Abstract
The cranial neural crest (CNC) undergoes complex molecular and morphological changes during embryogenesis in order to form the vertebrate skull, and nearly three quarters of all birth defects result from defects in craniofacial development. The molecular events leading to CNC differentiation have been extensively studied; however, the role of the cAMP-dependent protein kinase [protein kinase A (PKA)] during craniofacial development has only been described in palate formation. Here, we provide evidence that strict PKA regulation in postmigratory CNC cells is essential during craniofacial bone development. Selective inactivation of Prkar1a, a regulatory subunit of the PKA holoenzyme, in the CNC results in perinatal lethality caused by dysmorphic craniofacial development and subsequent asphyxiation. Additionally, aberrant differentiation of CNC mesenchymal cells results in anomalous intramembranous ossification characterized by formation of cartilaginous islands in some areas and osteolysis of bony trabeculae with fibrous connective tissue stabilization in others. Genetic interaction studies revealed that genetic reduction of the PKA catalytic subunit Cα was able to rescue the phenotype, whereas reduction in Cβ had no effect. Overall, these observations provide evidence of the essential role of proper regulation of PKA during the ossification of the bones of the skull. This knowledge may have implications for the understanding and treatment of craniofacial birth defects.
Loss of Prkar1a from the neural crest causes excess PKA signaling and interferes with cellular differentiation.
One of the most important and dynamic cell types involved in embryonic development is the neural crest. These cells are responsible for the proper development of a large number of adult structures ranging from the enteric ganglia to the bones of the face. The cranial neural crest (CNC) cells originate in the ectodermal-neuroectodermal ridges of the cephalic neural folds by embryonic d 8 (E8) and subsequently undergo epithelial to mesenchymal transition (1,2). This triggers the expression of soluble factors that mediate CNC migration to the frontonasal process (FNP) and the first and second branchial arches (BA1 and -2). As the FNP develops, it gives rise to the nose and cheeks, as well as to the bony structures of the sinus cavity. BA1 gives rise to the mandibular and maxillary processes and part of the external ear, whereas BA2 contributes to the hyoid bone, styloid process, and parts of the middle and external ear. Orchestrated development of all of these structures requires regulated differentiation of CNC-derived structures into bone, cartilage, smooth muscle, and glial fates.
The cAMP-dependent protein kinase [protein kinase A (PKA)] is a heterotetramer composed of a homodimer of regulatory subunits each bound to a catalytic subunit. There are four regulatory subunits, Prkar1a, Prkar1b, Prkar2a, and Prkar2b, that can interact with either of the two catalytic subunits, PrkaCα or PrkaCβ (hereafter referred to as Cα and Cβ, respectively). Although both catalytic isoforms are ubiquitously expressed, Cβ appears to be more prominently activated in the nervous system whereas Cα is the dominant signaling subunit in the remaining tissues (3). Mice with homozygous deletion of the Cα subunit are viable, but they are less fertile than their heterozygous counterparts (4). Moreover, when only one allele of either catalytic subunit is functional (e.g. Cα+/−;Cβ−/−, or Cα−/−;Cβ+/−), causing dramatic decreases in total PKA activity, severe developmental defects arise, including spina bifida and exencephaly (reviewed in Ref. 5). On the other hand, when PKA is abnormally activated, such as in the case of Prkar1a−/− embryos, aberrant primitive streak migration occurs causing defects in tissues derived from the mesenchyme, such as the BA, heart tube, somites, and portions of the head (6). As a result of these problems, Prkar1a−/− mice exhibit embryonic lethality before E9.5 (6,7). This phenotype was improved by loss of one or both alleles of Cα, but viable Prkar1a−/− mice have never been recovered (6).
Nearly three quarters of all birth defects described in humans result from problems with craniofacial development (8). One very common birth defect is cleft palate, which occurs in 1:500 to 1:1000 live births (9). PKA activation and regulation have been previously described to play important roles in cAMP response element-binding protein (CREB) phosphorylation during palate formation (10). However, its function in relation to the CNC has not been elucidated. The CREB transcription factor is directly phosphorylated by PKA, thereby promoting the transcription of a number of genes involved in palatogenesis. Although CREB is responsive to PKA activation, it can also be phosphorylated by other kinases in response to growth signals (11). It has been shown previously that Prkar1a and Cα expression is present at high levels in the mesenchymal cells of the embryonic palate throughout its development, and there is differential expression of Prkar2b as the palate forms (12). The levels of cAMP increase in the palate mesenchyme just before fusion of the palatal shelves, and this is accompanied by increased PKA activation (13,14,15). Upon fusion of the shelves, PKA activity decreases to baseline levels. Additional studies have shown that TGF-β signaling can induce CREB phosphorylation independent of PKA during palatogenesis, which suggests cross talk between the PKA- and TGF-β-signaling pathways (16).
Our interest in the role of Prkar1a in the neural crest also stems from studies of the Carney Complex, a human tumor predisposition syndrome caused by inactivating mutations in PRKAR1A. These patients exhibit manifestations in neural crest-derived tissues, including melanocyte hyperplasia (lentiginosis) and Schwann cell tumorigenesis (17). In Prkar1a+/− mice, Schwann cell tumors are observed in 33% of animals (7), and we recently reported that mice deficient for Prkar1a in a limited subset of facial Schwann cells develop schwannomas with even higher frequency (18).
