Abstract
Pancreatic β-cell failure underlies type 1 diabetes; it also contributes in an essential way to type 2 diabetes. β-Cell replacement is an important component of any cure for diabetes. The current options of islet and pancreas transplantation are not satisfactory as definitive forms of therapy. Here, we review strategies for induced de novo pancreatic β-cell formation, which depend on the targeted differentiation of cells into pancreatic β-cells. With this objective in mind, one can manipulate the fate of three different types of cells: 1) from terminally differentiated cells, e.g. exocrine pancreatic cells, into β-cells; 2) from multipotent adult stem cells, e.g. hepatic oval cells, into pancreatic islets; and 3) from pluripotent stem cells, e.g. embryonic stem cells and induced pluripotent stem cells, into β-cells. We will examine the pros and cons of each strategy as well as the hurdles that must be overcome before these approaches to generate new β-cells will be ready for clinical application.
Cell fate manipulation by directed differentiation is a promising new strategy for β-cell replacement therapy in diabetes.
Diabetes mellitus is an exploding health problem in the United States that affects 23 million people with overt diabetes and another one fourth of the population being glucose intolerant (1,2,3,4,5) and is the cause for much morbidity and mortality (6,7). Exogenous insulin administration has remained the standard of care for type 1 diabetes for nearly 90 yr! Over the last two decades, major advances in the development of new types of insulin, different delivery methods to mimic physiological insulin release by β-cells, and newer more user-friendly modes of glucose monitoring and islet transplantation have resulted in substantial improvements in morbidity and mortality as well as the quality of life of diabetic patients (8). Our knowledge on the pathogenesis of type 1 diabetes and the developmental biology of pancreatic islets has also been increasing steadily. Unfortunately, these advancements have not been translated into a cure for type 1 diabetes. The only treatment that was initially touted as a potential cure was pancreas and islet transplantation. Although whole pancreas transplantation, usually combined with kidney transplantation in select type 1 diabetic patients with end-stage renal disease, has good efficacy (9), it is a major surgical procedure and is not a preferred option for the majority of diabetic patients. Although the Edmonton protocol for islet transplantation was greeted initially with much hope and excitement as a definitive treatment (10,11), the primary hurdles with islet transplantation have been the limiting supply of cadaveric donor islets in relation to the high demand of eligible patients, the need for lifetime immunosuppression, and long-term graft dysfunction with consequent return to insulin dependence (12,13,14,15).
Renewed interest in β-cell replacement therapy for diabetes has occurred with the recent reports of successful in vivo induction of β-cell neogenesis by either transdifferentiation (16) or transdetermination (17) (see below) and the in vitro conversion of pluripotent cells (see Glossary of Terms), e.g. induced pluripotent stem (iPS) cells and embryonic stem (ES) cells, into insulin-secreting cells (18,19,20,21,22). With the creation of iPS cells in multiple laboratories using a cocktail of transcription factors that accord stemness to terminally differentiated cells (23,24,25,26,27,28,29,30), the hope for inducing islet neogenesis from pluripotent cells as a curative therapy for diabetes has been rekindled despite the limitations uncovered in early studies on ES cells (31,32).
Prerequisites for β-Cell Replacement Therapy
The ultimate objective for curative diabetes therapy is to generate a 1) nonlimiting source of 2) patient-derived, 3) nonimmunogenic β-cells that have 4) an intact stimulus-secretion coupling to enable them to secrete insulin appropriate to physiological demands, whereas they also self-renew 5) under strict physiological control in vivo. Some of these prerequisites are indispensable, whereas others are desirable.
A nonlimiting source of β-cells
A major limitation of islet transplantation restricting its wide applicability is a large disparity between supply and demand. Each successful transplant requires on average cadaveric islets isolated from up to three donors for each recipient (14), and any modest increase in donor availability in the future will be outstripped by the ever-increasing demand. Hence, the promise of reprogramming progenitor cells in vivo or in vitro has its appeal as a renewable and nonlimiting source of new β-cells.
Patient-derived β-cells
Current technology for allogeneic islet transplantation is based on the use of unrelated cadaveric donors and will always be hampered by alloimmune responses requiring immunosuppressive regimens with β-cell cytotoxic (33) and other potentially serious side effects. The possibility of reprogramming the patient’s own cells (in vivo by lineage switching or in vitro by lineage determination via iPS cells; see below) is enticing because it would circumvent alloimmunity entirely.
Nonimmunogenic β-cells
This property would be highly desirable, but could be dispensable, at least theoretically, if advances in encapsulation technology can prevent antigen presentation of the transplanted allograft while allowing for a rapid response to variations in blood glucose (34,35,36).
Physiologically regulated insulin secretion
Under physiological conditions, elevations in plasma glucose are the dominant stimulus for insulin secretion from the β-cell. Glucose-stimulated insulin secretion (GSIS) is the embodiment of a finely tuned stimulus-secretion coupling machinery, which is an indispensable function of normal β-cells. An intact stimulus-secretion coupling is necessary for appropriate insulin secretion and whole-body glucose homeostasis.
Self-renewal
One would envision that any β-cell induced via recapitulation of the normal β-cell developmental pathway would be endowed with a capacity for self-renewal (37,38,39,40) with intact cell cycle checkpoints. This is important, because with in vivo reprogramming, this ability to self-renew would obviate the need for repeated gene transfers. In the case of ES cells and iPS cells, self-renewal enables one to generate as much starting material as required for β-cell induction.
Directed Differentiation
Early attempts to cure diabetes were focused on restoring plasma insulin levels by using an approach similar to that used for the treatment of monogenic diseases such as glycogen storage disorders and hemophilia, viz., to express a secreted version of insulin controlled by glycemia-regulated promoters to lower the blood glucose (41,42,43,44,45). However, this approach was incapable of restoring physiological GSIS, because it is not possible to replace a process normally controlled at the posttranslational level with a transcriptionally regulated process. Such a strategy results in a markedly delayed insulin response, leading to uncontrolled early postprandial hyperglycemia. Similarly, a transcriptionally regulated insulin transgene also fails to promptly shut off insulin secretion in the presence of hypoglycemia, leading to prolonged delayed hypoglycemia. Consequently, many investigators have shifted their strategy to generating new β-cells, which are inherently armed with normal glucose-sensing and insulin-secreting machinery.
