Abstract
Neurons and endocrine cells package peptides in secretory granules (large dense-core vesicles) for storage and stimulated release. Studies of peptidylglycine α-amidating monooxygenase (PAM), an essential secretory granule membrane enzyme, revealed a pathway that can relay information from secretory granules to the nucleus, resulting in alterations in gene expression. The cytosolic domain (CD) of PAM, a type 1 membrane enzyme essential for the production of amidated peptides, is basally phosphorylated by U2AF homology motif kinase 1 (Uhmk1) and other Ser/Thr kinases. Proopiomelanocortin processing in AtT-20 corticotrope tumor cells was increased when Uhmk1 expression was reduced. Uhmk1 was concentrated in the nucleus, but cycled rapidly between nucleus and cytosol. Endoproteolytic cleavage of PAM releases a soluble CD fragment that localizes to the nucleus. Localization of PAM-CD to the nucleus was decreased when PAM-CD with phosphomimetic mutations was examined and when active Uhmk1 was simultaneously overexpressed. Membrane-tethering Uhmk1 did not eliminate its ability to exclude PAM-CD from the nucleus, suggesting that cytosolic Uhmk1 could cause this response. Microarray analysis demonstrated the ability of PAM to increase expression of a small subset of genes, including aquaporin 1 (Aqp1) in AtT-20 cells. Aqp1 mRNA levels were higher in wild-type mice than in mice heterozygous for PAM, indicating that a similar relationship occurs in vivo. Expression of PAM-CD also increased Aqp1 levels whereas expression of Uhmk1 diminished Aqp1 expression. The outlines of a pathway that ties secretory granule metabolism to the transcriptome are thus apparent.
A pathway through which Uhmk1 phosphorylation of the cytosolic domain of the peptide amidating enzyme links secretory granule metabolism to the transcriptome is identified.
Endocrine cells and neurons produce bioactive peptides that are packaged in large dense-core vesicles (LDCVs), stored, and secreted upon stimulation. Some LDCV membrane proteins, including peptidylglycine α-amidating monooxygenase (PAM), are recycled and reused in newly forming LDCVs (1,2,3). Little is known about how cells sense the luminal status or number of LDCVs, but essential granule membrane enzymes like PAM are likely candidates to play a role in these processes. Consistent with this hypothesis, increasing expression of PAM in a corticotrope tumor cell line engineered for inducible expression of PAM resulted in cytoskeletal rearrangement, reduced proopiomelanocortin (POMC) processing, and diminished secretagogue responsiveness (4).
The luminal enzymatic domains of PAM are required for amidated peptide production. The cytosolic domain (CD) of PAM (PAM-CD; residues 891–976) is essential for its entry into granules and for endocytic trafficking (5,6,7). PAM-CD is unstructured, protease sensitive, and multiply phosphorylated (8,9). Whereas phosphorylation of PAM-CD by protein kinase C is secretagogue responsive, phosphorylation at other sites occurs basally. Ser949, which is basally phosphorylated, can be phosphorylated by Uhmk1 (U2AF homology motif kinase 1) or casein kinase II (9). Mutation of Ser949 to Asp eliminated the ability of PAM to rearrange the cytoskeleton or inhibit regulated secretion, suggesting an important signaling role for phosphorylation and dephosphorylation at this site (9,10,11).
Uhmk1, also known as KIS (kinase interacting with stathmin) or P-CIP2 (PAM-CD interacting protein 2), consists of a kinase domain followed by a putative RNA-binding domain (RBD) homologous to RNA-splicing factor U2af65 (12). Genetic linkage studies revealed an association of Uhmk1 with schizophrenia (13,14). Uhmk1, which phosphorylates p27KIP1, a cyclin-dependent kinase inhibitor, has been linked to cell cycle regulation (15,16) and is expressed at high levels in endocrine and nervous tissues (9,17,18,19). Uhmk1 is localized in the nucleus of cultured fibroblast lines (19,20), a fact that is difficult to reconcile with a role for Uhmk1 in LDCV trafficking or stathmin-mediated microtubule stability (17,18,19).
We now know that a soluble fragment encompassing the C terminus of PAM (sf-CD) is released into the cytosol after proteolytic cleavage of PAM-1 (8). sf-CD is localized in the nuclei of AtT-20 cells expressing exogenous PAM-1 and in nuclei isolated from rat pituitary and atrium. This finding raised the possibility that Uhmk1 could interact with intact PAM in the cytosol and with sf-CD in the nucleus, perhaps participating in a signaling pathway linking LDCV metabolism to gene expression. In pancreatic β-cells, secretagogue stimulation results in cleavage of the CD of ICA512, a LDCV membrane protein, enabling nuclear translocation of its CD and altering gene expression (2). Cholesterol biosynthesis is controlled, in part, by the sterol-regulatory element-binding protein (SREBP), a membrane protein that signals to the nucleus after endoproteolytic cleavage (21,22). We present evidence of an important role for Uhmk1 in endocrine cells, where it moves quickly into and out of the nucleus, regulating sf-CD nuclear localization and altering the ability of PAM to affect gene expression.