In light of these previous findings, we sought to develop a neural crest-specific knockout (KO) mouse for Prkar1a to better understand the role of PKA regulation during the development of neural tissue. In the present study, we report that ablation of Prkar1a from postmigratory neural crest cells results in perinatal lethality due to asphyxiation caused by severe malformations of the craniofacial bones. Abnormalities associated with aberrant differentiation of CNC-derived mesenchymal cells in craniofacial tissues were consistently seen, and we observed clear evidence of defects in intramembranous ossification of the developing bones of the face and skull. Furthermore, we show that the excess PKA activity was due to the Cα subunit, because genetic reduction of this isoform rescued the mutant phenotype. These data indicate a novel role for proper PKA regulation during embryonic development in the CNC, which may be beneficial to the understanding and treatment of craniofacial birth defects.
Results
Loss of Prkar1a in the neural crest causes perinatal lethality
The Tyrosinase Expressing Cre 1 (TEC1) murine line was designed to express cre under the Tyrosinase promoter and enhancer, which is expressed specifically in the neural crest cells by E10.5 (19). TEC1 mice were mated with mice harboring the Prkar1a conditional allele [Prkar1aloxP/loxP; (7)] to generate a homozygous neural crest-specific KO, TEC1;Prkar1aloxP/loxP.
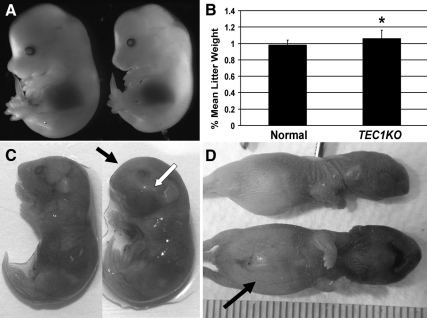
Genotyping of 21-d-old pups revealed that TEC1;Prkar1aloxP/loxP (hereafter referred to as TEC1KO) mice were not observed at expected Mendelian frequencies (Table 1). Thus, it was surmised that TEC1KO embryos died in utero, and time course experiments were conducted to determine the stage of embryonic demise. No significant morphological differences were noted between wild-type (WT) and TEC1KO littermates until E17.5 when mutants exhibited obvious swelling and rounding of the head (Fig. 1, A and C). Mutant embryos appeared generally larger than their WT littermates, and body weight comparisons revealed that TEC1KO embryos were significantly heavier at E17.5 than normal embryos (P = 0.0036; Fig. 1B). Late-stage TEC1KO embryos displayed normal embryonic movements and, surprisingly, survived through birth; however, immediately after birth, they were unable to breathe. Within 1–2 h after delivery, all TEC1KO pups exhibited cyanosis and severe abdominal distension, indicating ingestion of air while attempting to breathe (Fig. 1D), and they ultimately succumbed to asphyxiation.
Table 1.
Expected and observed frequencies from genetic interaction studies with TEC1KO
| Genotype | Observed | Expected | n | P value |
|---|---|---|---|---|
| TEC1KO | 0 | 22.50 | 90 | ≤0.0001 |
| Cα +/−;TEC1KO | 10 | 15.60 | 74 | 0.1489 |
| Cβ +/−;TEC1KO | 0 | 9.88 | 74 | ≤0.0001 |
Figure 1.
Gross phenotype of TEC1KO embryos. A, E14.5 TEC1KO embryos (right) are indistinguishable from WT littermates (left). Although the mutant appears slightly smaller than the WT type in this figure, this was not a consistent finding. B, E17.5 TEC1KO pups (n = 11) weighed significantly more than WT littermates (n = 35) (P = 0.0036). C, The mutants (right) exhibited increased swelling and rounding of the frontonasal (black arrow) and maxillary bones (white arrow) compared with WT embryos of the same age (left). D, Within 1–2 h after delivery, TEC1KO neonates showed signs of asphyxiation, such as facial cyanosis. Abdominal distention (arrow) results from swallowing air during attempts to breathe. *, P ≤ 0.01.
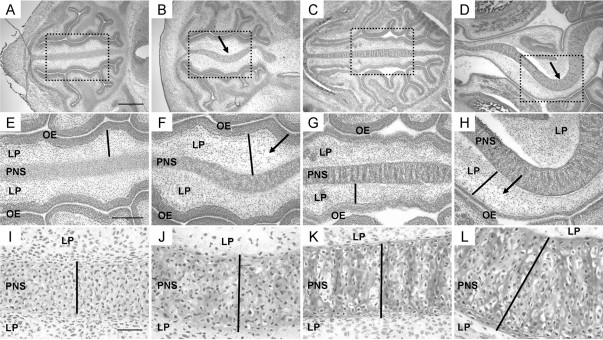
TEC1KO embryos were analyzed for malformations in the craniofacial region, specifically focusing on the sinus cavity. Histological analysis of normal and TEC1KO embryos at E12.5 showed no obvious differences (n = 6), but at E14.5 hematoxylin and eosin (HE) staining revealed mild deviation of the primordial nasal septum (PNS) in TEC1KO embryos (n = 8; Fig. 2, A and B). Some thickening of the lamina propria (LP) that separates the PNS from the olfactory epithelium occurred at this stage as well, which was attributed to edema (Fig. 2F, bar/arrow). The severity of deviation in the PNS and swelling in the LP increased with age to E17.5 (n = 14; Fig. 2, panels C and D and panels G and H). In addition, the PNS was significantly thicker than that of age-matched controls [Fig. 2, I–L (bars) and Supplemental Fig. 1 published on The Endocrine Society’s Journals web site at http://mend.endojournals.org; P < 0.0001]. Because mice are only able to breathe through their nasal passages, it is likely that obstruction of the airway caused by excessive swelling of the LP and septal deviation was the cause of death for these animals.