Stem cells have different capacities for regeneration and differentiation
In the early embryo, the cells isolated from the inner cell mass of the blastocyst are called ES cells. They are pluripotent and have the capacity to multiply and differentiate into different cell lineages (46,47,48,49). The lineage choices become progressively restricted as the embryo develops until the cells become terminally differentiated and lose their plasticity. However, to cope with the normal wear and tear, many tissues retain special cells, often referred to loosely as adult stem cells that have retained substantial plasticity. Subsequent differentiation of the adult stem cells is associated with a restriction in plasticity, a process thought to be due to epigenetic modification of homeotic genes and consequent repression of developmental programs toward other cell lineages (50,51,52,53).
The best-studied examples of adult stem cells are those from the hematopoietic system, wherein the hematopoietic stem cells at various stages of differentiation have the ability to differentiate into their respective previously determined lineage. For example, the most primitive hematopoietic stem cell can repopulate the entire bone marrow, whereas downstream in the hierarchy are myeloid progenitors that can differentiate into cells in the myeloid lineage and erythroid progenitors into cells in the erythroid lineage. Equivalent resident multipotent adult stem cells populate other organs and tissues, e.g. stellate cells in the skeletal muscle and oval cells in the liver. In the liver, multipotent adult stem cells exist in the portal triads that give rise to mature hepatocytes and biliary cells under specific circumstances when the hepatocytes themselves are unable to replicate. Despite some encouraging recent development (54), hepatic oval cells are relatively poorly defined; their paucity under normal circumstances and their seeming heterogeneity make them particularly difficult to study. In the pancreatic islet, β-cells normally replicate at a slow rate (37,38,39,40) and can proliferate after partial pancreatectomy. Under specific experimental conditions of injury, progenitor cells in the ducts have been shown to give rise to new islets as part of a regenerative process (55). At this time, these putative progenitors are also poorly defined.
The recent identification of the extrahematopoietic progenitors has opened up new avenues for directed differentiation; many laboratories have succeeded in coaxing these progenitors into functioning differentiated tissues that are either native to the organ or may be ectopic to the particular organ as their natural site of residence.
β-Cell Neogenesis
It is becoming clear that only intact β-cells that are fully armed with the capacity for glucose sensing, ready-to-release insulin granules, and the accompanying transcriptional, translational, and posttranslational regulatory mechanisms display normal GSIS (56,57). With increasing knowledge of β-cell ontogeny (58,59), many groups have attempted to recapitulate the process via the delivery of genes for various key transcription factors normally involved in β-cell development. Gene transduction experiments have been conducted on isolated stem cells and other cells at different stages of differentiation (derived from bone marrow, liver, pancreatic duct, salivary gland, and pancreatic exocrine or endocrine cells) to induce the formation of insulin-producing cells in vitro. Many of these genes have also been delivered to mice in vivo to induce new β-cell formation in the liver and pancreas (60,61).
The starting cell type targeted for β-cell development determines the path that needs to be taken. Mechanistically, the strategy aims at accomplishing one of two objectives: induced lineage determination or induced lineage switching.
Lineage determination
The barrier to successful reprogramming, posed by restrictive epigenetic modifications, is theoretically the highest with transdifferentiation, because the generation of a new cell type, such as β-cells, from an unrelated terminally differentiated cell of a different lineage necessitates that the latter shed its differentiated characteristics as it assumes a new differentiated state belonging to a different lineage. In contrast, this barrier is less when the original cell source is a multipotent adult stem cell, i.e. via transdetermination. Finally, the barrier would be lowest with ES cells or iPS cells, pluripotent stem cells that possess the potential to differentiate to all cells of the body (23,24,25,26,27,28,29,30). However, the genes for maintaining cell cycle are in the active state in these pluripotent cells, whereas those that lead to cell cycle exit and subsequent differentiation are repressed. This state has to be changed to allow directed reprogramming of these pluripotent cells, a technology under intense investigation by many groups. Experiments on directed differentiation of ES and iPS cells into pancreatic β-cells are conducted in vitro. Because there is an unlimited supply of the starting material, the successful production of mature β-cells from these pluripotent cells would fill an important void in the availability of donor-derived islets for transplantation. Recently, iPS cells were generated from the skin cells of patients with type 1 diabetes that could be converted into insulin-producing cells in vitro (63).
Early attempts to induce insulin production from ES cells using transcription factors in an ad hoc manner led to poor outcomes. Subsequent research showed that a strategy that relies on an orderly differentiation process from ES cells into definitive endoderm and then into the pancreatic lineage offers the most robust results. Targeted differentiation protocols are complex because they depend on the addition of exogenous factors to the culture media and vector-driven expression of transcription factors as well as coculture methods with different substratum. Some laboratories have produced far superior results than others, but even the best results fall short of satisfying all the prerequisites outlined in a previous section. Nonetheless, considerable progress has been made in the field in the last few years (21,22,64). Some of the best results were reported by Kroon et al. (22) who used a four-staged differentiation protocol to guide human ES cells to a pancreatic endodermal state. They first exposed the ES cells to activin A and Wnt3a to induce the formation of definitive endoderm and then to keratinocyte growth factor [KGF or fibroblast growth factor-7 (FGF7)] that led to the formation of the primitive gut tube. Subsequent addition of all-trans retinoic acid, cyclopamine (which antagonizes the hedgehog signaling pathway), and noggin [which antagonizes the TGFβ superfamily signaling, especially of bone morphogenetic protein-4 (BMP-4)] led to differentiation of the cells toward the posterior foregut, the source of the pancreatic buds in the developing embryo. These cells were subsequently cultured for 3 more days to allow them to acquire the characteristics of the pancreatic endoderm. When grafted into immunodeficient mice, these cells developed into functional islets that produced insulin in a sustained fashion for more than 100 d. They not only displayed the histological and ultrastructural appearance of pancreatic islets but also acquired some degree of GSIS, although a high basal C-peptide and a trend toward hypoglycemia have raised concern (65). The strategy of completing the final steps of the differentiation process in vivo seemed to be a key advance, because an earlier study (21) using a similar protocol omitting the in vivo steps led to the formation of insulin-expressing cells that displayed no GSIS. However, complete reversal of hyperglycemia did not happen with the improved protocol, although the cells did protect against streptozotocin (STZ)-induced diabetes and removal of the implanted cells resulted in hyperglycemia. One major drawback of the treatment strategy is the substantial risk for teratoma formation (>15% in this study), which in theory could be prevented by an efficient protocol for selecting only fully differentiated cells. It is unclear at this time whether a sufficiently stringent protocol could be developed for this purpose.