Results
Uhmk1 alters proopiomelanocortin metabolism in corticotropes
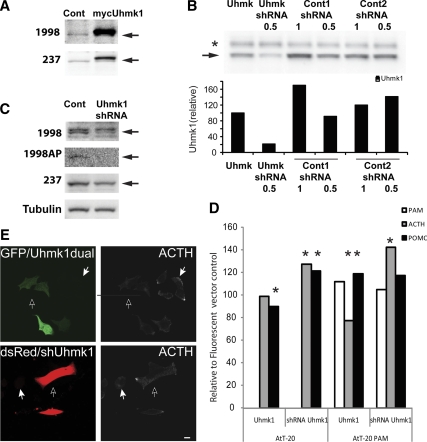
We used AtT-20 corticotrope tumor cells, which produce POMC and store its cleaved products, ACTH and β-endorphin, as our model system to examine the role of Uhmk1 in LDCV metabolism. Endogenous AtT-20 Uhmk1 (46 kDa) was detected by two antibodies (Fig. 1A). Transient expression of mycUhmk1 increased Uhmk1 levels several fold over endogenous levels. To validate the efficacy of our Uhmk1 short hairpin RNA (shRNA), we expressed exogenous Uhmk1 in a fibroblast line along with shRNA specific for Uhmk1 or two control shRNAs of similar composition that do not correspond to known mouse transcripts (Fig. 1B); quantification of Western blots confirmed the efficacy and specificity of the Uhmk1 shRNA. Expression of Uhmk1 shRNA in AtT-20 cells reduced the intensity of the 46-kDa band, confirming its identity (Fig. 1C).
Figure 1.
Uhmk1 is expressed in AtT-20 cells and affects POMC metabolism. A, Control AtT-20 cells or AtT-20 cells transiently expressing mycUhmk1 were subjected to Western blot analysis using the indicated Uhmk1 antibodies; arrows mark endogenous Uhmk1. B, pEAK Rapid cells transiently expressing mycUhmk1 were cotransfected with vectors (1 or 0.5 μg, as indicated) encoding Uhmk1 shRNA or two control shRNAs; Western blot analysis was performed using JH1998. Arrow, endogenous Uhmk1; *, nonspecific band. Quantification is shown below. C, Control AtT-20 cells or AtT-20 cells expressing Uhmk1 shRNA for 48 h were subjected to Western blot analysis using the indicated Uhmk1 or tubulin antisera; JH1998 AP, affinity-purified antibody; arrows, endogenous Uhmk1. D, Ratiometric analysis of AtT-20 and AtT-20 PAM-1 cells expressing mycUhmk1 (along with GFP in a dual promoter vector) vs. the GFP control or Uhmk1 shRNA/DsRed vs. the average of the two control shRNA/DsRed vectors. Fixed cells stained for cleaved ACTH or total POMC were visualized with Cy5-tagged secondary antibody. *, Ratios of samples with significant differences (P < 0.05 by t test and Kolmogorov-Smirnov). E, Representative AtT-20 PAM-1 cells expressing GFP and Uhmk1 from the dual promoter vector or dsRed/shUhmk1 24 h after transfection using antibody to ACTH; open arrows mark transfected cells; solid arrows mark nontransfected cells. Scale bar, 10 μm. Cont, Control.
The effects of Uhmk1 expression on POMC metabolism were evaluated by staining transiently transfected AtT-20 cells and AtT-20/PAM-1 cells with antisera that recognize the C terminus of ACTH or with antisera that recognize intact POMC and any processed products that include the N-terminal region of ACTH (total POMC) (Fig. 1D). AtT-20/PAM-1 cells express much higher levels of PAM than AtT-20 cells (4). The images in Fig. 1E are representative of those used to generate the data in Fig. 1D. When Uhmk1 levels in AT-20 cells were increased by transient expression of mycUhmk1, total POMC levels decreased slightly and ACTH levels were unchanged compared with the green fluorescent protein (GFP) controls. When Uhmk1 levels in these cells were reduced using Uhmk1 shRNA, total POMC and ACTH levels were both increased. AtT-20/PAM-1 cells responded differently to alterations in Uhmk1 expression. When Uhmk1 levels were increased, ACTH levels decreased by 33%, and total POMC levels increased to 119%, indicative of inhibited POMC processing. When Uhmk1 levels in AtT-20/PAM-1 cells were reduced, ACTH levels increased to 142% of control, but total POMC levels did not change significantly (Fig. 1D). PAM expression in this stably transfected cell line was not affected by changes in Uhmk1 levels.
In AtT-20 cells, Uhmk1 is present in both nucleus and cytoplasm
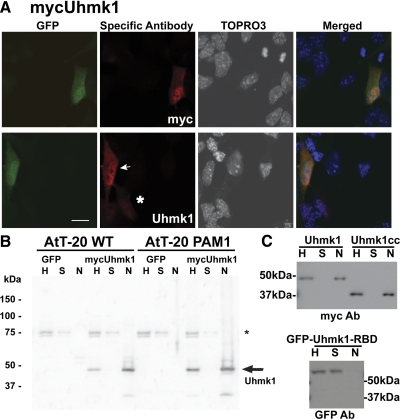
To determine how Uhmk1 affects POMC metabolism, we needed to know where it was localized. Because none of our Uhmk1 antibodies reliably visualized endogenous Uhmk1 for immunocytochemistry, AtT-20 cells were transfected with a dual promoter vector encoding GFP and mycUhmk1 or with a vector encoding a GFP-Uhmk1 fusion protein (Fig. 2A). In cells expressing GFP and mycUhmk1, Uhmk1 was concentrated in the nucleus but excluded from the nucleolus. Cytosolic Uhmk1 staining in these cells was diffusely distributed; this pattern was observed in 92% of the cells examined (Fig. 2A). Visualization of mycUhmk1 with antibody to Uhmk1 or myc yielded the same pattern. Nuclear localization of Uhmk1 was apparent at both low (Fig. 2A, asterisk) and moderate (Fig. 2A, arrow) levels of Uhmk1 expression. At higher levels of expression, AtT-20 cells rounded up and appeared to be unhealthy (data not shown).
Figure 2.