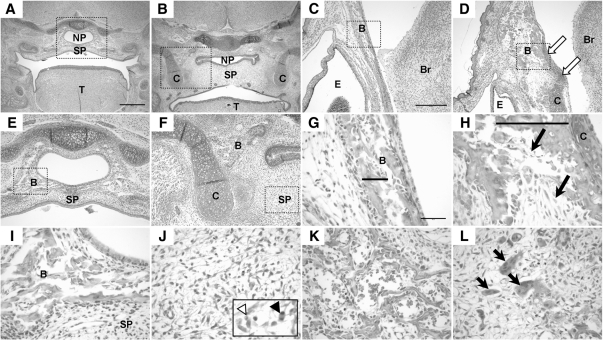
Figure 2.
Histological progression of PNS defects found in TEC1KO embryos. A–D, Magnified views (×4) of transverse sections of embryonic sinuses. For orientation, the brain is to the right in each panel, and the nasal openings are to the left. A, WT E14.5 embryo. Scale bar represents 500 μm and applies to images in panels A–D. B, E14.5 TEC1KO sinus showing slight deviation (arrow) of the PNS. C, WT E17.5 embryo. D, E17.5 TEC1KO embryo. Note that septal deviation is exacerbated at this stage (arrow). E–H, Magnified views (×10) of the boxed areas from panels A–D, respectively. E, WT E14.5 embryo. Note the thickness of the LP (bar). Scale bar represents 250 μm and applies to images in panels E–H. F, E14.5 TEC1KO sinus showing edema (arrow) and thickening (bar) of the LP. G, WT E17.5 embryo; the bar indicates thickness of the LP. H, Mutant E17.5 sinus showing increased edema (arrow) and thickening (bar) of the LP. I–L, Views (×40) of the PNS from panels E–H, respectively. The black bars represent thickness of the septum, which was significantly increased in mutants by E17.5 (panel L, P < 0.0001). Scale bar in panel I represents 50 μm and applies to images in panels I–L. OE, Olfactory epithelium.
Histological sections of E14.5 and E17.5 embryos that carried a copy of the Rosa26LacZ cre reporter (20) were stained for X-gal to confirm cre expression in the affected tissues of TEC1KO embryos. Staining was observed throughout the PNS and LP, but not in the olfactory epithelium (Supplemental Fig. 2). These results directly paralleled the HE staining that showed severe alterations in the PNS and LP of TEC1KO embryos, whereas the olfactory epithelium was unaffected.
Micro-CT imaging of TEC1KO mice reveals both qualitative and quantitative craniofacial bone defects
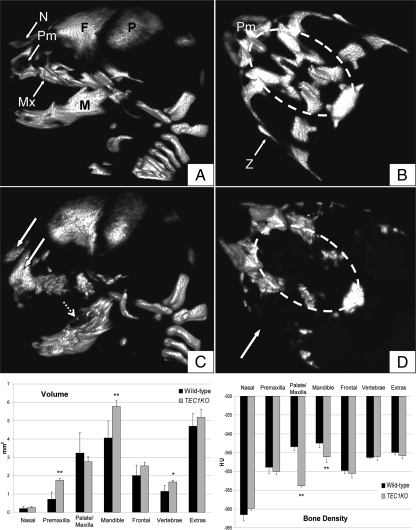
Micro-CT scans were performed to more completely visualize the malformations of the craniofacial bones in TEC1KO embryos. Total body scans showed no difference in the appendicular skeleton or portions of the axial skeleton of E17.5 mutant embryos, consistent with the fact that those bones are not neural crest derivatives. However, there were remarkable malformations in the neural crest-derived bones of the face, such that the premaxilla and nasal bones showed increased radiodensity in the mutants (Fig. 3C, white arrows). This equated to a statistically significant increase in premaxilla volume (P ≤ 0.01), but the density of the two bones was essentially unchanged (Fig. 3, bottom). In addition, the mandibles of TEC1KO embryos had variably radiolucent areas (Fig. 3C, dashed arrow), and they were significantly larger and less dense than those of the WT controls (P ≤ 0.01; Fig. 3, bottom). Perhaps most striking was the apparent loss of palate bones and the zygomatic arch in TEC1KO animals (Fig. 3D, dashed oval/arrow). In this analysis, it was not clear whether the bone was truly absent or rather not calcified enough to be visualized by micro-CT scanning. Surprisingly, there was no statistically significant difference in volume between TEC1KO and WT palates, although this may be due to variability in the WT samples. The small amount of bone that was present in the TEC1KO palate was much less dense than that of WT (P ≤ 0.01). These results indicate that proper PKA regulation in the developing bones of the skull is required for normal bone formation.
Figure 3.
Micro-CT scans of E17.5 embryos. A, Sagittal view of WT skull. B, Axial view of WT palate. The dashed oval signifies the region of the palate. C, Sagittal view of TEC1KO skull. Note the increased opacity of the premaxilla (Pm) and nasal (N) bones (solid white arrows). These bones, in addition to the frontal (F), parietal (P), mandible (M, dashed arrow), and maxilla (Mx) are irregularly shaped with variably radiolucent areas. D, Axial view through TEC1KO palate. Note the absence of a zygomatic arch (Z, arrow) and central palate bones (dashed oval). Bottom, Volumetric (left) and densitometric (right) measurements for individual craniofacial bones. Volume is expressed as mm3, and density is expressed in Hounsfield units (HU). Note that Palate/Maxilla measurements included all of the bones found in panels B and D with the exception of the premaxilla. Also, the parietal, occipital, and other small bones of the face and neck were collectively measured and designated as Extras in the graphs. *, P ≤ 0.05; **, P ≤ 0.01.