Other laboratories have worked with iPS cells using a staged differentiation protocol with minor variations, which led to induction of insulin-producing cells that displayed limited GSIS but falling short of the normal islet response (18,19). The recent report of the generation of viable, live-born mice from iPS cells by tetraploid complementation (30) indicates that, like ES cells, these cells are competent to differentiate into all cell types in the body, including pancreatic β-cells.
Another approach that holds considerable promise is the use of small molecules identified by high-throughput screening to achieve directed differentiation of ES cells. For example, Zhu et al. (66) identified stauprimide as a molecule that decreases c-myc expression in ES cells, directing the cell out of its pluripotent state and down the differentiation pathway. A high-throughput screen in another laboratory identified two compounds that had the capacity to induce a highly efficient conversion of human and mouse ES cells into endoderm (in 70–80% of the cells) (67). A second set of screens identified indolactam V as a compound that could induce a large proportion of the definitive endodermal cells to express PDX1 (20), although the number of insulin-positive cells remained low.
Lineage switching
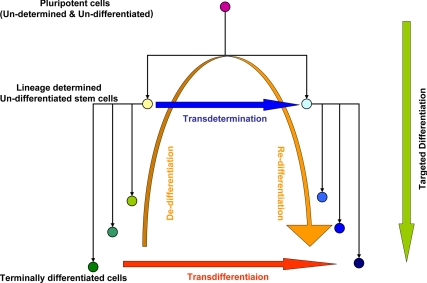
To produce lineage switching, the treatment maneuver targets cells that are committed to a certain lineage and redirects them to a lineage that is different from the one that they were originally programmed for. There are many reports on the attempted reprogramming of terminally differentiated cells from one linage into another, e.g. from liver to pancreas. The maneuvers that do not involve dedifferentiation and redifferentiation have traditionally been called transdifferentiation (68). However, recently, others have shown that it may be possible to effectuate the loss of a mature phenotype (e.g. loss of albumin in hepatocytes) and the attainment of the characteristics of another lineage (gain of C-peptide or insulin, akin to β-cells), a process of dedifferentiation followed by redifferentiation, which has also been referred to as transdifferentiation (69,70,71,72). Alternatively, one can start with a multipotent adult stem cell population that is normally committed to certain lineages, for example, hepatic oval cells that are destined to become hepatocytes and cholangiocytes under appropriate conditions, and redirect them to differentiate into a different but related lineage, pancreatic islets. This process of redirected differentiation of determined multipotent adult stem cells is called transdetermination (Fig. 1).
Figure 1.
Schematic representation of the various pathways of reprogramming by directed differentiation. Lineage determination by targeted differentiation of pluripotent (ES and iPS) cells is represented by the green arrow. Lineage switching between progenitor cells by transdetermination is represented by the horizontal blue arrow. Lineage switching between terminally differentiated cells can occur either via dedifferentiation and redifferentiation with an intervening progenitor state (orange arrow) or via direct transdetermination (red arrow).
From mature terminally differentiated cells (transdifferentiation)
Transdifferentiation, the redirection of an existing terminally differentiated cell type to a different cell lineage, if technically feasible, would be an ideal method for inducing β-cell neogenesis for the treatment of diabetes, because terminally differentiated cells are abundant and easily available from many different organs. Proof of transdifferentiation depends on the fulfillment of some stringent criteria (68). Many reports of β-cell transdifferentiation from cells of other lineages, especially hepatocytes (70,73,74,75) and bone marrow-derived cells (76,77,78) exist; however, as noted by Rizzino (79), cellular mimicry and hybrid cell types must be excluded in such cases.
Many laboratories have reported the transdifferentiation of pancreatic exocrine cells both in vitro and in vivo into insulin-expressing β-cells (80,81,82). One recent study demonstrated convincingly directed transdifferentiation of pancreatic exocrine cells into insulin-expressing β-cells in mice in vivo (16). The authors first screened nine transcription factors involved in islet development and selected a combination of three transcription factors, Ngn3, Pdx1, and MafA, as the reprogramming cocktail. They delivered adenoviruses expressing the three factors by direct injection into the exocrine pancreas of nondiabetic mice. The treatment led to the induction of insulin-positive cells, occurring singly, within the exocrine tissue in about 20% of vector-infected cells. When the treatment was applied to STZ-induced diabetic mice, there was a sustained (up to 3 months) partial correction of the fasting hyperglycemia in these animals. The regimen raised the nonfasting insulin to about 15% of that in nondiabetic mice, a significant rise when compared with the control-vector-treated mice, indicating an increase in total body insulin output in mice treated by targeted transdifferentiation, with some improvement in glucose tolerance tests. The insulin response to glucose was not reported, and thus it was not possible to determine whether some GSIS was effected. Importantly, the study conclusively demonstrated targeted transdifferentiation, being supported by lineage tracing experiments showing that the insulin-producing cells in the acinar pancreas came from carboxypeptidase A1-expressing exocrine cells. These cells appeared to lose their exocrine properties and attain the characteristics of β-cells as documented by morphology, immunocytochemistry, gene expression, and ultrastructure.
