Abstract
The homeobox transcription factor OTX2 plays an essential role during embryonic brain development. It is normally silenced in the adult brain, but is overexpressed by genomic amplification or other mechanisms in the majority of medulloblastomas (MBs). Retinoic acids (RAs) can suppress OTX2 expression and inhibit MB growth. In this study, 9-cis RA most potently inhibited MB cell growth. 9-cis RA functions through the downregulation of OTX2 expression, which subsequently induces neuronal differentiation of OTX2-expressing cells. Treatment with 9-cis RA reduced the growth of D425 flank xenograft tumors in mice. In an intracranial model, however, MB tumors showed resistance to 9-cis RA treatment, and we implicated fibroblast growth factor (FGF) as a potential mediator of resistance to RA therapy. These findings suggest a mechanism for RA-mediated anti-tumor effect on OTX2-positive MB cells and indicate that therapeutic targeting of OTX2 might be effective if FGF pathway-mediated resistance can be overcome.
Keywords: FGF, medulloblastoma, OTX2, retinoic acid
Medulloblastoma (MB), the most common pediatric brain cancer, is an aggressive primitive neuroectodermal tumor that arises in the cerebellum. There are several subtypes or related histological classifications that include classic MB, desmoplastic/nodular MB, large-cell MB, and the rare medullomyoblastoma.1 Classic MBs are the most common subtype and normally arise in the vermis of the cerebellum, whereas the desmoplastic MBs normally arise in one of the hemispheres.
The treatment for MBs involves combination chemo- and radiotherapy and has led to significant survival increases. Unfortunately, though, MB still remains a significant cause of cancer-related death in children, with most survivors experiencing subsequent learning delays.2 Therefore, research has been aimed at molecular pathways activated by cancer-related genomic DNA alterations and mutations, in the hopes of identifying tumor-specific therapeutic targets that are less toxic to normal brain cells.
Our laboratory and others have identified the genomic amplification and/or overexpression of OTX2 in over 60% of MBs.3–7 A recent high-resolution SNP analysis revealed genomic amplification of the locus containing OTX2 in 2 of the 123 primary MB cases.8 The frequent overexpression of OTX2 in MBs is significant since expression of OTX2 is not present in the cerebellum beyond the late postnatal period and because it appears that this is a pathological mechanism to activate OTX2 in MB cells.
OTX2 is a member of a highly conserved family of the bicoid-like homeobox transcription factors that control brain morphogenesis.9 During embryogenesis, OTX2 is required for specification and regionalization of the developing brain, and is expressed in restricted areas of the forebrain and midbrain and throughout the posterior cerebellum, particularly within the external granular layer (EGL) and the emerging internal granular layer.10 Although OTX2 expression in children and adults is still found in the retina, OTX2 is mainly silenced in the central nervous system and the rest of the body (for examples of its gene expression pattern, see the Cancer Genome Anatomy Project data at http://cgap.nci.nih.gov/SAGE/AnatomicViewer).4
The oncogenic role of OTX2 in MB is supported by genomic amplification in 3 MB cell lines, two of which, D425 and D458, were derived from the same patient at different times. Recently, Northcott et al.8 showed in a survey of copy number changes in MBs, additional OTX2 amplifications, further supporting its role in MB pathogenesis. It has been shown that siRNA-mediated knockdown of OTX2 resulted in slower growth of MB cells.5 Even genomic amplification is an infrequent means of OTX2 activation in MB; our previous work has shown that the majority of MBs overexpress OTX24 and that it is the most commonly overexpressed transcription factor known in MBs. Although the exact mechanism by which OTX2 is activated in the majority of MB remains unknown, its genomic amplification and other published data suggest that the aberrant expression of the wild-type gene product is oncogenic for MBs.
Retinoic acids (RAs) are a group of vitamin A-related compounds that regulate a variety of processes during development, including cell growth, differentiation, and apoptosis. They inhibit proliferation in several cancer types, such as gastric cancer, ovarian cancers, and neuroblastoma.11–13 Previous reports indicate that all trans-retinoic acid (ATRA) downregulates OTX2 expression via its promoter, and inhibits the growth of OTX2-positive MB cells.5,14,15 The value of RA for in vivo use, however, has not been satisfactorily investigated in MBs.
In this study, we identified the 9-cis RA as the most effective retinoid among a panel of RAs in suppressing MB cells. We further revealed the underlying mechanism of how this suppression is achieved. In developing a therapeutic approach with RA, we tested the efficacy of 9-cis RA in both flank and intracranial xenograft MB tumor models and investigated a possible mechanism of resistance to intracranial RA use.
Materials and Methods
Cell Lines and Cell Culture
The following MB cell lines were used in the study: D283 Med (D283), D341 Med (D341), D384 Med (D384), D425 Med (D425), D458 Med (D458), D487 Med (D487), D556 Med (D556), DAOY, MCD1, Mhh-Med-1 (Mhh1), and UW228-2 (UW228). All cells were maintained in Dulbecco's modified Eagle's medium (DMEM) media supplemented with 10% fetal bovine serum (FBS) and antibiotics. Stem/progenitor cell media containing 20 ng/mL EGF and 10 ng/mL basic fibroblast growth factor (bFGF) was purchased from Cambrex. Human EGF and recombinant basic fibroblast growth factor (bFGF) were purchased from PeproTech, Inc.