TEC1KO embryos exhibit defects in CNC mesenchymal cell differentiation and intramembranous ossification
Because proper proliferation, apoptosis, migration, and differentiation of the neural crest are all critical to normal CNC development, each of these functions was investigated in TEC1KO CNC tissues to evaluate the role of Prkar1a in those cells. Results from BrdU incorporation assays and cleaved caspase-3 immunolabeling experiments showed no significant differences between mutant and WT embryos, indicating that the proliferative and apoptotic indices were unaffected (Supplemental Fig. 3). Additionally, tracking of the neural crest cells revealed no changes in CNC migration between WT and TEC1KO embryos. This observation may be partly explained by the fact that the majority of the neural crest has already migrated before E10.5 when cre expression initiates.
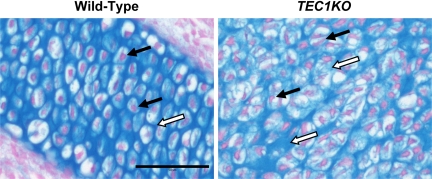
To determine whether proper differentiation of the CNC-derived tissues was achieved in TEC1KO, histological sections from E14.5 and E17.5 embryos were stained with Alcian blue to evaluate the morphology of the cartilage in the PNS. Although no significant changes in the cartilage were observed at E14.5, the staining revealed distinct differences between TEC1KO and WT embryos at E17.5 (n = 4; Fig. 4). The mutant cartilage consisted of disorganized chondrocyte lacunae with elongated, crescent-shaped nuclei (Fig. 4, black arrows) and variable amounts of glycosaminoglycan matrix formation (Fig. 4, white arrows). Although the septae were thicker, there was no difference in the number of chondrocytes present in TEC1KO PNS compared with WT. This result is consistent with our observations that there were no alterations in proliferation or apoptosis in that tissue. Based on these observations, we propose that the increased thickness of the mutant cartilage was due to disorganization of the glycosaminoglycan matrix and chondrocyte dysplasia, which may have promoted pliability and therefore deviation of the PNS.
Figure 4.
Alcian blue staining of the PNS. WT (left) and TEC1KO (right) sections through the PNS were stained with Alcian blue for the presence of cartilage. Note that WT chondrocytes contain rounded nuclei and are uniformly aligned, whereas the disorganized mutant chondrocyte nuclei are typically elongated and crescent shaped (black arrows). In addition, the amount of glycosaminoglycan matrix (white arrows) is highly variable in the TEC1KO PNS, which likely contributed to the overall flexibility of the tissue causing severe deviation. Scale bar, 50 μm.
In addition to cartilage, we also examined the palate and craniofacial bones histologically to identify possible differentiation defects present in those tissues. Although cleft palate was anticipated based on the micro-CT scans, HE staining of coronal sections revealed no sign of cleft palate in the mutants (n = 8; Fig. 5, A and B). Despite this, there were still obvious abnormalities in the secondary palate, including edema, apoptotic cells, and mitotic figures (Fig. 5J, inset). Additionally, whereas the WT mice exhibited bone formation encircling the nasopharynx, this bone was only present lateral to the airway in TEC1KO animals (Fig. 5, E and F). As a result, the nasopharynx lacked the necessary support to maintain an open airway, which may have further contributed to the inability of these animals to draw air into their lungs.
Figure 5.
Histology of the E17.5 palate and craniofacial bones. A and B, View of coronal sections through the embryonic palate. A, WT embryo showing completely fused secondary palate (SP). Scale bar represents 500 μm and applies to images in panels A and B and E and F. Panel B, TEC1KO palate. Note that the secondary palate is fused; however, it is dramatically thicker than normal. Also note that the nasopharynx (NP) is collapsed due to loss of bony support. C and D, Views (×10) of the frontal bone. Note the presence of cartilage in the mutant (panel D, arrows) that is not found in the WT (panel C). Scale bar in panel C represents 250 μm and applies to images in panels C and D. Panels E and F, The boxed areas in panels A and B, respectively, are shown at ×10 magnification. Note the erroneous cartilage flanking the mutant (panel F). Also, note that the bone found in WT (panel E) encircling the nasopharynx is found only lateral to the palate in TEC1KO. G and H, Magnification (×40) of the areas boxed in panels C and D, respectively. Bone trabeculation (bars) was thicker and disorganized in mutant (panel H) compared with normal (panel G), and there was increased deposition of fibrous connective tissue (arrows). Scale bar in panel G represents 50 μm and applies to the images in panels G–L. Panels I and J, Magnified view of the boxed areas in panels E and F, respectively. Panel I, WT bone adjacent to the nasopharynx. Panel J, TEC1KO secondary palate. Note the absence of bone and the presence of apoptotic cells (inset, white arrowhead) and mitotic figures (inset, black arrowhead). Panels K and L, View (×40) of WT (panel K) and TEC1KO (panel L) mandible. Note the increased fibrous connective tissue and numerous osteoclasts (arrows) indicating osteolysis in the TEC1KO. B, Bone; Br, brain; C, cartilage; E, eye; T, tongue.