From multipotent adult stem cells (transdetermination)
During the normal course of development and organogenesis, some pluripotent stem cells self-renew, whereas others go down the path of determination by differentiating into their destined lineage. Determination is a moderate restriction of cell fate choices in a multipotent population, as compared with the pluripotent state. Compared with redirecting a terminally differentiated cell type into a differentiated cell of a different lineage, there is substantially much less barrier to the induced differentiation of a multipotent cell such as adult stem cells of one lineage to a developmentally related differentiated cell lineage, a process known as transdetermination. The transdetermination process has been well studied in Drosophila larva, in which an imaginal disc that is destined to differentiate into a wing gives rise to a leg instead, a developmentally closely related appendage (83,84,85,86,87,88,89,90). Mechanistically, transdetermination occurs in the imaginal discs when differentiation programs in the progenitor cells are unmasked by the regulation of repressive polycomb proteins, activation of homeobox transcription factors, or Wnt signaling (87,89,91,92,93,94,95).
Transdetermination has also been observed in mice in which bronchial stem cells were found to give rise to gastric and intestinal cell types under the influence of an overactive Wnt signaling (96). A probable example of transdetermination in humans has been reported in a case of atrophic gastritis in which the presence of lung-specific transcription factors (TTF-1) and ciliated intestine-specific transcription factor (HFH-4) was detected in the gastric mucosa, which also harbored bronchopulmonary cells (97). In addition, it has been proposed that the commonly observed metaplastic changes in histopathological sections of human tissues may be the result of transdetermination events (72). Recently, targeted transdetermination was explored as a possible therapeutic approach toward β-cell replacement in mice in vivo (17,98).
The endocrine islet and the exocrine acini share the common pancreatic progenitor that arises from the distal foregut endoderm. During fetal development, this part of the endoderm, under the influence of fibroblast growth factor and bone morphogenetic protein signaling coming from the cardiac mesoderm and the septum transversum, also gives rise to the liver progenitors. Thus, the liver progenitors and the pancreatic progenitors are developmentally closely related (59,99,100,101). The liver progenitors in the embryo (the fetal hepatoblasts) give rise to all the liver cells that are not hematopoietic derived. However, in the adult animal, liver progenitor cells are less well defined and have been generally thought to be represented by the oval cells residing in the portal triads adjacent to the canals of Hering. After acute loss of liver mass, e.g. after partial hepatectomy, the liver cells (hepatocytes) regenerate themselves rapidly and with high capacity. However, if hepatectomy occurs in the presence of another insult that limits the capacity of hepatocytes to proliferate, the liver remnant still regenerates itself rapidly, but the source of new hepatocytes and cholangiocytes under these conditions comes from hepatic oval cells (102,103,104,105).
Because oval cells are closely related to the pancreatic progenitors and have the capacity to rapidly expand, they comprise an attractive starting cell type to be nudged down the path of the pancreatic islet lineage, i.e. via transdetermination. Suggestive evidence for their transdetermination into an islet lineage in vitro was demonstrated by a prolonged culture of oval cells in a high-glucose medium that led to the acquisition of insulin expression by these cells (106) or by culturing them with extracellular matrix proteins, such as fibronectin and laminin (107). Others have shown a different cell population, the intrahepatic biliary epithelial cell population, to also have the capacity to express many β-cell characteristics including insulin, when transduced with Pdx1 or NeuroD1 (108). The existence of cells within the biliary tree that have the capacity to display the islet phenotype in vivo has also been reported. Occasional cells have been reported in the biliary tree that display a β-cell phenotype (109) and in Hes1-null mice, the consequent derepression of Ngn3 leads to the appearance of many islet cell types along the biliary tree (110,111). Furthermore, oval cell activation by the chemical DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine) has been reported to protect against STZ-induced hyperglycemia not only by stimulating the differentiation of oval cells into insulin-producing cells but also by increased islet regeneration in the pancreas (112). Interestingly, hepatic oval cell activation has been reported in STZ-induced diabetic mice with expression of proinsulin but not insulin (113).
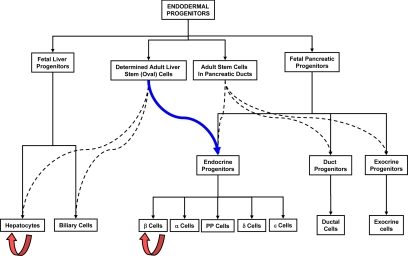
We recently reported the induction of insulin-producing neo-islets by targeted transdetermination of oval cells in vivo using the islet lineage-determining factor Ngn3 (114,115), combined with an islet growth factor, betacellulin, resulting in a complete and sustained correction of hyperglycemia, reversal of carbohydrate and lipid metabolic disruptions, and restoration of GSIS in an STZ-induced insulin-deficient diabetic mouse model (17,116). On electron microscopic observation, the neo-islet cell clusters display electron-dense granules surrounded by a halo inside secretory vesicles, typical of those seen on ultrastructural analysis of pancreatic β-cells (116). They secrete insulin in a glucose-responsive manner that is indistinguishable from the pattern seen in nondiabetic mice, a response that could have occurred only via an intact stimulus-secretion coupling (17). These islet clusters harbor cells that produce all four major pancreatic islet hormones, insulin, glucagon, somatostatin, and pancreatic polypeptide. Lineage tracing and other analyses showed that the periportal neo-islets originate from hepatic oval cells by a process consistent with transdetermination (Fig. 2). The degree to which the transdetermination process recapitulates the development and function of islets suggests that, using therapeutic reprogramming via transdetermination and selecting the appropriate receptive multipotent adult stem cell population, one could harness the power of lineage-defining transcription factors to induce therapeutic organogenesis for the treatment of different diseases, including diabetes (98).
