escherichia coli by 31P nuclear magnetic resonance. Biochim Biophys Acta. 1978 Apr 11;502(1):45–50. doi: 10.1016/0005-2728(78)90130-5. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Patel L., Schuldiner S., Kaback H. R. Reversible effects of chaotropic agents on the proton permeability of Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3387–3391. doi: 10.1073/pnas.72.9.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Grollman E. F., Lazo P. S., Dyer S. A., Habig W. H., Hardegree M. C., Kaback H. R., Kohn L. D. Effect of tetanus toxin on the accumulation of the permeant lipophilic cation tetraphenylphosphonium by guinea pig brain synaptosomes. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4783–4787. doi: 10.1073/pnas.76.10.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. pH-dependent changes in proton:substrate stoichiometries during active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Sep 20;16(19):4270–4275. doi: 10.1021/bi00638a022. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rottenberg H., Avron M. Determination of pH in chloroplasts. 2. Fluorescent amines as a probe for the determination of pH in chloroplasts. Eur J Biochem. 1972 Jan 31;25(1):64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent methyl 1-thio-beta-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977 May 17;16(10):2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]