Uhmk1 is present in both nucleus and cytosol. A, AtT-20 cells expressing mycUhmk1 and GFP were examined 24 h after transfection using antibody to myc or Uhmk1. Primary antibody was visualized using Cy3-tagged secondary antibody whereas nuclei were visualized using TOPRO3. Arrow marks moderate and asterisk marks low expressing cell; scale bar, 10μm. B, MycUhmk1 was transiently expressed in AtT-20 or PAM-1 AtT-20 cells; nuclei were isolated and 1% of the homogenate (H), supernatant (S), and nuclear (N) fractions was analyzed by SDS-PAGE; exogenous Uhmk1 was visualized using rabbit polyclonal antibody JH1998. *, Nonspecific band. C, AtT-20 PAM-1 cells were transfected with vectors encoding mycUhmk1, mycUhmk1cc, or GFP-Uhmk1-RBD. Cells were harvested the next day, and nuclei were isolated and analyzed. Antibodies used for Western blots are indicated below each blot. Ab, Antibody.
We used a biochemical approach to confirm the nuclear localization of Uhmk1. Nuclei were purified from AtT-20 cells and from AtT-20/PAM-1 cells transiently transfected with dual promoter vector encoding GFP and mycUhmk1 (Fig. 2B). An equal percentage of the initial homogenate, supernatant, and nuclear fraction was subjected to Western blot analysis, revealing the presence of 47-kDa mycUhmk1 in the nuclear fraction (Fig. 2B). The efficacy of the fractionation scheme was verified using antibodies specific for histone and calnexin (data not shown).
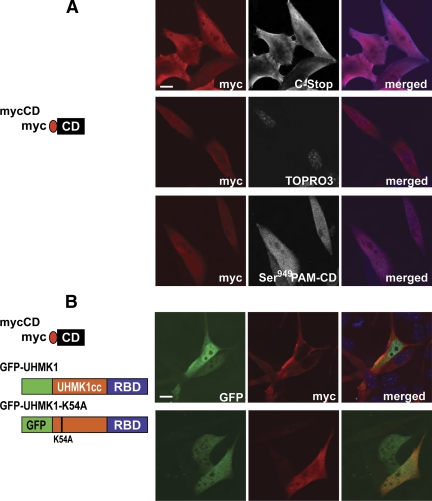
In addition to its kinase domain, Uhmk1 has a putative RBD. To determine whether both domains were required for nuclear localization, Uhmk1 was separated into its catalytic core (residues 1–303) and RNA binding domain (residues 304–419). The catalytic core was tagged with myc (mycUhmk1cc) (9), and the RBD was tagged with GFP (GFP-Uhmk1-RBD). When expressed in AtT-20 cells and in AtT-20/PAM-1 cells, mycUhmk1, mycUhmk1cc, and GFP-Uhmk1 (data not shown) were recovered in the nuclear fraction; in contrast, GFP-RBD was recovered in the supernatant fraction (Fig. 2C). These results for corticotrope tumor cells are consistent with previous studies of Uhmk1 in 3T3 and Chinese hamster ovary cells (19,20) and indicate that its kinase domain is sufficient for nuclear localization; the RBD had no significant affinity for the nucleus. When cells expressing inactive GFP-Uhmk1K54A were fractionated, most of the GFP-Uhmk1K54A was still recovered from the nuclear fraction (data not shown).
Uhmk1 shuttles between nucleus and cytoplasm
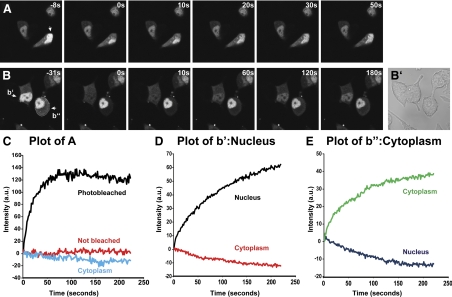
Because some Uhmk1 substrates are localized to the nucleus whereas others, such as PAM-1, are not, we wondered whether Uhmk1 might shuttle between these compartments (23,24,25,26). To address this question, we used fluorescence recovery after photobleaching (FRAP) to examine GFP-Uhmk1 expressed in AtT-20 cells. As observed with Uhmk1, when expressed transiently in AtT-20 cells, GFP-Uhmk1 was predominantly nuclear (Fig. 3A, left), although excluded from nucleoli. Lower levels of GFP-Uhmk1 were diffusely distributed throughout the cytoplasm.
Figure 3.
Uhmk1 shuttles into and out of the nucleus. FRAP experiments were performed on AtT-20 cells transfected the previous day with vector encoding GFP-Uhmk1. A, In the cell marked by the arrow, the indicated region of the nucleus was photobleached; cells were imaged for the next 4 min. B, The nucleus of one cell (b’) and the cytoplasm of another cell (b”) were photobleached as indicated by the white lines and recorded as in A. B’, Phase image after FRAP experiment was completed. C, Fluorescence intensities were quantified in photobleached nuclei, nonbleached nuclei, and cytoplasm. The recovery curve was fit by a single exponential function; f = 78.4 + 3.9(1 − e(−0.06 X)); r = 0.96; P < 0.0001; in this case significant photobleaching was detected, and the curve fitting includes a linear component; f = [41.5 − 0.05 X] + 48.6(1 − e(−0.0424 X)); r = 0.97; P < 0.0001. D, Fluorescence intensities in the entire nucleus and entire cytoplasm of cells b′ (panel D) and b″ (panel E) were quantified. The nuclear recovery curve (D) was fit by a single exponential function: f = 29.5 + 9.8(1 − e(−0.0113 X)); r = 0.99; P < 0.0001. The cytoplasmic recovery curve (panel E) was fit by a single exponential function: f = 53.4 + 22.2(1 − e(−0.0064 X)); r = 0.998; P < 0.0001. Representative experiments are shown.