Figure 2.
Schematic representation of transdetermination (blue arrow) of hepatic oval cells into islet progenitors. The black continuous lines represent normal differentiation and the interrupted lines the paths taken for regeneration and repair. The orange arrows signify the normal replication process of β-cells and hepatocytes.
Outstanding Questions and Limitations of Current Technology
Recent advances in our understanding of islet development and stem cell biology notwithstanding, there remain many gaps in both knowledge and current technology that need to be bridged before we can contemplate clinical application of any of the induced β-cell neogenesis protocols reviewed above. A bottleneck for successful β-cell replacement therapy using these approaches is the inability to generate new β-cells that display the finely tuned secretion-stimulus coupling of native pancreatic islets unfettered by unwanted constitutive insulin secretion.
As these technologies move toward ultimate clinical application, many issues need to be resolved. What are the characteristics, including but not limited to states of chromatin, epigenetic modification, and presence or absence of specific coactivators and repressors as well as micro-RNAs, that determine whether and which cells are susceptible for robust lineage-specific programming? Once cellular reprogramming appears to have occurred, what are the parameters that need to be measured to ensure that it is a complete or near-complete differentiated phenotype and not merely cellular mimicry or a hybrid state (79)? These could include profiling of the genomic state, transcriptome, proteome, kinome, etc. but would have to be based on, yet unavailable, evidence of defining the normal state for these parameters for the β-cell. Many of the approaches described result in the production of heterogeneous cell populations and lineages with varying degrees of differentiation potential (117). Some may retain a potential for oncogenic transformation (24,25,26,65,118), a risk that must be weighed against the requirement for appropriate self-renewal, a characteristic of normal β-cells. In addition, use of iPS and ES cells is associated with teratoma formation in a high enough frequency to preclude the unmodified clinical application of the currently available protocols (24,25,26,118). A stringent negative selection process that weeds out the poorly or partly differentiated cells, together with one that positively selects for differentiated β-cells, needs to be in place to prevent teratoma formation. Nonetheless, it is not clear that such selection can completely eliminate teratoma formation because it may be an inherent property of iPS and ES cells (118,119). These and other long-term safety issues need to be addressed in future experiments.
A proper niche that fosters appropriate growth and physiological responses would need to be in place for the induced β-cells. The use of the liver as an ectopic location for newly formed β-cell may be associated with problems seen with islet transplantation (120,121), although they may be specific to transplanted cells and may or may not apply to periportal islets generated in situ.
Finally, all of the newly generated β-cells must be shielded against immune-mediated cell death in the context of a resurgent autoimmune attack and destruction when the treatment is applied to patients with type 1 diabetes, an issue that has been discussed in other reviews (62,122) and is beyond the scope of this article. With the resolution of these limitations, we believe that an optimized induced islet differentiation strategy, via one approach or another, will become part of our armamentarium for the treatment of patients with type 1 diabetes.
Glossary of Terms
Totipotent cells: Cells, such as the zygote, capable of giving rise to all cells including embryonic and extra-embryonic tissues. These cells have no lineage restriction and are fully plastic. There is no known advantage over pluripotent cells for therapeutic use and hence of questionable clinical utility.
Pluripotent cells: Cells capable of giving rise to all the three germ layers (ectoderm, mesoderm and endoderm) and their derived tissues. These are also fully plastic and have no lineage restriction and can give rise to the whole embryo. Cells from the inner cell mass (ICM) of the blastocyst and the ES cells (see below) derived from them are pluripotent. Recently developed iPS cells (see below) are also pluripotent and can give rise to the whole embryo. These cells can be used for therapeutic purposes after appropriate directed differentiation.
Multipotent cells: Cells that have some restriction in their ability to give rise to all lineages. They are less plastic than the totipotent and pluripotent cells. These typically can give rise to many different cell types usually derived from the same germ layer but cannot give rise to the whole embryo. These have been loosely termed adult stem cells; hematopoietic stem cells, intestinal stem cells, dermal stem cells typify this group. These cells can be used for therapeutic purposes after appropriate directed differentiation.
Embryonic stem cells (ES cells): Pluripotent cells derived from the ICM of the blastocyst that have the capacity to remain in culture indefinitely and have the ability upon implantation into the blastocyst to give rise to the whole embryo. Many different ES cell lines have been produced, each with subtle differences in property and differentiation capabilities.
Induced pluripotent stem cells (iPS cells): Cells that have been demonstrated to be pluripotent but are derived from terminally differentiated cells by the overexpression of specific transcription factors that accord them pluripotency. These have been demonstrated to behave like ES cells, and can give rise to all cell lineages and the whole embryo.
Terminal differentiation: Cells upon full differentiation attain specialized properties and functions that are not reversible under normal circumstances. These cells may (e.g. hepatocytes) or may not (e.g. neurons) have the ability to divide, but they do not give rise to any other cell type upon replication.
Transdetermination: A process whereby multipotent cells switch their lineage commitment to a different but closely related lineage.
Transdifferentiation: A process whereby terminally differentiated cells switch their lineage commitment to a completely different lineage without an intervening stage of de-differentiation to a multipotent cell.
Footnotes
The work in the authors’ laboratories described in this review was supported by National Institutes of Health Grants K08DK068391 (to V.Y.), 1R03DK078716 (to V.Y.), R01DK068037, R21DK075002, and the Diabetes and Endocrinology Research Center P30DK079638 and by the Betty Rutherford Chair in Diabetes Research and St. Luke’s Episcopal Hospital, the Iacocca Foundation, and the T. T. and W. F. Chao Global Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 10, 2010
Abbreviations: ES, Embryonic stem; GSIS, glucose-stimulated insulin secretion; iPS, induced pluripotent stem; STZ, streptozotocin.