In addition to the palatal defects, atypical cartilaginous structures flanked the palate region in the mutants (Fig. 5, B and F), and excess cartilage extended into the region of the frontal bone, which typically is devoid of cartilage at this stage of development (Fig. 5, C and D). The mutant frontal bone had thicker trabeculation (Fig. 5H, bar), and there was a dramatic increase in fibrous connective tissue deposition in both the frontal bone and the mandible (Fig. 5, H and L). This phenotypic change was accompanied by marked osteolysis, as evidenced by the presence of numerous osteoclasts (Fig. 5L, arrows). Combined, these features contributed to the increased volume of the bones, and directly paralleled the micro-CT densitometry data, which showed decreased radiodensity in the mandible and palate.
Taken together, these unusual observations indicated that the loss of Prkar1a had a dramatic effect on CNC cell differentiation, causing interference with intramembranous ossification of the craniofacial bones. Specifically, we observed that CNC-derived mesenchymal cells that normally would differentiate directly into osteoblast precursors produced a mix of cartilage and disordered bone, whereas other cells produced ectopic cartilage only (Fig. 5).
Proper regulation of PrkaCα is crucial for normal craniofacial development
To better define which catalytic isoform of PKA is most important for craniofacial development, TEC1;Prkar1aloxP/+ mice were mated to Cα+/−;Prkar1aloxP/loxP and Cβ+/−;Prkar1aloxP/loxP animals to generate TEC1KO pups carrying a mutation in either the Cα or Cβ subunit. The Cα crosses yielded no significant deviation from expected Mendelian frequencies (P = 0.1489), indicating that a 50% reduction in Cα was sufficient to rescue the TEC1KO phenotype (Table 1). Cα+/−;TEC1KO animals survived to adulthood and were fertile, and they generally appeared to have a normal phenotype. Conversely, the Cβ crosses did not yield any rescue of the mutant phenotype (P < 0.0001), suggesting that the bulk of PKA signaling in the CNC comes from Cα.
Although Cα+/−;TEC1KO animals were indistinguishable from WT littermates as adults, we also studied these animals during late embryogenesis. Phenotypically, Cα+/−;TEC1KO animals examined at E17.5 demonstrated a spectrum of phenotypes ranging between indistinguishable from WT littermates to those animals that appeared outwardly similar to KO animals. At the histopathological level, all Cα+/−;TEC1KO animals studied (n = 4) demonstrated modest bowing of the nasal septum (Fig. 6C), intermediate between WT (Fig. 6A) and KO (Fig. 6B), associated with reduced edema. Cartilage and bone structure near the palate were similarly improved, although in no case did the phenotype appear normal (data not shown).
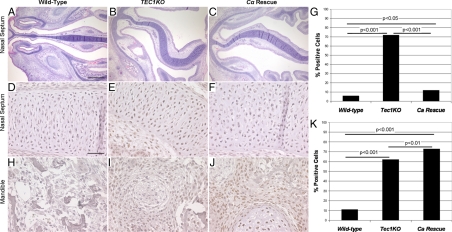
Figure 6.
Partial phenotypic reversion and reduction in pCREB caused by genetic reduction of Prkaca. A–C, Low power view of transverse sections of embryonic sinuses, orientated as in Fig. 2. A, E17.5 WT sinus. Scale bar represents 500 μm and applies to images in this row. B, E17.5 TEC1KO sinus showing deviation of the nasal septum. C, E17.5 Cα+/−;TEC1KO sinus, showing partial reversion of the curvature of the septum. D–F, High-power view of pCREB staining in E17.5 nasal septa from WT (panel D), TEC1KO (panel E), and Cα+/−;TEC1KO (panel F) embryos. Scale bar represents 50 μm and applies to panels in this row and lower row. G, Quantitation of percent pCREB-positive cells in WT (n = 224), TEC1KO (n = 225), and Cα+/−;TEC1KO (n = 226) nasal septa. H–J, High-power view of pCREB staining in E17.5 mandibles from WT (H), TEC1KO (I), and Cα+/−;TEC1KO (J) embryos. K, Quantitation of percent pCREB-positive cells in WT (n = 209), TEC1KO (n = 227), and Cα+/−;TEC1KO (n = 294) mandibles.
Based on the experiments presented in this study, it was concluded that proper Prkar1a regulation of Cα activity after E12.5 in the primordial craniofacial structures derived from the CNC is crucial for normal differentiation of the cartilaginous and bony tissues that make up the face.
Ablation of Prkar1a is associated with increased PKA signaling
To understand signaling changes associated with loss of Prkar1a, we studied signaling events involved in CNC differentiation. In our previous tissue-specific KOs of Prkar1a, we observed enhanced PKA activity (21,22). To confirm PKA activity in the CNC, we performed immunostaining for the phosphorylated (activated) form of CREB and found increased phospho-CREB (pCREB) staining in the cartilage of the FNP and in the abnormal CNC-derived bone (Fig. 6, D–F and H–J). The enhanced staining for pCREB was reduced in the nasal septa of Cα+/−;TEC1KO animals, although the percentage of pCREB-positive cells remained slightly but significantly higher than in the WT (Fig. 6G). In the bone of the mandible, reduction of Cα subunits did not decrease the number of cells staining for pCREB (Fig. 6K). Knowing that TGF-β signaling could also account for increased CREB phosphorylation, we stained for phospho-Smad 1/5/8, which is also a marker of bone morphogenetic protein (BMP) signaling required for bone formation. There was no difference in staining between WT and TEC1KO sections, indicating that TGF-β and BMP signaling was not altered in these animals (Supplemental Fig. 4). Additionally, previous reports have shown the importance of PTHrP down-regulation of the Runx2 transcription factor during bone development, which is dependent of PKA activation (23,24). Staining for PTHrP and Runx2 also revealed no differences between WT and mutant embryos (Supplemental Fig. 5). Finally, staining for Sox9, a marker for cartilage differentiation, was also not changed between WT and TEC1KO (Supplemental Fig. 6). Taken as a whole, these data indicate that loss of Prkar1a causes dysregulated PKA activation leading to enhanced CREB phosphorylation. This subsequently interferes with normal CNC differentiation via a mechanism independent of other known signaling pathways involved in craniofacial development.