To determine whether the GFP-Uhmk1 in the nucleus were mobile, half the nucleus was photobleached (Fig. 3A). Images were collected every 1.6 sec for the next 4 min. Total fluorescence intensities in defined areas of bleached and nonbleached regions of the nucleus and in the cytoplasm were quantified (Fig. 3C). The photobleached region of the nucleus recovered, reaching a plateau in about 60 sec. The recovery curve was fit by a single exponential function with a half-time (τ1/2) of 16 sec; for comparison, freely diffusing GFP has a diffusion τ1/2 of approximately 2 sec (27). This difference suggests that a significant fraction of the GFP-Uhmk1 in the nucleus could be in rapid equilibrium with nuclear binding sites (Fig. 3, A and C).
To address the possibility that GFP-Uhmk1 was shuttling between the nucleus and the cytoplasm, we photobleached the entire nucleus (Fig. 3B, panel b’) or the entire cytoplasm (Fig. 3B, panel b”) of AtT-20 cells transiently expressing GFP-Uhmk1, and quantified fluorescence intensity over time (Fig. 3, D and E). As GFP-Uhmk1 returned to the nucleus, the cytoplasm lost intensity; recovery of fluorescence was much slower than when a portion of the nucleus was bleached. The nuclear recovery curve was fit by a single exponential function with a τ1/2 of 88 sec, presumably reflecting nuclear import. The cytoplasmic recovery curve was fit by a single exponential function with τ1/2 of 156 sec, presumably reflecting nuclear export. Although most of the GFP-Uhmk1 was localized to the nucleus at steady state, it is clear that the protein shuttles back and forth between nucleus and cytoplasm. Uhmk1 would have access to PAM-1 in the cytosol and to sf-CD in the nucleus.
Nuclear localization of PAM-CD is inhibited by phosphomimetic mutations
PAM is subject to endoproteolytic cleavage in LDCVs and as it traverses the endocytic pathway. Antibody specific for the C terminus of PAM (8) identifies full-length PAM-1, peptidyl-α-hydroxyglycine α-amidating lyase (PAL)-transmembrane domain (TMD)-CD, and TMD-CD along with sf-CD, a soluble, cytosolic 16-kDa protein thought to be produced by cleavage within the TMD, which extends from Val867 to Ile890 (8). sf-CD was identified in nuclei purified from adult rat pituitary and atrium (8). When the Uhmk1/casein kinase II sites in the CD of PAM-1 were replaced with phosphomimetic mutations (Thr946Ser949→ Asp946Asp949), levels of sf-CD were greatly reduced (8).
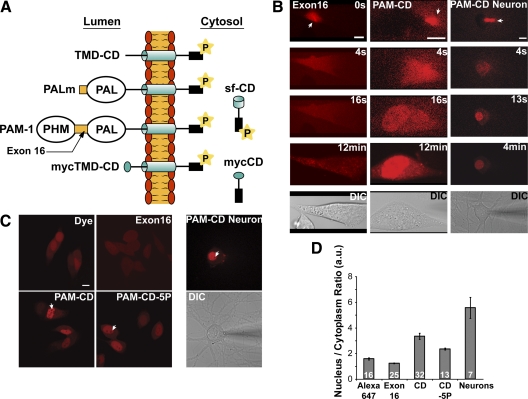
To study the nuclear localization of sf-CD, we prepared fluorescently tagged recombinant PAM-CD (Arg896 to Ser976) for injection into AtT-20 cells (Fig. 4A). As a control, we prepared fluorescently tagged recombinant exon 16, a noncatalytic luminal linker region of PAM of similar size and hydrophilicity. Fluorescently tagged PAM-CD and exon 16 were injected into AtT-20 cells; white arrows mark the point at which the pipette penetrated the cell and fluorescently tagged protein began to enter the cytosol (Fig. 4B). Labeled Exon 16 was evenly distributed between nucleus and cytoplasm for the time period examined (Fig. 4B). At 16 sec, fluorescently tagged PAM-CD was seen in the nucleus and in the cytosol at approximately equal levels. After longer times, fluorescently tagged PAM-CD was more concentrated in the nucleus than in the cytosol and remained so for at least 12 min, the longest time examined (Fig. 4B). Similar results were obtained when hippocampal neurons were injected with PAM-CD; these images were taken at lower power to include neuronal processes, which were not heavily labeled (Fig. 4B and Supplemental Video 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Figure 4.
Recombinant PAM-CD localizes to the nucleus. A, Cleavage of PAM-1 in exon 16 occurs in LDCVs, generating membrane-anchored PAL (PALm); cleavage after PAL produces 22-kDa TMD-CD (8). Intramembrane proteolysis is thought to create soluble, cytosolic sf-CD (16 kDa) (8). The structures of mycTMD-CD and mycCD, which were stably expressed in AtT-20 cells, are shown for comparison. B, Alexa647 labeled PAM-exon16 or PAM-CD was microinjected into AtT-20 cells or neurons; white arrow marks the injection site. Representative confocal images taken at the times indicated are shown along with a differential interference contrast (DIC) image. Scale bars, 10 μm. C, AtT-20 cells or neurons were microinjected with free Alexa647 dye, Alexa647-Exon16, Alexa647-PAM-CD, or Alexa647-PAM-CD-5P (with five phosphomimetic mutations). Images were taken no longer than 2 min after injection. Scale bar, 10 μm. D, Quantification of the nuclear/cytoplasmic ratio of images taken as in panel C; mean ± sem. The number of cells analyzed is indicated. a.u., Arbitrary units.