Retinoic Acids
CD437 was purchased from Sigma. MDI-301 was a kind gift from Dr. William P. Purcell of Molecular Design International. All the other retinoids were purchased from Biomol.
Plasmids and Constructs
OTX2 cDNA was fused with 3 copies of FLAG sequence on the N-terminus and subcloned in pCMV-TAG-2B (Stratagene). This construct was transfected in D425 cells by electroporation at 200 volts/25 ms with a GenePulser (Bio-Rad) and selected by 1 mg/mL of G418 (Invitrogen). Single clones were selected by limiting dilution.
Isolation of RNA and RT-PCR
Total RNA was isolated by SV Total RNA Isolation Kit (Promega). cDNA was made by cDNA Synthesis System (Invitrogen). PCR was performed using Platinum Taq polymerase (Invitrogen) for 27 cycles using gene-specific custom primers.
Western Blotting
Cells were lysed in lysis buffer as described previously.16 Cell lysates were heated for 5 minutes with Invitrogen's LDS Sample Buffer supplemented with 100 mM DTT before loading on a 4%–12% NuPAGE Bis-Tris Gel (Invitrogen). After transfer to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad), immunostaining was performed according to standard procedures. The following antibodies were used in this study: mouse anti-OTX2 and anti-β-TubIII clone Tuj1 (R&D Systems), rabbit anti-OTX2 and anti-synapsin I (Chemicon), anti-GAPDH (Santa Cruz Biotech), and rabbit anti-REST (Upstate). Signals were visualized by SuperSignal chemiluminescent system (Pierce).
Immunohistochemistry
The mouse brain was first fixed by formalin and embedded in paraffin. For hematoxylin and eosin (H&E) staining, the section was deparaffinized and stained by the standard H&E procedure to visualize tissue structures. For immunostaining, rabbit anti-RA antibody was purchased from Abcam, and rabbit anti-basic FGF antibody was purchased from Millipore. The section was deparrafinized using a standard procedure and blocked using 4.5% H2O2 in methanol at room temperature for 10 minutes. The section was heated at 100°C for 10 minutes in antigen retrieval citra solution (BioGenex) and blocked using 10% goat serum for 15 minutes at room temperature. After incubation, with the rabbit anti-OTX2 or anti-RA antibody diluted 1:500 overnight at 4°C, polymer-HRP anti-rabbit antibody was applied for 30 minutes at room temperature. Antibody binding was visualized by DAB-Chromogen system (DakoCytomation). Subsequently, the section was stained by H&E to help view the tissue structure.
Immunofluorescence
D425 cells were incubated with 2 mM 9-cis RA for 10 days in a 6-well plate and fixed with 4% paraformaldehyde solution for 10 minutes with 3 subsequent washes in PBS. The cells were permeabilized with methanol for 2 minutes and washed 4 times. Then they were incubated with Tuj1 antibody in 10% goat serum at room temperature for 2 hours. Following 3 washes, Texas Red-conjugated goat anti-mouse antibody was added for 30 minutes at room temperature with 3 subsequent washes and visualized on a fluorescence microscope.
RA Reporter Assay
Three hours after the 9-cis RA injection, mice were sacrificed and brain samples were collected. Hundred milligram brain sample was homogenized in hypotonic buffer containing 20 mM NaCl and 10 mM phosphate buffer at pH 7.3. Brain tissues were lysed by sonification and repeated freeze and thaw. The luciferase reporter construct RARE-luc (pRARE3-TK-luc) with 3 RA-responsive element repeats was kindly provided by Dr. Rene Bernands of the Netherlands Cancer Institutes. It was transfected into P19 mouse embryonal carcinoma cells by Lipofectamine 2000, with pRL-CMV from Promega encoding Renilla luciferase as the transfection control. After 24 hours, 50 µL of brain sample lysates were added to the cells for 24 hours. Luciferase assay was performed with the dual-luciferase reporter assay system from Promega using the FB12 luminometer (Zylux). Three independent brain samples in each group were measured. Luciferase activity was normalized to Renilla luciferase activity.
Measurement of Cell Growth
Cells were plated in 96-well flat bottom plates and mixed with 10% of WST-1 solution (Alexis). After incubation for 1–2 hours at 37°C, the plates were measured by a Victor3 plate reader at an absorbance of 450 nm (PerkinElmer). Wells filled with WST-1 and media were used as blank controls.
Caspase Assay
Caspase-Glo 3/7 assay kit from Promega was used to evaluate the apoptotic activity of treated cells. The cells were incubated with retinoids for 48 hours in a 96-well plate, and the caspase 3/7 activities were measured with a luminescence plate reader according to the manufacturer's instructions (PerkinElmer).