References
- National Institute of Diabetes and Digestive and Kidney Diseases 2008 National Diabetes Statistics, 2007 fact sheet. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health [Google Scholar]
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ 2006 Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 29:2114–2116 [DOI] [PubMed] [Google Scholar]
- Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF 2003 Lifetime risk for diabetes mellitus in the United States. JAMA 290:1884–1890 [DOI] [PubMed] [Google Scholar]
- Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, Thompson TJ 2001 Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 24:1936–1940 [DOI] [PubMed] [Google Scholar]
- Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, Imperatore G, Narayan KM 2004 The evolving diabetes burden in the United States. Ann Intern Med 140:945–950 [DOI] [PubMed] [Google Scholar]
- Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC 2007 Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 147:149–155 [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation 2006 Diabetes atlas. 3rd ed. Brussels: International Diabetes Federation [Google Scholar]
- Benhamou PY, Milliat-Guittard L, Wojtusciszyn A, Kessler L, Toso C, Baertschiger R, Debaty I, Badet L, Penfornis A, Thivolet C, Renard E, Bayle F, Morel P, Morelon E, Colin C, Berney T 2009 Quality of life after islet transplantation: data from the GRAGIL 1 and 2 trials. Diabet Med 26:617–621 [DOI] [PubMed] [Google Scholar]
- Lipshutz GS, Wilkinson AH 2007 Pancreas-kidney and pancreas transplantation for the treatment of diabetes mellitus. Endocrinol Metab Clin North Am 36:1015–1038; x [DOI] [PubMed] [Google Scholar]
- Robertson RP 2000 Successful islet transplantation for patients with diabetes: fact or fantasy? N Engl J Med 343:289–290 [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV 2000 Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238 [DOI] [PubMed] [Google Scholar]
- Vaithilingam V, Sundaram G, Tuch BE 2008 Islet cell transplantation. Curr Opin Organ Transplant 13:633–638 [DOI] [PubMed] [Google Scholar]
- Berney T, Toso C 2006 Monitoring of the islet graft. Diabetes Metab 32:503–512 [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR 2006 International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330 [DOI] [PubMed] [Google Scholar]
- Feng S, Barr M, Roberts J, Oberbauer R, Kaplan B 2006 Developments in clinical islet, liver thoracic, kidney and pancreas transplantation in the last 5 years. Am J Transplant 6:1759–1767 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA 2008 In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H, Chan L 2009 Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell 16:358–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H 2009 Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19:429–438 [DOI] [PubMed] [Google Scholar]
- Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y 2008 Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem 283:31601–31607 [DOI] [PubMed] [Google Scholar]
- Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D 2009 A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5:258–265 [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE 2006 Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401 [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE 2008 Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S 2006 Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S 2007 Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S 2007 Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ 2008 Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146 [DOI] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ 2008 Disease-specific induced pluripotent stem cells. Cell 134:877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S 2008 Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321:699–702 [DOI] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ, Dausman JA, Jaenisch R 2008 Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133:250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XY, Li W, Lv Z, Liu L, Tong M, Hai T, Hao J, Guo CL, Ma QW, Wang L, Zeng F, Zhou Q 2009 iPS cells produce viable mice through tetraploid complementation. Nature 461:86–90 [DOI] [PubMed] [Google Scholar]
- Meier JJ, Bhushan A, Butler PC 2006 The potential for stem cell therapy in diabetes. Pediatr Res 59:65R–73R [DOI] [PubMed] [Google Scholar]
- Blyszczuk P, Wobus AM 2004 Stem cells and pancreatic differentiation in vitro. J Biotechnol 113:3–13 [DOI] [PubMed] [Google Scholar]
- Nir T, Melton DA, Dor Y 2007 Recovery from diabetes in mice by β-cell regeneration. J Clin Invest 117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P 2009 Human β-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation 87:983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort A, Fort N, Ricordi C, Stabler CL 2008 Biohybrid devices and encapsulation technologies for engineering a bioartificial pancreas. Cell Transplant 17:997–1003 [DOI] [PubMed] [Google Scholar]
- Pickup JC, Zhi ZL, Khan F, Saxl T, Birch DJ 2008 Nanomedicine and its potential in diabetes research and practice. Diabetes Metab Res Rev 24:604–610 [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA 2004 Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- Brennand K, Huangfu D, Melton D 2007 All β-cells contribute equally to islet growth and maintenance. PLoS Biol 5:e163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA 2005 Very slow turnover of β-cells in aged adult mice. Diabetes 54:2557–2567 [DOI] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA 2007 Growth and regeneration of adult β-cells does not involve specialized progenitors. Dev Cell 12:817–826 [DOI] [PubMed] [Google Scholar]
- Kolodka TM, Finegold M, Moss L, Woo SL 1995 Gene therapy for diabetes mellitus in rats by hepatic expression of insulin. Proc Natl Acad Sci USA 92:3293–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulé PM, Liu JM 2000 Regulated hepatic insulin gene therapy of STZ-diabetic rats. Gene Ther 7:1744–1752 [DOI] [PubMed] [Google Scholar]
- Olson DE, Paveglio SA, Huey PU, Porter MH, Thulé PM 2003 Glucose-responsive hepatic insulin gene therapy of spontaneously diabetic BB/Wor rats. Hum Gene Ther 14:1401–1413 [DOI] [PubMed] [Google Scholar]
- Kozlowski M, Olson DE, Rubin J, Lyszkowicz D, Campbell A, Thulé PM 2007 Adeno-associated viral delivery of a metabolically regulated insulin transgene to hepatocytes. Mol Cell Endocrinol 273:6–15 [DOI] [PubMed] [Google Scholar]