Alexa dyes are known to have nucleophilic properties. To eliminate the possibility that the dye caused accumulation of PAM-CD in the nucleus, we injected Alexa Fluor 647 alone (Fig. 4C). To quantify the ability of different labeled proteins to accumulate in the nucleus, cells were imaged within 2 min of injection, and the nuclear/cytoplasmic ratio of the fluorescent signal was quantified (Fig. 4, C and D). Whereas exon 16 had a nuclear/cytoplasmic ratio of 1.2 ± 0.02 (sem) and Alexa Fluor 647 had a ratio of 1.6 ± 0.1, PAM-CD gave a ratio of 3.3 ± 0.2 in pituitary cells and 5.5 ± 0.8 in neurons. The ability of PAM-CD to localize to the nucleus varied with cell type.
Under basal conditions, the CD of PAM is phosphorylated at more than six sites (8); to evaluate the possibility that its phosphorylation state might alter the ability of PAM-CD to accumulate in the nucleus, recombinant PAM-CD with five phosphomimetic mutations at established sites of phosphorylation was examined. In PAM-CD-5P, Ser/Thr→Asp/Glu mutations were made at Ser932, Ser937, Ser945, Thr946, and at the Uhmk1 target site, Ser949. The nuclear/cytoplasmic ratio of PAM-CD-5P was lower than that of PAM-CD, 2.3 ± 0.1, but higher than that of Alexa 647 alone (Fig. 4, C and D). These data suggest that phosphorylation of PAM-CD would reduce its nuclear localization. Thus the possibility that Uhmk1 might reduce PAM-CD nuclear localization was tested.
Uhmk1 causes exclusion of PAM-CD from the nucleus
To determine whether Uhmk1 affected PAM-CD nuclear localization, we transiently expressed soluble mycCD (PAM-1:Arg891 to Ser976; Fig. 4A) in AtT-20 cells in the absence or presence of exogenous, GFP-tagged Uhmk1. At steady state without exogenous Uhmk1, mycCD was detected in the nucleus and in the cytoplasm using antibodies to myc or the C terminus of PAM (Fig. 5A, upper panels); staining was more intense at the edges of the cell. Although mycCD was present in the nucleus, it was not concentrated there, as observed immediately after acute injection of recombinant PAM-CD. This difference may reflect an equilibrium among phosphorylation/dephosphorylation or nuclear import/export/degradation in the transiently transfected cells that does not have time to occur in the few minutes after microinjection of recombinant PAM-CD. When the subset of mycCD phosphorylated at Ser949 (the Uhmk1 site) was visualized using antibody specific for P-Ser949, staining was again observed throughout both nucleus and cytosol (Fig. 5A, bottom panel).
Figure 5.
PAM-CD nuclear localization is affected by Uhmk1 in a kinase activity-dependent manner. A, AtT-20 cells were transfected with vector encoding mycCD alone or (B) cotransfected with vectors encoding mycCD and active GFP-Uhmk1 or inactive GFP-Uhmk1K54A. In both panels A and B, mycCD was detected with myc antibody (red). In panel A, mycCD was also visualized with C-Stop antibody or P-Ser949 antibody, and nuclei were visualized with TOPRO; these images are shown in white in the middle panels and in blue in the right panels. In panel B, GFP-tagged proteins are shown in green. Scale bar, 10 μm.
To determine whether active Uhmk1 could regulate the nuclear localization of PAM-CD, AtT-20 cells were cotransfected with vectors encoding mycCD and GFP-Uhmk1 or its inactive mutant, GFP-Uhmk1K54A (Fig. 5B). Both GFP-Uhmk1 and its inactive mutant were excluded from regions of the nucleus. In 85% of the cells coexpressing mycCD and active Uhmk1, nuclear mycCD staining was markedly decreased compared with the surrounding cytosol. In contrast, nuclear mycCD staining was lower than surrounding cytosolic levels in only 30% of the cells not expressing GFP-Uhmk1 (Fig. 5B). Nuclear mycCD staining was lower than cytosolic levels in only 17% of the cells coexpressing inactive GFP-Uhmk1K54A. These results indicate that reducing the concentration of PAM-CD in the nucleus requires the kinase activity of Uhmk1.
Uhmk1 does not need to enter the nucleus to cause exclusion of PAM-CD
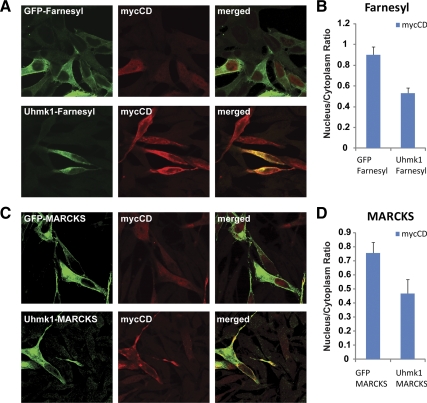
To determine whether Uhmk1 needs to enter the nucleus to alter the distribution of the PAM-CD, Uhmk1 was tethered to membranes using the lipid-anchoring sequence from MARCKS at its N terminus or the lipid-anchoring sequence from K-Ras at its C terminus (28,29). Membrane tethering was effective, as judged by the redistribution of GFP and Uhmk1 with both methods of anchoring (Fig. 6A, C). Membrane-tethered Uhmk1 was not localized to the nucleus. The nuclear/cytoplasmic distribution of mycCD was compared in cells expressing membrane-tethered GFP or membrane-tethered Uhmk1; membrane-tethered Uhmk1 effectively excluded mycCD from the nucleus (Fig. 6, B and D). The fact that cytosolic Uhmk1 can diminish mycCD nuclear localization does not mean that Uhmk1 does not phosphorylate PAM-CD when both proteins are in the nucleus.