Testing of 9-cis RA on Flank and Intracranial Xenograft Models
Female 6–8 weeks old athymic nu/nu mice were purchased from the National Cancer Institute (NCI). D425 cells were grown in culture flasks and harvested. For the flank xenograft experiment, 4 million cells were mixed with growth factor-reduced Matrigel (BD) and 200 µL of this mixture were injected subcutaneously in the flank of each mouse. After 7 days, when the tumor reached about 300 mm3, mice were treated by daily intraperitoneal (IP) injection of 15 mg/kg of 9-cis RA (Biomol) dissolved in 50 µL of DMSO. In the control group, DSMO alone was injected. The animal weight and tumor size were followed daily. We observed a moderate 10% weight loss in the 9-cis RA-treated mice and slight skin flaking and moderate flaking around the neck. For the intracranial tumor model, 1 million D425 cells were injected intracranially as described before.17 Fifty microliters of 9-cis RA dissolved in DMSO were IP injected in 13 mice in a dose of 20 mg/kg. The same volume of DMSO was injected in 10 mice as control. Due to the side effects after the first 6 daily treatments, we took a pause of 2 days and resumed the treatment for 5 additional days.
Results
OTX2 in MB Cell lines
We found that the MB cell lines D283, D341, D384, D425, D458, D487, and D556 expressed OTX2, while DAOY, MCD1, Mhh1, and UW228 did not (Fig. 1A), which is consistent with previous reports.4,6 In D425, D458, and D487, the OTX2 locus is genomically amplified and it should be noted that D425 was derived from the primary MB tumor, and D458 was established from a cerebrospinal metastasis from the same patient at a later date.5,6 We chose D425, a cell line that grows rapidly as clusters in suspension, as our cell line model due to its well-defined OTX2 amplification.
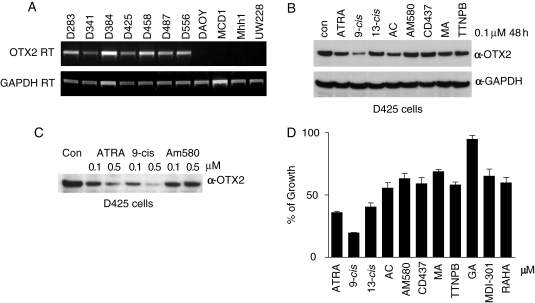
Fig. 1.
9-cis retinoic acid (RA) suppresses OTX2 expression better than other available retinoids. (A) Expression of OTX2 in MB cell lines evaluated by RT-PCR. OTX2 or GAPDH primers were used with cDNA from the 11 MB cell lines for 28 cycles of PCR. (B) 9-cis RA suppresses OTX2 expression better than other retinoic acids. A panel of RAs at 0.1 µM was incubated with D425 cells for 48 hours. Protein level of OTX2 was analyzed by Western blotting. Equal loading was confirmed by probing with an anti-GAPDH antibody. Other retinoids tested were: ATRA, all trans-RA; 13-cis, 13-cis RA; AC, acitretin; MA, methoprene acid; TTNPB, 4-[E-2-(5,6,7,8-Tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid. (C) Comparison of ATRA, 9-cis RA, and AM580 in suppressing OTX2 expression. D425 cells were incubated with 0.1 or 0.5 µM of the indicated retinoids for 48 hours. OTX2 protein level was detected by Western blotting. (D) Comparison of RAs in inhibiting the growth of D425 cells. A volume of 0.1 µM of the indicated RAs was incubated with D425 cells for 6 days with a re-addition of RAs after 3 days. Viable cells were monitored by cell proliferation assay reagent WST-1 and calculated in percentage to the DMSO control. RAHA, retinoic acid p-hydroxyanilide; GA, geranylgeranoic acid.
9-cis RA Most Effectively Suppresses OTX2 Expression
All trans-retinoic acid suppresses OTX2 at the promoter level during embryonic development and inhibits the growth of OTX2-positive MB cell lines, when compared with OTX2-negative lines.5,14 In order to determine if other retinoic acids will inhibit MB growth, we compared a panel of commercially available retinoids based initially on the downregulation of OTX2 expression. In this study, 9-cis RA, a naturally occurring derivative of ATRA, was the most effective suppressor of OTX2 expression (Fig. 1B) in replicate experiments at different concentrations (Fig. 1C). In addition to the retinoids shown in Fig. 1B, we also tested RA p-hydroxyanilide (RAHA), geranylgeranoic acid (GA), docosahexaenoic acid, and MDI-301, a derivative of 9-cis RA kindly provided by Dr. William P. Purcell of Molecular Design International. None of these RAs were as effective as 9-cis RA in the downregulation of OTX2 in D425 cells (data not shown). Among the retinoids tested, 9-cis RA also displayed its strongest potency in reducing OTX2 expression in D283 and D341 MB cells (Supplementary Material, Fig. S1).
9-cis RA Suppresses Growth of OTX2-expressing MB Cells
Compared with a panel of RAs, 9-cis RA showed the most potent growth inhibition with D425 cells (Fig. 1D). In the experiments with different MB cell lines, cells treated with 9-cis RA exhibited improved growth inhibition and suppression of OTX2 compared with cells treated with ATRA; since this phenomenon might have translational implications, we investigated this aspect further. It was noted that growth inhibition affected only OTX2-positive MB cell lines, especially D425, and to a much lesser extent D341 and D283 cells, but that OTX2-negative cells were largely not affected by any RA (Fig. 2A). However, at the high concentration of 10 µM, ATRA did exhibit a marginal inhibition on DAOY, an OTX2-negative cell line. Part of the growth reduction by RAs in OTX2-positive MB cells can be attributed to the increased apoptosis as reflected by the elevated caspase 3/7 activities (Supplementary Material, Fig. S2). This observation implied a relationship between OTX2 expression and RA response.