Discussion
The TEC1KO phenotype is complex, and it involves defects associated with several tissue types. The most detrimental deformations were found in the nasal region where edema and severe deviation of the PNS prevented these animals from breathing upon birth. Dysplastic chondrocytes, paired with disorganization of the glycosaminoglycan matrix, contributed to an increased thickness of the PNS, and loss of structural integrity led to dramatic curvature of the tissue. Loss of skull shape caused by defects in CNC-derived bone likely also contributed to this phenotype by removing a rigid support structure for the developing nasal septum. Additionally, increased swelling in the LP and deficient nasopharyngeal support further constricted the airway in these animals, ultimately resulting in asphyxiation. This conclusion is further strengthened by the observation that increased PKA activity, as judged by pCREB staining, was reduced to near-normal levels in the nasal septum in the Cα+/−;TEC1KO animals, which do not exhibit perinatal lethality.
At the developmental level, there are two mechanisms by which bone can be formed: intramembranous ossification and endochondral ossification. The former mechanism is used during the formation of the majority of the flat bones of the skull and face, and this same process appears to play a role in the healing of bone fractures (25). During intramembranous ossification of the skull, mesenchymal cells of the CNC differentiate into osteoprogenitor cells, which further differentiate directly into osteoblasts (reviewed in Ref. 26). Little is known about the molecular basis of intramembranous ossification, and PKA signaling has not been previously reported to play a role. In TEC1KO mice, we observed that dysregulated PKA activation in the neural crest was sufficient to cause impaired intramembranous ossification. As a result, in some areas bone was inappropriately resorbed, whereas in other areas the trabecular bone was thicker and dysmorphic. Additionally, Prkar1a−/− CNC cells gave rise to ectopic cartilage in locations such as the frontal bone and near the palate. Our observations suggest that a portion of Prkar1a−/− mesenchymal cells that would normally undergo intramembranous ossification are instead triggered to differentiate into cartilage. Those cells that are able to initiate intramembranous ossification, however, do so inefficiently and are unable to produce normal bone. An alternative possibility is that defective cross talk between those cells undergoing intramembranous ossification and those in the process of endochondral ossification may be responsible, in part, for those islands of cartilage flanking the palate in E17.5 TEC1KO embryos.
In the areas where bone is present, such as the mandible and frontal bones, there was a marked increase in osteolysis. There are at least two possibilities to explain this observation: either the bone never formed properly causing it to be resorbed, or bone development occurred prematurely and remodeling started prenatally. Examination of E14.5 bone in the mutants revealed mild changes in bone development; however, mature bone was not present, consistent with an unaffected timing of mineralization. This evidence supports the likelihood that there was improper bone formation and substantiates the hypothesis that PKA plays a role in the early events that lead up to CNC mesenchymal cell differentiation into osteoprogenitor cells during intramembranous ossification.
This study showcases a range of defects associated with the inability of CNC cells to undergo proper differentiation in the process of intramembranous ossification. As our laboratory has previously reported, Prkar1a+/− mice exhibit an increased risk of bone tumors in the tail vertebrae (7). This location suggested to us that the tumors might initiate at the site of local trauma. Because intramembranous ossification is part of the healing process for bone fractures (25), we now believe that these tumors may reflect the same defect observed in intramembranous ossification in the TEC1KO model. Specifically, Prkar1a+/− tail tumors exhibited increased fibrous connective tissue, similar to that observed in TEC1KO frontal and mandibular bones (7,27). Additionally, although Prkar1a+/− tail tumors were of osteoblastic nature, there was significant turnover of the bone marked by decreased mineralization of the tissue and increased osteoclasts (27). In the TEC1KO mandibles, the presence of osteoclasts suggested increased osteolysis as well. Similar bone tumors have also been reported in Carney Complex patients, primarily in the sinus region (28). Notably, these lesions were often congenital abnormalities and, given their location in the sinus region, there may be similarities to the TEC1KO model. In TEC1KO mice, however, the bone defects are not necessarily associated with tumorigenesis. Taken as a whole, the previous studies, combined with the observations in TEC1KO embryos, suggest that proper PKA regulation is essential for both normal bone development as well as homeostasis of adult bone.
Here, we show new data suggesting a novel role for PKA in CNC differentiation, but PKA is already known to play an important role in palate development. Loss of PKA activity can cause cleft palate, and in TEC1KO embryos the dysregulated activation of the enzyme actually increased palate thickness. CREB phosphorylation was increased in these and other CNC-derived tissues in the TEC1KO sections, independent of TGF-β or BMP signaling alterations. In addition, given that there were no changes in PTHrP and Runx2 staining, we postulate that the TEC1KO phenotype is indeed PKA dependent and does not involve alterations in other known pathways involved in craniofacial development.
It was shown recently that epigenetic control of the transcription factors Otx2 and Lhx1 by histone deacetylase 8 (Hdac8) plays an important role in neural crest cell specification of the craniofacial bones (29). Conventional Hdac8 KO mice, as well as neural crest-specific Hdac8 KOs, exhibited ossification defects of the frontal and interparietal bones that led to brain trauma and perinatal lethality. Although the pathology described in the Hdac8 KO mice was distinct from that of TEC1KO animals, the previous study may provide clues for future studies aimed at characterizing the molecular basis for PKA-related ossification defects.