Figure 6.
Uhmk1 tethering to membranes does not prevent nuclear expulsion of PAM-CD. A, AtT-20 cells were cotransfected with vectors encoding mycCD and GFP-Farnesyl or mycCD and Uhmk1-Farnesyl. B, Ratio between the nuclear and the cytoplasmic intensities of the cotransfected cells in panel A; P < 0.001. C, AtT-20 cells were cotransfected with vectors encoding mycCD and GFP-MARCKS or mycCD and Uhmk1-MARCKS. D, Ratio between the nuclear and the cytoplasmic intensities of the cotransfected cells in panel C; P < 0.05. Even when tethered to a membrane anchor, Uhmk1 diminished the ability of mycCD to accumulate in the nucleus. Mean ± sem, nine to 16 cells per bar. MARCKS, Myristoylated alanine-rich C-kinase substrate.
PAM alters gene expression
The Uhmk1-regulated presence of a C-terminal fragment of PAM in the nucleus raised the possibility that PAM might alter gene expression, perhaps contributing to the observed ability of both PAM-1 and mycTMD-CD to alter cytoskeletal organization and the function of the regulated secretory pathway in corticotrope tumor cells (4,9,10,11,30). The doxycycline-inducible iPAM (doxycycline-inducible rat PAM-1) AtT-20 cell line was used to assess the effects of PAM-1 on gene expression while avoiding secondary effects due to transfection or accommodation to overexpression. iPAM cells were grown in medium with or without doxycycline for 48 h, a treatment previously shown to increase exogenous PAM-1 expression 20- to 60-fold over basal levels (4); RNA prepared from three subclones of iPAM cells grown under both conditions was subjected to microarray analysis using the Illumina Mouse6 chip.
After induction by doxycycline, the expected large increase in PAM-1 expression was confirmed by Western blot analysis and immunocytochemical staining (4) (data not shown). Transcripts encoding ubiquitin (Ubb), proopiomelanocortin (POMC), ATP5b (mitochondrial F1 ATPase subunit, nuclear gene), and several mitochondrial and ribosomal proteins were among those most highly expressed (Supplemental Table 1). To establish background levels of expression, genes not known to be expressed in corticotropes were examined. Immunoglobulins and several olfactory receptors gave expression values close to 120, which was chosen as background and subtracted. This cut off eliminated 10,000 of the 35,000 genes in the screen. Endogenous mouse PAM was expressed at levels above this background (338 before background subtraction) and was not regulated by doxycycline. Exogenous rat PAM-1 was not detected by the 50-nucleotide Illumina oligonucleotide targeted to mouse PAM.
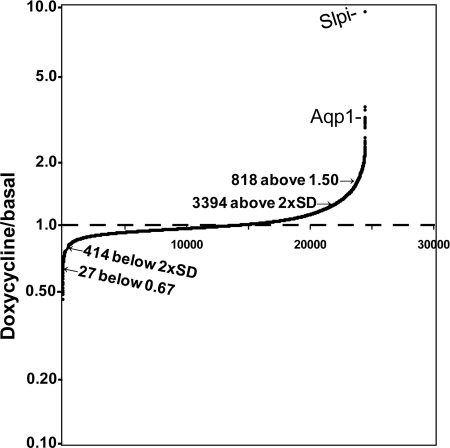
The doxycycline to basal ratio for the remaining 25,000 transcripts was calculated for each iPAM subclone, identifying a set of up-regulated transcripts and a smaller set of down-regulated transcripts (Fig. 7 and Supplemental Table 1). Only 13.5% of the transcripts were induced to a doxycycline/basal ratio more than twice the average standard deviation of the doxycycline/basal ratio (12%), and only 3% had a doxycycline/basal ratio above 1.50. Only 1.6% of the transcripts had a doxycycline/basal ratio more than 2 sds below 1.00, and only 27 transcripts (0.1%) yielded a doxycycline/basal ratio below 0.67. Doxycycline-induced expression of PAM-1 altered the expression of a small subset of the genes expressed in corticotrope tumor cells.
Figure 7.
PAM alters expression of a subset of genes in AtT-20 cells: array analysis. Replicate wells of three iPAM subclones were grown in control medium or medium containing 4 μg/ml doxycycline for 48 h; RNA prepared from these six samples was used to probe an Illumina MouseWG-6v2.0 Expression BeadChip. Background was subtracted before calculating the doxycycline/basal expression ratios for genes expressed in each subclone. Average ratios were ordered to yield the cumulative plot shown. The sd in the doxycycline/basal ratio within samples was 12%; the points for 2 sds above and below 1.00 are indicated, with the number of transcripts in each pool. The full data set is provided in Supplemental Table 1.