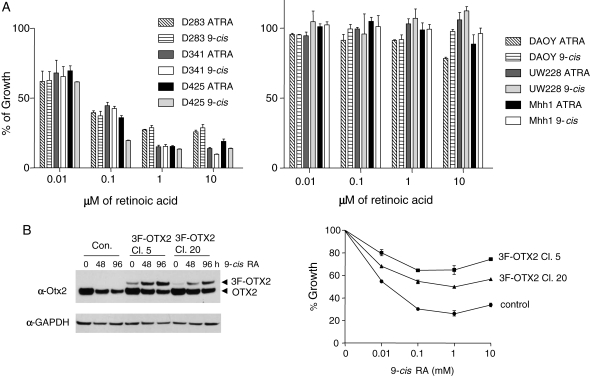
Fig. 2.
Suppression of MB cells by RA is found only in cells expressing OTX2, and RA sensitivity is lost with exogenous OTX2 expression. (A) Comparison of the growth inhibition from ATRA and 9-cis RA on MB cell lines. Medulloblastoma cell lines (D283, D341, D425, DAOY, UW228, and Mhh1) were incubated with DMSO (control), 0.01, 0.1, 1, or 10 µM of the indicated RA for 6 days. After 3 days, we re-added RA. Viable cells were monitored by WST-1 and calculated in percentage to the DMSO control. All the OTX2-positive cells (D283, D341, and D425) were inhibited by ATRA and 9-cis RA, while 9-cis RA generally exhibited inhibition at slightly lower concentration in D425 cells. (B) Overexpression of exogenous OTX2 reduces the growth inhibitory effect of 9-cis RA on D425 cells. OTX2 was subcloned in fusion with 3 copies of FLAG tag (3F-OTX2) and stably transfected in D425 cells. Single clones were selected by limited dilution and tested for 3F-OTX2 expression by Western blotting. D425 cells with vector alone were used as a negative control. On the anti-OTX2 Western blot, 3F-OTX2 showed a slower migration pattern compared with that of the endogenous OTX2 protein. Clone 5 (Cl. 5), Clone 20 (Cl. 20), and control cells were incubated with DMSO, 0.01, 0.1, 1, or 10 mM 9-cis RA for 96 hours. Viable cells were measured by WST-1 and graphed in percentage to the DMSO control (0 hour).
To see if growth suppression by RA operated via control of OTX2 expression, we attempted to overcome the suppression of endogenous OTX2 expression with an artificial construct. To do this, we transfected D425 cells with a 3xFLAG-tagged OTX2 (3F-OTX2) expression construct with a cytomegalovirus (CMV) constitutive expression promoter and obtained two independent clones expressing the chimeric protein (Fig. 2B, left panel). During normal growth, both clones expressed relatively low levels of 3F-OTX2 compared with the endogenous OTX2, whereas Clone 5 showed slightly higher 3F-OTX2 expression. In comparison with the control, both clones demonstrated significant resistance to 9-cis RA-mediated growth inhibition (Fig. 2B, right graph). Clone 5 displayed a higher degree of resistance to 9-cis RA than Clone 20, which could be attributed to the higher level of 3F-OTX2 expressed in Clone 5 than that in Clone 20. Interestingly, in both Clones 5 and 20, treatment with 9-cis RA led to increased exogenous 3F-OTX2 while endogenous OTX2 expression was reduced by RA incubation. Taken together, these results indicate that the constitutive expression of OTX2 can overcome the RA-mediated growth suppression in MBs, supporting OTX2 as a mediator of RA response.
9-cis RA Induces Neuronal Differentiation in MB Cells
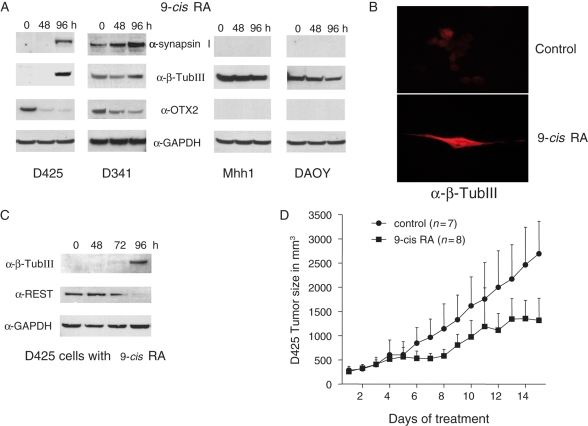
9-cis RA treatment induced expression of neuronal markers, such as synapsin I and β-TubIII in D425 and D341 cells but not in the OTX2-negative Mhh1 and DAOY cells (Fig. 3A). It is noteworthy that D341 cells expressed significant levels of these neuronal markers prior to the RA treatment, which only slightly increased the expression of β-TubIII. 9-cis RA also induced morphological changes in D425 cells that were stained by α-β-TubIII antibody (Fig. 3B). Expression of the neuronal terminal differentiation markers, such as TubIII and Synaspsin I, is suppressed by REST in non-neural cells and undifferentiated neural cells. 9-cis RA silenced the expression of REST and gave rise to the neuronal marker TubIII (Fig. 3C).