- Yoon JW, Jun HS 2002 Recent advances in insulin gene therapy for type 1 diabetes. Trends Mol Med 8:62–68 [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH 1981 Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156 [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM 1998 Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- Jones JM, Thomson JA 2000 Human embryonic stem cell technology. Semin Reprod Med 18:219–223 [DOI] [PubMed] [Google Scholar]
- Martin GR 1981 Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78:7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst AV, Dunleavy E, Almouzni G 2009 Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol 10:192–206 [DOI] [PubMed] [Google Scholar]
- Mohn F, Schübeler D 2009 Genetics and epigenetics: stability and plasticity during cellular differentiation. Trends Genet 25:129–136 [DOI] [PubMed] [Google Scholar]
- Morgan HD, Santos F, Green K, Dean W, Reik W 2005 Epigenetic reprogramming in mammals. Hum Mol Genet 14(Spec No 1):R47–R58 [DOI] [PubMed] [Google Scholar]
- Hemberger M, Dean W, Reik W 2009 Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol 10:526–537 [DOI] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR, Grompe M 2008 Surface markers for the murine oval cell response. Hepatology 48:1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H 2008 β-Cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132:197–207 [DOI] [PubMed] [Google Scholar]
- Halban PA, Kahn SE, Lernmark A, Rhodes CJ 2001 Gene and cell-replacement therapy in the treatment of type 1 diabetes: how high must the standards be set? Diabetes 50:2181–2191 [DOI] [PubMed] [Google Scholar]
- Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB 2008 Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295:E1287–E1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver-Krasinski JM, Stoffers DA 2008 On the origin of the β-cell. Genes Dev 22:1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Grompe M 2008 Generation and regeneration of cells of the liver and pancreas. Science 322:1490–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechoor V, Chan L 2005 Gene therapy progress and prospects: gene therapy for diabetes mellitus. Gene Ther 12:101–107 [DOI] [PubMed] [Google Scholar]
- Samson SL, Chan L 2006 Gene therapy for diabetes: reinventing the islet. Trends Endocrinol Metab 17:92–100 [DOI] [PubMed] [Google Scholar]
- Waldron-Lynch F, Herold KC 2009 Advances in type 1 diabetes therapeutics: immunomodulation and β-cell salvage. Endocrinol Metab Clin North Am 38:303–317, viii [DOI] [PubMed] [Google Scholar]
- Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA 2009 Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA 106:15768–15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE 2005 Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541 [DOI] [PubMed] [Google Scholar]
- Ricordi C, Edlund H 2008 Toward a renewable source of pancreatic β-cells. Nat Biotechnol 26:397–398 [DOI] [PubMed] [Google Scholar]
- Zhu S, Wurdak H, Wang J, Lyssiotis CA, Peters EC, Cho CY, Wu X, Schultz PG 2009 A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell 4:416–426 [DOI] [PubMed] [Google Scholar]
- Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA 2009 Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell 4:348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL 2004 Plasticity of adult stem cells. Cell 116:639–648 [DOI] [PubMed] [Google Scholar]
- Tosh D, Slack JM 2002 How cells change their phenotype. Nat Rev Mol Cell Biol 3:187–194 [DOI] [PubMed] [Google Scholar]
- Horb ME, Shen CN, Tosh D, Slack JM 2003 Experimental conversion of liver to pancreas. Curr Biol 13:105–115 [DOI] [PubMed] [Google Scholar]
- Meivar-Levy I, Sapir T, Gefen-Halevi S, Aviv V, Barshack I, Onaca N, Mor E, Ferber S 2007 Pancreatic and duodenal homeobox gene 1 induces hepatic dedifferentiation by suppressing the expression of CCAAT/enhancer-binding protein β. Hepatology 46:898–905 [DOI] [PubMed] [Google Scholar]
- Slack JM 2007 Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol 8:369–378 [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A 2000 Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 6:568–572 [DOI] [PubMed] [Google Scholar]
- Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, Benvenisti-Zarum L, Meivar-Levy I, Ferber S 2003 Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem 278:31950–31957 [DOI] [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, Umayahara Y, Watada H, Yamasaki Y, Magnuson MA, Miyazaki J, Hori M 2003 Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun 310:1017–1025 [DOI] [PubMed] [Google Scholar]
- Ianus A, Holz GG, Theise ND, Hussain MA 2003 In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest 111:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JB, Uchino H, Azuma K, Iwashita N, Tanaka Y, Mochizuki H, Migita M, Shimada T, Kawamori R, Watada H 2003 Little evidence of transdifferentiation of bone marrow-derived cells into pancreatic β-cells. Diabetologia 46:1366–1374 [DOI] [PubMed] [Google Scholar]
- Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF 2004 No evidence for significant transdifferentiation of bone marrow into pancreatic β-cells in vivo. Diabetes 53:616–623 [DOI] [PubMed] [Google Scholar]
- Rizzino A 2007 A challenge for regenerative medicine: proper genetic programming, not cellular mimicry. Dev Dyn 236:3199–3207 [DOI] [PubMed] [Google Scholar]
- Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L 2005 In vitro generation of insulin-producing β-cells from adult exocrine pancreatic cells. Diabetologia 48:49–57 [DOI] [PubMed] [Google Scholar]
- Lardon J, Huyens N, Rooman I, Bouwens L 2004 Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Arch 444:61–65 [DOI] [PubMed] [Google Scholar]
- Zhao M, Amiel SA, Christie MR, Rela M, Heaton N, Huang GC 2005 Insulin-producing cells derived from human pancreatic non-endocrine cell cultures reverse streptozotocin-induced hyperglycaemia in mice. Diabetologia 48:2051–2061 [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G 1998 A molecular basis for transdetermination in Drosophila imaginal discs: interactions between wingless and decapentaplegic signaling. Development 125:115–124 [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G 1999 Cell determination and transdetermination in Drosophila imaginal discs. Curr Top Dev Biol 43:115–151 [DOI] [PubMed] [Google Scholar]