Aquaporin 1 (Aqp1) and secretory leukocyte peptidase inhibitor (Slpi) expression are increased by PAM
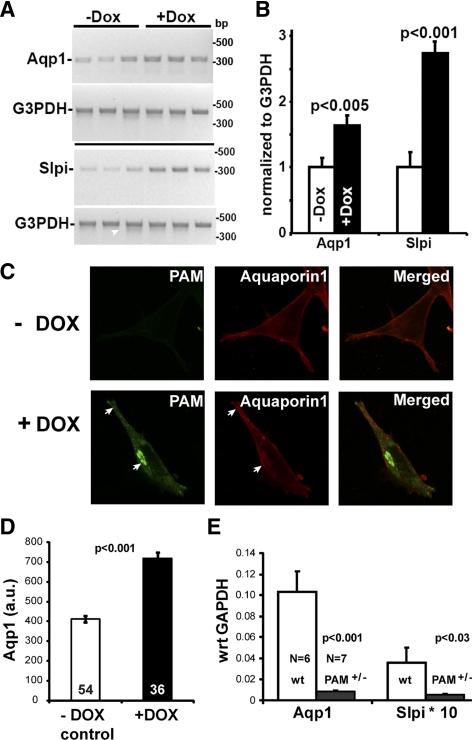
Among transcripts encoding known proteins, expression of Slpi showed the greatest increase (up 9.5 ± 2.1 fold) in response to increased PAM expression (Fig. 7). Slpi, a member of the whey acidic protein four-disulfide core family of protease inhibitors, inhibits elastase, cathepsin G, trypsin, and chymotrypsin (31). Aqp1 transcripts rose 3.1 ± 0.2 fold after doxycycline treatment. Aqp1, a water channel, has been localized to the LDCVs of AtT-20 cells, other endocrine cells, and exocrine cells and is known to play an essential role in LDCV biogenesis (32,33). Changes in Slpi and Aqp1 expression were not induced in doxycycline-treated wild-type AtT-20 cells, in AtT-20 cells expressing only the tetracycline activator, or in AtT-20 cells expressing a different doxycycline-inducible transcript, RESP18 (34). Slpi and Aqp1 were therefore selected for further study.
The doxycycline-induced changes in Aqp1 and Slpi mRNA levels in iPAM cells were confirmed by RT-PCR (Fig. 8, A and B) and by quantitative real-time PCR (data not shown). Doxycycline-induced changes in Aqp1 expression in iPAM cells were also observed by Western blot analysis (data not shown) and immunostaining (Fig. 8C). In the absence of doxycycline, staining for PAM was not detectable, and staining for Aqp1 was most prominent near the cell surface. In doxycycline-induced cells, PAM staining became apparent, with signal concentrated in the trans-Golgi network (TGN) region and in LDCVs at the distal tips of processes (Fig. 8C, arrows). Although Aqp1 staining was still detectable at the cell surface, Aqp1 staining appeared in the TGN area and at the tips of processes (Fig. 8C, arrows). The total intensity of Aqp1 staining in each cell was quantified in the absence and presence of doxycyline, revealing a 74% increase in cells expressing PAM-1 (Fig. 8D).
Figure 8.
Verification of array analysis. The cDNA prepared from noninduced and induced iPAM subclones was subjected to RT-PCR using PCR primers for aquaporin1 (Aqp1, NM_007472) and secretory leukocyte peptidase inhibitor (Slpi, NM_011414). G3pdh (NM_008084.2) transcript levels were evaluated simultaneously. A, Ethidium bromide-stained gels are shown. B, RT-PCR signals from Aqp1 or Slpi were normalized to G3PDH for each subclone. Data are mean ± sd; P values calculated using Excel (t test, unequal variances). C, iPAM cells grown in the absence or presence of doxycycline (72 h) were stained simultaneously for PAM-1 (green) and Aqp1 (red). Arrows mark colocalization and accumulation of PAM-1 and Aqp1 at the TGN and at the distal tips of processes, where LDCVs accumulate. D, Aqp1 staining intensity in iPAM cells grown in the absence (n = 54) or presence (n = 36) of doxycycline (as in panel C) was quantified; data are mean ± sem (t test, unequal variance). E, The expression of Aqp1 and Slpi was analyzed by quantitative PCR using pituitary RNA from wild-type control mice (wt; n = 5) and PAM heterozygote mice (PAM+/−; n = 6); G3PDH was analyzed simultaneously, and data were calculated relative to G3PDH (GAPDH) for each sample using the ΔCT method (65); data are mean ± sem. Dox, Doxycycline.
If iPAM cells are representative of pituitary endocrine cells, we reasoned that the 2-fold decrease in PAM levels observed in PAM+/− mice would decrease pituitary levels of Aqp1 and Slpi mRNA. Pituitary RNA from five wild-type mice and six PAM+/− mice (35) was used to prepare cDNA for analysis by quantitative PCR (qPCR). Based on this analysis, levels of Aqp1 mRNA were reduced 12-fold (P < 0.0004), and levels of Slpi mRNA were reduced 6.6-fold (P < 0.035) in PAM+/− vs. wild-type mice (Fig. 8E). These data strongly support the hypothesis that PAM plays an important role in regulating gene expression in the pituitary.
Uhmk1 alters the ability of mycCD to increase gene expression
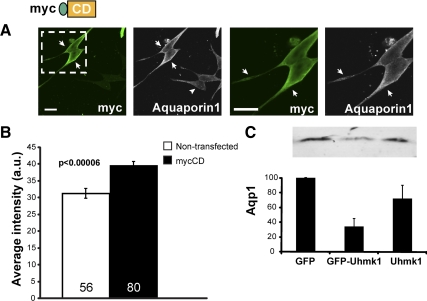
As a means of examining the effects of the CD of PAM on gene expression, we used transient transfection of mycCD (Fig. 9). As noted above (Fig. 5A), mycCD was distributed throughout the cytosol and the nucleus and was especially concentrated along the margins of the cell (Fig. 9A). In cells expressing high levels of mycCD, staining for endogenous Aqp1 increased 26 ± 4% in intensity (Fig. 9B), Although much remains to be done to determine how expression of PAM-1 leads to alterations in gene expression and the role of PAM-CD in this response, we focused here on whether Uhmk1 played a role in the response.
Figure 9.