Fig. 3.
9-cis RA induces neuronal differentiation and reduces growth in a flank xenograft tumor. (A) Induction of neuronal differentiation markers in OTX2-positive MB cells by 9-cis RA. D425, D341, Mhh1, and DAOY cells were incubated with 1 µM 9-cis RA up to 96 hours. Control (0 hour) is done with incubation with DMSO for 96 hours. Western blots revealed the induction of synapsin I and β-tubulin III (β-TubIII). (B) β-TubIII immunofluorescence staining of D425 cells untreated or treated by 9-cis RA. D425 cells were incubated with 2 µM 9-cis RA for 10 days and stained with α-β-TubIII (Tuj1) antibody and Texas Red-conjugated goat anti-mouse secondary antibody. (C) 9-cis RA suppresses REST and induces β-tubulin III (β-TubIII). D425 cells were incubated with 1 µM 9-cis RA in a time course of 48, 72, and 96 hours. Western blotting reveals that 9-cis RA will suppress REST and induce β-tubulin III expression. (D) Treatment of 9-cis RA reduces flank D425 xenograft tumors. Nude mice were injected with 4 million D425 cells. After 7 days, the mice were treated with 9-cis RA dissolved in DMSO via intraperitoneal injection. Mice in the control group were treated with DMSO alone. The daily treatment of the first 7 days was paused for 1 day and resumed in the following days. The tumor size and the weight of mice were measured. The P-value at day 15 is .0006.
Reduction of Flank Xenograft Tumor by 9-cis RA
A previous study reported successful treatment of murine flank xenografts from D283 cells by ATRA and 13-cis RA,15 which has been subsequently shown to be an OTX2-positive line. To test 9-cis RA in a different OTX2-positive tumor, we started with D425 flank xenograft tumors, which were grown for 12 days, prior to treatment, to a visible size of between 200 and 300 mm3. A Phase I clinical trial of 9-cis RA given systemically recommended a daily dose below 140 mg/m2 to avoid adverse effects, which can be translated to 16–20 mg/kg in mice.18 We used 15 mg/kg to test what would be predicted to be a nontoxic dose in humans. In our testing in a subcutaneous flank model, the intraperitoneal administration of 9-cis RA significantly slowed tumor growth. The average tumor size was reduced from 2692 to 1317 mm3 after 14 days of treatment (P = .0006) before the experiment was terminated due to continued tumor growth, although growth slowed in the treated group. Despite a predicted nontoxic dose, the 15 mg/kg treatment of 9-cis RA did result in some moderate side effects frequently associated with systemic administration of RA, such as skin irritation.19 Consistent with the in vitro data, 9-cis RA was able to reduce the OTX2 level and increase the neuronal marker tubulin III in D425 flank tumors (Supplementary Material, Fig. S3).
RA Therapy of Intracranial MB and Resistance to RA
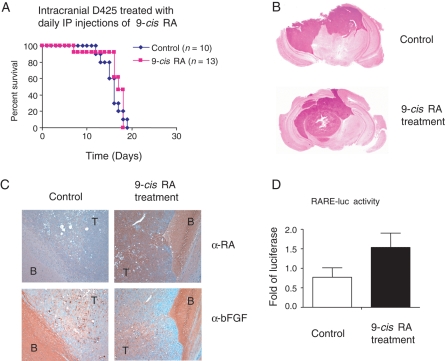
To explore the therapeutic application of RA, we tested 9-cis RA in mice intracranially injected with D425 cells. D425 cells quickly formed well-demarcated tumors in the mouse brain (Fig. 4B) and caused death within 2–3 weeks. However, 9-cis RA treatment of a higher 20 mg/kg dose via IP injection failed to prolong survival compared with the control-treated animals (Fig. 4A). The intracranial delivery of RA via systemic treatment has been confirmed before,20,21 and the mice in our study displayed typical side effects of RA, such as loss of weight and local skin irritation.22,23 These observations suggest that dosing or intracranial drug levels are unlikely the problem rendering 9-cis RA ineffective in the mouse brain. We next stained mouse brain slides with RA antibody and found the elevated level of RA in the xenograft tumor with 9-cis RA treatment, while bFGF, a potential antagonistic factor of RA, showed a constant expression in both the tumor and the brain areas of the RA-treated and untreated samples (Fig. 4C). An RA reporter assay also confirmed the increased RA levels in the mouse brains treated with 9-cis RA (Fig. 4D).
Fig. 4.