We demonstrate here that a fine balance of activation and repression of Cα (the predominant PKA-C isoform in the developing CNC) exists in order for craniofacial features to develop normally. It is still unclear, however, whether the individual TEC1KO defects occur independently or whether they result from problems in a coordinated aspect of development that has not been previously understood. Future work in this model may identify mechanisms by which tissues previously thought to differentiate independently may, in fact, rely more heavily upon surrounding tissues for proper differentiation and maturation. Additionally, this model could serve as a useful tool for better understanding the molecular basis of craniofacial bone development, which may, in turn, be beneficial for treatment strategies for craniofacial birth defects found in humans.
Materials and Methods
Mouse experiments
The generation of Prkar1aloxP/loxP and TEC1 mice has been previously described (7,19). TEC1 and Prkar1aloxP/loxP mice were mated to produce the neural crest-specific KO mouse, TEC1;Prkar1aloxP/loxP (TEC1KO). Embryonic experiments were carried out via timed matings and observation of vaginal plugs. Six litters (11 TEC1KO embryos, 35 WT embryos) were weighed at E17.5, and the results were analyzed using a two-sample t test for statistical relevance.
The Cα+/− and Cβ+/− mice have been described previously (4,30) and were obtained from the Mutant Mouse Regional Resource Centers (www.mmrrc.org). TEC1;Prkar1aloxP/+ mice were mated with Cα+/−;Prkar1aloxP/loxP and Cβ+/−;Prkar1aloxP/loxP animals to generate Cα+/−;TEC1;Prkar1aloxP/loxP (Cα+/−-TEC1KO) and Cβ+/−;TEC1;Prkar1aloxP/loxP (Cβ+/−-TEC1KO) animals.
Histology
All embryos were fixed in 10% formalin, processed with ethanol and xylene, and embedded in paraffin wax. Sections were taken using a microtome and mounted on glass slides for further staining. HE staining was performed using a Leica autostainer (Leica Microsystems Inc., Bannockburn, IL). Samples were stained with hematoxylin (Thermo Scientific, Waltham, MA) for 8 min, eosin (Sigma Aldrich, St. Louis, MO) for 30 sec, dehydrated in ethanol and xylenes, and coverslipped.
Alcian blue staining was performed manually in 1% Alcian blue (Sigma Aldrich) prepared in 0.1 n HCl for 30 min. Nuclei were counterstained using Nuclear Fast Red (Sigma) for 5 min before dehydration and coverslipping.
Micro-CT scans
Micro-CT images were obtained on an Inveon micro-CT in vivo scanner (Siemens USA, Malvern, PA) for four WT and four TEC1KO E17.5 embryos. Full-body scans were done using two projections per degree, a 650-msec exposure, and 0.5 mm of Al filtration. These settings were selected to maximize soft tissue contrast without sacrificing the scan to data artifacts. A settle time of 500 msec was used to keep the sample and acquisition steady at maximum magnification. Initial resolution acquisitions were 10.1 μm; however, this was decreased to 20.2 μm to reduce noise in the scan. Densitometric and volumetric measurements of individual craniofacial bones were obtained using Inveon Research Workplace software (Siemens), and the results were compared using a standard two-sample t test to check for statistical relevance.
Supplementary Material
Acknowledgments
We thank Alan Fletchner, HTL (ASCP), Ohio State University, for his help with processing and sectioning the embryos for this study; Brandy Marlow, Ohio State University, for her help with the PTHrP and Sox9 immunohistochemistry; and Lisa Rawahneh, Ohio State University, for her help with HE staining. We also thank Don Stredney and the Wright Center of Innovation in Biomedical Imaging at The Ohio State University for their contributions with the micro-CT imaging and analysis. Finally, we thank Dr. Ian Tonks and Dr. Graham Kay of the Queensland Institute of Medical Research, Queensland, Australia, for generously supplying us with the TEC1 transgenic mice.
Footnotes
This work was supported in part by a Children’s Tumor Foundation Young Investigator Award 2006-01-026 (to G.N.J.), and by National Institutes of Health grants CA112268-02 (to L.S.K.) and CA16058 (to the Ohio State University Comprehensive Cancer Center).
Current address for G.N.J.: Building 560, Room 32-31B, National Cancer Institute-Frederick, Frederick, Maryland 21702.
Disclosure Summary: All authors declare no conflicts of interest.
First Published Online June 9, 2010
Abbreviations: BA, Branchial arch; CNC, cranial neural crest; CREB, cAMP response element binding protein; E8, embryonic day 8; FNP, frontonasal process; Hdac8, histone deacetylase 8; HE, hematoxylin and eosin; KO, knockout; LP, lamina propria; pCREB, phospho-CREB; PKA, cAMP-dependent protein kinase; PNS, primordial nasal septum; TEC1, Tyrosinase expressing cre 1; TEC1KO, TEC1;Prkar1aloxP/loxP; WT, wild type.