- Wei G, Schubiger G, Harder F, Müller AM 2000 Stem cell plasticity in mammals and transdetermination in Drosophila: common themes? Stem Cells 18:409–414 [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G 2003 Transdetermination in Drosophila imaginal discs: a model for understanding pluripotency and selector gene maintenance. Curr Opin Genet Dev 13:472–479 [DOI] [PubMed] [Google Scholar]
- Klebes A, Sustar A, Kechris K, Li H, Schubiger G, Kornberg TB 2005 Regulation of cellular plasticity in Drosophila imaginal disc cells by the polycomb group, trithorax group and lama genes. Development 132:3753–3765 [DOI] [PubMed] [Google Scholar]
- McClure KD, Schubiger G 2007 Transdetermination: Drosophila imaginal disc cells exhibit stem cell-like potency. Int J Biochem Cell Biol 39:1105–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg JC 2006 Divided loyalties: transdetermination and the genetics of tissue regeneration. Bioessays 28:574–577 [DOI] [PubMed] [Google Scholar]
- Johnston LA 2005 Regeneration and transdetermination: new tricks from old cells. Cell 120:288–290 [DOI] [PubMed] [Google Scholar]
- Lee N, Maurange C, Ringrose L, Paro R 2005 Suppression of polycomb group proteins by JNK signalling induces transdetermination in Drosophila imaginal discs. Nature 438:234–237 [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V 2007 Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet 8:9–22 [DOI] [PubMed] [Google Scholar]
- Gehring WJ 1987 Homeo boxes in the study of development. Science 236:1245–1252 [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G 1995 Wingless induces transdetermination in developing Drosophila imaginal discs. Development 121:1263–1272 [DOI] [PubMed] [Google Scholar]
- Johnston LA, Schubiger G 1996 Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development 122:3519–3529 [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL 2004 Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol 3:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau T, Dimmler A, Häfner M, Brabletz T, Kirchner T, Faller G 2005 Aberrant expression of TTF-1 and forkhead factor HFH-4 in atrophic gastritis and ciliated metaplasia suggests gastric broncho-pulmonary transdetermination. J Pathol 206:383–387 [DOI] [PubMed] [Google Scholar]
- Manohar R, Lagasse E 2009 Transdetermination: a new trend in cellular reprogramming. Mol Ther 17:936–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS 1999 Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 284:1998–2003 [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lóra J, Zaret KS 2001 A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128:871–881 [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS 2009 Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science 324:1707–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M 2003 The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA 100(Suppl 1):11881–11888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N, Campbell JS 2003 The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev 120:117–130 [DOI] [PubMed] [Google Scholar]
- Cantz T, Manns MP, Ott M 2008 Stem cells in liver regeneration and therapy. Cell Tissue Res 331:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erker L, Grompe M 2007 Signaling networks in hepatic oval cell activation. Stem Cell Res 1:90–102 [DOI] [PubMed] [Google Scholar]
- Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB 2002 In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci USA 99:8078–8083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite AR, Corrêa-Giannella ML, Dagli ML, Fortes MA, Vegas VM, Giannella-Neto D 2007 Fibronectin and laminin induce expression of islet cell markers in hepatic oval cells in culture. Cell Tissue Res 327:529–537 [DOI] [PubMed] [Google Scholar]
- Nagaya M, Katsuta H, Kaneto H, Bonner-Weir S, Weir GC 2009 Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrinol 201:37–47 [DOI] [PubMed] [Google Scholar]
- Dutton JR, Chillingworth NL, Eberhard D, Brannon CR, Hornsey MA, Tosh D, Slack JM 2007 β-Cells occur naturally in extrahepatic bile ducts of mice. J Cell Sci 120:239–245 [DOI] [PubMed] [Google Scholar]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R, Kageyama R, Wright CV, Chiba T 2006 Ectopic pancreas formation in Hes1-knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest 116:1484–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A 2004 Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet 36:83–87 [DOI] [PubMed] [Google Scholar]
- Kim S, Shin JS, Kim HJ, Fisher RC, Lee MJ, Kim CW 2007 Streptozotocin-induced diabetes can be reversed by hepatic oval cell activation through hepatic transdifferentiation and pancreatic islet regeneration. Lab Invest 87:702–712 [DOI] [PubMed] [Google Scholar]
- Vorobeychik M, Bloch K, Zemel R, Bachmetov L, Tur-Kaspa R, Vardi P 2008 Immunohistochemical evaluation of hepatic oval cell activation and differentiation toward pancreatic β-cell phenotype in streptozotocin-induced diabetic mice. J Mol Histol 39:463–468 [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F 2000 neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA 2002 Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129:2447–2457 [DOI] [PubMed] [Google Scholar]
- Yechoor V, Liu V, Paul A, Lee J, Buras E, Ozer K, Samson S, Chan L 2009 Gene therapy with neurogenin 3 and betacellulin reverses major metabolic problems in insulin-deficient diabetic mice. Endocrinology 150:4863–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA 2008 Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol 26:313–315 [DOI] [PubMed] [Google Scholar]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, Ogawa D, Ikeda E, Okano H, Yamanaka S 2009 Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 27:743–745 [DOI] [PubMed] [Google Scholar]
- Yamanaka S 2009 A fresh look at iPS cells. Cell 137:13–17 [DOI] [PubMed] [Google Scholar]
- Lee Y, Ravazzola M, Park BH, Bashmakov YK, Orci L, Unger RH 2007 Metabolic mechanisms of failure of intraportally transplanted pancreatic β-cells in rats: role of lipotoxicity and prevention by leptin. Diabetes 56:2295–2301 [DOI] [PubMed] [Google Scholar]
- van der Windt DJ, Echeverri GJ, Ijzermans JN, Cooper DK 2008 The choice of anatomical site for islet transplantation. Cell Transplant 17:1005–1014 [PubMed] [Google Scholar]
- Bagley J, Paez-Cortez J, Tian C, Iacomini J 2008 Gene therapy in type 1 diabetes. Crit Rev Immunol 28:301–324 [DOI] [PubMed] [Google Scholar]