9-cis RA does not extend survival in mice with intracranial MB tumors. (A) No survival advantage in treatment by 9-cis RA. One million D425 cells were intracranially injected into mice and treated by IP injection of 50 µL of 9-cis RA dissolved in DMSO (n = 13) or treated with DMSO as control (n = 10). The daily treatment of the first 6 days was followed by a pause of 2 days and 5 additional daily injections. (B) Sections of H&E staining of mouse brains with or without the treatment by 9-cis RA. The darker nucleus-rich area represents the tumor formed by D425 cells. (C) α-RA and α-bFGF staining of mouse brains with or without the 9-cis RA treatment. Brain slides shown in (B) were stained with α-RA or α-bFGF and the positive staining was visualized by DAB colored in brown with hematoxylin in blue as counterstaining. Slides of serial cut of 10 µm apart were used and shown in ×10 magnification. Compared with the control, the brain with 9-cis RA treatment revealed an increased level of RA and generally unchanged bFGF expression. T, tumor; B, brain. (D) Luciferase reporter assay with the brain lysates. RARE-luc reporter construct with RA-responsive element were transfected in P19 cells and incubated with lysates of brain samples treated with or without 9-cis RA. The relative luciferase activity was graphed as the fold increase compared with the control. Three brain samples were used in each group.
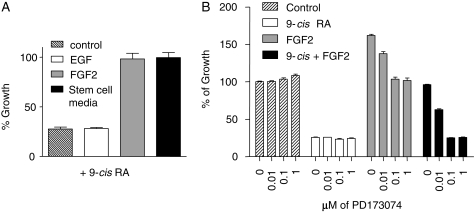
In light of the notion that cancer cells with an undifferentiated phenotype are involved in tumor initiation or propagation of MB24 and the stem cell-like features of D425 cells, such as the lack of differentiation markers and the expression of CD133 in over 60% of cells, we tested RA in serum-free neural stem cell media. This media, which contains bFGF and EGF, rendered 9-cis RA completely ineffective on D425 cells (Fig. 5A). By incubating D425 cells in the conventional DMEM media containing bFGF or EGF, we found that bFGF, and not EGF, is the factor blocking the RA-mediated growth inhibition in vitro. If the serum-free neural stem cell media more accurately reproduce in vivo conditions, then it is possible that bFGF signaling is responsible for the lack of response in our animal trials.
Fig. 5.
FGF2 renders the MB cells insensitive to RA treatment. (A) FGF-2 was capable of overriding the growth-inhibitory effect of 9-cis RA on D425 cells. D425 cells were incubated with 20 ng/mL of EGF, 10 ng/mL of FGF-2 in DMEM media containing 10% FBS or stem/progenitor cell media for 96 hours in the presence of 1 µM 9-cis RA. Viable cells were determined by cell proliferation assay reagent WST-1 and graphed in percentage to the control that was cultured without 9-cis RA. (B) FGFR inhibitor PD173074 restored the RA-sensitivity of D425 cells. D425 cells were incubated for 96 hours with the indicated combinations of 9-cis RA (1 µM) and FGF2 (10 ng/mL) in the presence of the FGFR inhibitor PD173074 at the concentration from 0 to 1 µM. Viable cells were determined by WST-1 and graphed in percentage to the control that was cultured without 9-cis RA, FGF2, and PD173074.
Next, we used an FGFR inhibitor, PD173074, which has been shown to be a specific inhibitor of FGFR at concentrations below 0.1 µM,25 and determined that the FGF2-mediated resistance to 9-cis RA can be overcome by this FGFR inhibitor (Fig. 5D).
Discussion
The homeobox transcription factors OTX2 and its homologue OTX1 are essential in early brain development, but are not expressed in postnatal brains. In the embryonic cerebellum, OTX1 and OTX2 are found in the EGL. During postnatal development, OTX2 is localized in the posterior portion, while OTX1 is expressed within the anterior portion, and a region of overlapping expression in mid-cerebellum defines a third domain.10 de Haas et al.6 observed that most MB tumors expressed OTX2, OTX1, or both. OTX2 expression was found in 75% of tumors and was associated with classic MBs localized in the vermis. OTX1 was found almost exclusively in desmoplastic MBs in the hemispheres.
Retinoids have been widely studied in the treatment of various kinds of tumors, mainly based on RA's ability to induce differentiation of tumor cells.26 A study reported that ATRA reduced growth of D283 and D341 as well as the size of D283 flank xenograft tumors.15 Di et al.5 showed that only OTX2-positive MB cell lines responded to RA with reduced growth, while OTX2-negative MB cell lines tested in the study were resistant to RA. By comparing the 12 available retinoids, we found that 9-cis RA was the most potent RA for downregulating OTX2 and inhibiting MB cell growth. We observed that 9-cis RA induced neuronal-specific gene expression and slowed cell growth only in OTX2-positive MB cells. Stable over-expression of 3F-OTX2 rendered D425 cells largely resistant to RA-mediated growth inhibition, while 9-cis RA still induced downregulation of endogenous OTX2 in these cells. Taken together, these data indicate that the RA responsiveness in MB cells is associated with the endogenous OTX2 expression and the RA-induced growth inhibition is mediated by first downregulating OTX2 expression.