References
- Nichols DH 1986 Formation and distribution of neural crest mesenchyme to the first pharyngeal arch region of the mouse embryo. Am J Anat 176:221–231 [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M 1995 Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development 121:525–538 [DOI] [PubMed] [Google Scholar]
- Uhler MD, Chrivia JC, McKnight GS 1986 Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem 261:15360–15363 [PubMed] [Google Scholar]
- Skålhegg BS, Huang Y, Su T, Idzerda RL, McKnight GS, Burton KA 2002 Mutation of the Cα subunit of PKA leads to growth retardation and sperm dysfunction. Mol Endocrinol 16:630–639 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Yin Z, Jones GN, Mahoney E 2009 Mouse models of altered protein kinase A signaling. Endocr Relat Cancer 16:773–793 [DOI] [PubMed] [Google Scholar]
- Amieux PS, Howe DG, Knickerbocker H, Lee DC, Su T, Laszlo GS, Idzerda RL, McKnight GS 2002 Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J Biol Chem 277:27294–27304 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Kusewitt DF, Matyakhina L, Towns II WH, Carney JA, Westphal H, Stratakis CA 2005 A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65:4506–4514 [DOI] [PubMed] [Google Scholar]
- Hall BK 1999 The neural crest in development and evolution. New York: Springer [Google Scholar]
- Marazita ML, Mooney MP 2004 Current concepts in the embryology and genetics of cleft lip and cleft palate. Clin Plast Surg 31:125–140 [DOI] [PubMed] [Google Scholar]
- Weston WM, Greene RM 1995 Developmental changes in phosphorylation of the transcription factor CREB in the embryonic murine palate. J Cell Physiol 164:277–285 [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME 1999 CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68:821–861 [DOI] [PubMed] [Google Scholar]
- Greene RM, Lloyd MR, Uberti M, Nugent P, Pisano MM 1995 Patterns of cyclic AMP-dependent protein kinase gene expression during ontogeny of the murine palate. J Cell Physiol 163:431–440 [DOI] [PubMed] [Google Scholar]
- Greene RM 1989 Signal transduction during craniofacial development. Crit Rev Toxicol 20:137–152 [DOI] [PubMed] [Google Scholar]
- Greene RM, Pratt RM 1979 Correlation between cyclic-AMP levels and cytochemical localization of adenylate cyclase during development of the secondary palate. J Histochem Cytochem 27:924–931 [DOI] [PubMed] [Google Scholar]
- Olson FC, Massaro EJ 1980 Developmental pattern of cAMP, adenyl cyclase, and cAMP phosphodiesterase in the palate, lung, and liver of the fetal mouse: alterations resulting from exposure to methylmercury at levels inhibiting palate closure. Teratology 22:155–166 [DOI] [PubMed] [Google Scholar]
- Potchinsky MB, Weston WM, Lloyd MR, Greene RM 1997 TGF-β signaling in murine embryonic palate cells involves phosphorylation of the CREB transcription factor. Exp Cell Res 231:96–103 [DOI] [PubMed] [Google Scholar]
- Carney TA 1985 A national code for drugs used in general practice—an identified need? J R Coll Gen Pract 35:198 [PMC free article] [PubMed] [Google Scholar]
- Jones GN, Tep C, Towns II WH, Mihai G, Tonks ID, Kay GF, Schmalbrock PM, Stemmer-Rachamimov AO, Yoon SO, Kirschner LS 2008 Tissue-specific ablation of Prkar1a causes schwannomas by suppressing neurofibromatosis protein production. Neoplasia 10:1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks ID, Nurcombe V, Paterson C, Zournazi A, Prather C, Mould AW, Kay GF 2003 Tyrosinase-Cre mice for tissue-specific gene ablation in neural crest and neuroepithelial-derived tissues. Genesis 37:131–138 [DOI] [PubMed] [Google Scholar]
- Soriano P 1999 Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- Nadella KS, Kirschner LS 2005 Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res 65:10307–10315 [DOI] [PubMed] [Google Scholar]
- Yin Z, Jones GN, Towns II WH, Zhang X, Abel ED, Binkley PF, Jarjoura D, Kirschner LS 2008 Heart-specific ablation of Prkar1a causes failure of heart development and myxomagenesis. Circulation 117:1414–1422 [DOI] [PubMed] [Google Scholar]
- Li TF, Dong Y, Ionescu AM, Rosier RN, Zuscik MJ, Schwarz EM, O'Keefe RJ, Drissi H 2004 Parathyroid hormone-related peptide (PTHrP) inhibits Runx2 expression through the PKA signaling pathway. Exp Cell Res 299:128–136 [DOI] [PubMed] [Google Scholar]
- Tsutsui TW, Riminucci M, Holmbeck K, Bianco P, Robey PG 2008 Development of craniofacial structures in transgenic mice with constitutively active PTH/PTHrP receptor. Bone 42:321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton CT, Hunt RM 1991 Early histological and ultrastructural changes in medullary fracture callus. J Bone Joint Surg Am 73:832–847 [PubMed] [Google Scholar]
- Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R 1995 Toward a molecular understanding of skeletal development. Cell 80:371–378 [DOI] [PubMed] [Google Scholar]
- Pavel E, Nadella K, Towns II WH, Kirschner LS 2008 Mutation of Prkar1a causes osteoblast neoplasia driven by dysregulation of protein kinase A. Mol Endocrinol 22:430–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JA, Boccon-Gibod L, Jarka DE, Tanaka Y, Swee RG, Unni KK, Stratakis CA 2001 Osteochondromyxoma of bone: a congenital tumor associated with lentigines and other unusual disorders. Am J Surg Pathol 25:164–176 [DOI] [PubMed] [Google Scholar]
- Haberland M, Mokalled MH, Montgomery RL, Olson EN 2009 Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev 23:1625–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Zhuo M, Skålhegg BS, Brandon EP, Kandel ER, McKnight GS, Idzerda RL 1996 Impaired hippocampal plasticity in mice lacking the Cβ1 catalytic subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA 93:1571–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.