One interesting observation we made in Fig. 2B was that in the two D425 clones overexpressing 3F-OTX2, treatment with 9-cis RA led to increased exogenous 3F-OTX2 while endogenous OTX2 expression was reduced by RA incubation. Although the causality of the elevated CMV promoter-driven 3F-OTX2 level cannot be determined in this report, further investigations are planned. According to current literature, the following scenarios are possible: (i) The expression level of 3F-OTX2 was maintained differently among cells in various growth stages. Therefore, the killing of lower OTX2-expressing cells via 9-cis RA led to accumulation of high 3F-OTX2 expressing cells among the total viable cells, which was reflected by the higher 3F-OTX2 protein detected on anti-OTX2 Western blot that was loaded with an equal number of viable cells. (ii) The degradation of OTX2 protein changed in light of the downregulation of endogenous OTX2 promoter by 9-cis RA and subsequent growth arrest. This might lead to higher levels of 3F-OTX2 protein even when its CMV promoter activity remains constant.
9-cis RA seemed to be the logical choice for clinical trials, especially since it is reported to have less toxic side effects than ATRA.18,22 Previous studies showed ATRA and 13-cis RA were capable of reducing the growth of subcutaneous D283 xenograft tumors in mice when these RAs were used immediately after tumor implantation.15 Our treatment of D425 flank xenograft tumors with 15 mg/kg 9-cis RA showed a tumor-inhibitory effect on established tumors. Surprisingly, 9-cis RA failed to prolong survival of mice injected intracranially with D425 cells, most likely due to its inability to inhibit intracranial tumor growth. To our knowledge, this phenomenon has not been described yet, since this is the first published report of treating intracranial brain tumors with RAs. Our further experiments revealed that bFGF was capable of completely blocking the RA-mediated growth inhibition in D425 cells in vitro. Additionally, FGFR inhibition helped overcome the FGF-mediated resistance to this retinoid. Past studies have determined the pharmacokinetics of RA in the brain via systemic delivery.20,21 Both the RA reporter assay and immunohistochemistry staining confirmed the higher RA level in the brain samples of treated mice than that of control, concluding that 9-cis RA did reach the brain and the intracranial tumor. We therefore hypothesize that, based on our results, certain factors such as bFGF from intra-tumoral surroundings may render RA treatment ineffective.
FGF and RA oppose each other during body axis extension in embryos. In a mutually inhibitory control, RA attenuates FGF8 in neuroepithelium and paraxial mesoderm, while FGF8 in turn downregulates the RA-synthesizing Raldh2 in the paraxial mesoderm and is able to block RA-mediated differentiation in neuroepithelium.27,28 In essence, FGF can maintain an undifferentiated cell state and RA can drive differentiation in a different context. It is noteworthy that FGF was able to override RA-mediated growth inhibition in D425 cells in vitro, while the repression of OTX2, a direct effect of RA on the promoter of OTX2, remained undisturbed. It is unclear whether the mechanism by which the RA represses FGF at the transcriptional level during somatogenesis was in any degree active in the mouse brain in our 9-cis RA treatment of intracranial D425 tumor. Both bFGF and FGFR1 are present in most parts of embryonic and postnatal rat brain, including postnatal cerebellar cortex, purkinje layer, and internal granular layer.29 The NIH's SAGE Genie database has documented that bFGF and FGFRs (1, 2, or 3) were widely expressed in MB (http://cgap.nci.nih.gov/SAGE/AnatomicViewer). The staining of the mouse brain sampled with implanted D425 tumor demonstrated a widespread presence of bFGF in both the tumor and the brain parts. If MBs arise from or have a component of undifferentiated or ‘stem-like’ bFGF-responsive cells, these cells will be unresponsive to RA in an environment with the presence of bFGF. If this is true, RA therapy will stand little chance of success if not combined with an effective method of blocking the FGF pathway.
In our in vitro study, the FGFR inhibitor PD173074 was able to completely restore the 9-cis RA-responsiveness of D425 cells, at concentrations reported to be specific for FGFR.25 With a molecular weight of 524 Da, it remains to be determined whether this compound is capable of crossing the blood brain barrier and thereby providing therapeutic value for systemic in vivo use.
Based on these studies we propose that the downregulation of OTX2 by RA mediates the RA-induced growth inhibition of MB cells and that 9-cis RA is the most potent among the common retinoids. While 9-cis RA showed tumor-reducing efficacy in the flank xenograft MB tumors, its intracranial application is precluded by possible local resistance, which appears to be at least partially mediated by bFGF. Either bFGF resistance must be targeted separately in combination with RA therapy or small molecules not subject to this effect should be developed to increase the chances that an OTX2 pathway-targeted therapy will be successful. Using a successful OTX2-targeted therapy instead of more cytotoxic therapies for the treatment of patients affected by MBs raises the urge of creating an overall therapy with better survival benefits and fewer side effects in the majority of MBs with elevated OTX2 levels.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Conflict of interest statement: None declared.
Funding
This project was supported by NIH Grant R01 NS052507, the Virginia and D.K. Ludwig Fund for Cancer Research, and the Children's Cancer Foundation. G.J.R. is supported by the Irving J. Sherman M.D. Neurosurgery Research Professorship.
References
- 1.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 2.Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32:1040–1049. doi: 10.1093/jpepsy/jsl056. [DOI] [PubMed] [Google Scholar]
- 3.Yokota N, Mainprize TG, Taylor MD, et al. Identification of differentially expressed and developmentally regulated genes in medulloblastoma using suppression subtraction hybridization. Oncogene. 2004;23:3444–3453. doi: 10.1038/sj.onc.1207475. [DOI] [PubMed] [Google Scholar]
- 4.Boon K, Eberhart CG, Riggins GJ. Genomic amplification of orthodenticle homologue 2 in medulloblastomas. Cancer Res. 2005;65:703–707. [PubMed] [Google Scholar]
- 5.Di C, Liao S, Adamson DC, et al. Identification of OTX2 as a medulloblastoma oncogene whose product can be targeted by all-trans-retinoic acid. Cancer Res. 2005;65:919–924. [PubMed] [Google Scholar]
- 6.de Haas T, Oussoren E, Grajkowska W, et al. OTX1 and OTX2 expression correlates with the clinicopathologic classification of medulloblastomas. J Neuropathol Exp Neurol. 2006;65:176–186. doi: 10.1097/01.jnen.0000199576.70923.8a. [DOI] [PubMed] [Google Scholar]
- 7.Michiels EM, Oussoren E, Van Groenigen M, et al. Genes differentially expressed in medulloblastoma and fetal brain. Physiol Genomics. 1999;1:83–91. doi: 10.1152/physiolgenomics.1999.1.2.83. [DOI] [PubMed] [Google Scholar]
- 8.Northcott PA, Nakahara Y, Wu X, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–472. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simeone A. Otx1 and Otx2 in the development and evolution of the mammalian brain. Embo J. 1998;17:6790–6798. doi: 10.1093/emboj/17.23.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantz GD, Weimann JM, Levin ME, McConnell SK. Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum. J Neurosci. 1994;14:5725–5740. doi: 10.1523/JNEUROSCI.14-10-05725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Q, Chen ZM, Su WJ. Anticancer effect of retinoic acid via AP-1 activity repression is mediated by retinoic acid receptor alpha and beta in gastric cancer cells. Int J Biochem Cell Biol. 2002;34:1102–1114. doi: 10.1016/s1357-2725(02)00030-4. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003;197:185–192. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 13.Recchia F, Saggio G, Cesta A, et al. Interleukin-2 and 13-cis retinoic acid as maintenance therapy in advanced ovarian cancer. Int J Oncol. 2005;27:1039–1046. [PubMed] [Google Scholar]
- 14.Simeone A, Acampora D, Mallamaci A, et al. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallahan AR, Pritchard JI, Chandraratna RA, et al. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med. 2003;9:1033–1038. doi: 10.1038/nm904. [DOI] [PubMed] [Google Scholar]
- 16.Bai RY, Dieter P, Peschel C, Morris SW, Duyster J. Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-gamma to mediate its mitogenicity. Mol Cell Biol. 1998;18:6951–6961. doi: 10.1128/mcb.18.12.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson RC, Pardoll DM, Jaffee EM, et al. Systemic and local paracrine cytokine therapies using transduced tumor cells are synergistic in treating intracranial tumors. J Immunother Emphasis Tumor Immunol. 1996;19:405–413. doi: 10.1097/00002371-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Miller VA, Benedetti FM, Rigas JR, et al. Initial clinical trial of a selective retinoid X receptor ligand, LGD1069. J Clin Oncol. 1997;15:790–795. doi: 10.1200/JCO.1997.15.2.790. [DOI] [PubMed] [Google Scholar]
- 19.Cheng C, Michaels J, Scheinfeld N. Alitretinoin: a comprehensive review. Expert Opin Investig Drugs. 2008;17:437–443. doi: 10.1517/13543784.17.3.437. [DOI] [PubMed] [Google Scholar]
- 20.Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS. Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem. 1995;270 doi: 10.1074/jbc.270.30.17850. 17 850–17 857. [DOI] [PubMed] [Google Scholar]
- 21.Werner EA, Deluca HF. Retinoic acid is detected at relatively high levels in the CNS of adult rats. Am J Physiol Endocrinol Metab. 2002;282:E672–E678. doi: 10.1152/ajpendo.00280.2001. [DOI] [PubMed] [Google Scholar]
- 22.Varani J, Fligiel H, Zhang J, et al. Separation of retinoid-induced epidermal and dermal thickening from skin irritation. Arch Dermatol Res. 2003;295:255–262. doi: 10.1007/s00403-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponthan F, Borgstrom P, Hassan M, et al. The vitamin A analogues: 13-cis retinoic acid, 9-cis retinoic acid, and Ro 13-6307 inhibit neuroblastoma tumour growth in vivo. Med Pediatr Oncol. 2001;36:127–131. doi: 10.1002/1096-911X(20010101)36:1<127::AID-MPO1030>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 24.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi M, Froum S, Hamby JM, et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamata H, Tachibana M, Fujimori T, Imai Y. Differentiation-inducing therapy for solid tumors. Curr Pharm Des. 2006;12:379–385. doi: 10.2174/138161206775201947. [DOI] [PubMed] [Google Scholar]
- 27.Diez del Corral R, Breitkreuz DN, Storey KG. Onset of neuronal differentiation is regulated by paraxial mesoderm and requires attenuation of FGF signalling. Development. 2002;129:1681–1691. doi: 10.1242/dev.129.7.1681. [DOI] [PubMed] [Google Scholar]
- 28.Diez del Corral R, Olivera-Martinez I, Goriely A, et al. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]