Verification of a role for PAM-CD. A, AtT-20 cells transiently transfected with a vector encoding mycCD were fixed 48 h later and stained simultaneously for endogenous Aqp1 (white) and exogenous mycCD (green; C-stop antibody); boxed area in left panel is enlarged in right panel. Arrows indicate transfected cells; arrowhead indicates nontransfected cell. In cells expressing high levels of mycCD, staining for endogenous Aqp1 increased, especially near the cell surface. Scale bar, 20 μm. B, Aqp1 staining intensity in nontransfected (n = 56) and mycCD-expressing (n = 80) cells was quantified with the Nikon imaging software NIS-Elements; data are mean ± sem (t test, unequal variance). C, AtT-20 cells expressing GFP, GFP-Uhmk1, or GFP and Uhmk1 expressed from a dual promoter vector were FACS sorted; for each sample, 30,000 cells were subjected to Western blot analysis for Aqp1. Error bars, sd (STDEV).
Based on cotransfection studies (Fig. 5), catalytically active Uhmk1 reduced the nuclear localization of mycCD. AtT-20 cells, which express low levels of endogenous PAM, exhibited increased POMC processing when Uhmk1 levels were reduced (Fig. 1). We therefore asked whether expression of Uhmk1 altered Aqp1 expression in these cells (Fig. 9C). AtT-20 cells transiently expressing GFP, GFP-Uhmk1, or both GFP and Uhmk1 were sorted by fluorescent-activated cell sorting (FACS), and Aqp1 levels were evaluated by Western blot. Aqp1 levels were reduced to 34 ± 10% of the GFP control in cells expressing the GFP-Uhmk1 fusion protein and to 72 ± 16% of the GFP control in cells expressing GFP and mycUhmk1 encoded by a dual promoter vector (Fig. 9C). This confirms the stimulatory effects of PAM and its soluble CD fragment on Aqp1 expression and demonstrates a negative regulatory role for Uhmk1-mediated phosphorylation of PAM-CD. These data suggest that sf-CD must be nuclear to exert its modulatory effects on gene expression.
Discussion
Organelle to nucleus signaling
Endocrine feedback loops utilize secreted hormones to coordinate the actions of various target tissues. It is clear from studies of cholesterol biosynthesis (21) and the endoplasmic reticulum stress response (36) that feedback loops operating within a single cell coordinate steps that occur in different subcellular organelles. Because PAM catalyzes one of the final steps in peptide biosynthesis, it seemed well suited for a signaling role from granules.
Several previous observations supported this hypothesis and suggested a role for Uhmk1. First, an increase in PAM expression in corticotrope tumor cells resulted in granule relocalization from the tips of processes and the cell periphery to the TGN area, inhibited prohormone cleavage, and eliminated the ability of secretagogues to stimulate granule exocytosis (4). Second, PAM bearing a phosphomimetic mutation at the site phosphorylated by Uhmk1 (Ser949) failed to cause these changes whereas PAM with Ala at this position continued to cause these changes. Third, Uhmk1 interacts with the CD of PAM at sites more proximal to the TMD (Lys919, Leu926, Phe929, and Phe930), and mutation of these sites blocked the ability of PAM to affect granule localization and release (37).
Signaling from the regulated secretory pathway
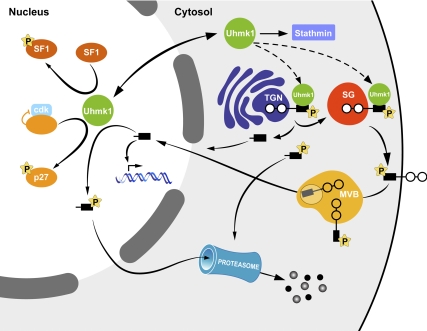
Our working model (Fig. 10) outlines selected steps in what is likely one part of a complex regulatory system. Under basal conditions, PAM is phosphorylated at multiple sites, including the Uhmk1 site, Ser949 (10). Both phosphorylation and dephosphorylation of Ser949 play essential roles (10,38). PAM with a phosphomimetic Asp949 mutation enters secretory granules more efficiently than wild-type PAM (10,38) but enters the intraluminal vesicles of multivesicular bodies more slowly than wild-type PAM or PAM with Ala at this site (66). Endoproteolytic cleavage of PAM releases sf-CD, a soluble, cytosolic fragment that is rapidly degraded by the proteasome; production of sf-CD is increased when granule content is depleted by prolonged exposure to secretagogue (8). sf-CD is detected in nuclei purified from rat pituitary and atrium, tissues that express high levels of PAM (8), leading to the idea that sf-CD interacts with nuclear targets that selectively alter gene expression. Neuronal nuclei concentrate PAM-CD to a greater extent than AtT-20 nuclei, suggesting the existence of cell type-specific binding.
Figure 10.
Model outlining the LDCV/nuclear signaling pathway in which PAM and Uhmk1 participate. Under basal conditions, the Uhmk1 site in the CD of PAM (Ser949) is phosphorylated, expediting PAM entry into LDCVs; late stages in the endocytic trafficking of PAM require both phosphorylation and dephosphorylation at this site (8,10). The endoproteolytic cleavage that releases sf-CD is diminished in PAM with a phosphomimetic mutation at Ser949. In addition, Uhmk1 phosphorylation at this site reduces PAM-CD accumulation in the nucleus. Nuclear PAM-CD, acting through pathways that remain to be described, alters the expression of a limited subset of genes, including Aqp1, which is known to affect LDCV metabolism. In the nucleus, Uhmk1, which interacts with nuclear binding sites, can phosphorylate PAM-CD, leading to nuclear exit and rapid proteasomal degradation. PAM-1 with the phosphomimetic Asp949 mutation fails to traverse the endocytic compartment normally, fails to alter cytoskeletal organization or regulated secretion, and fails to generate sf-CD efficiently. Uhmk1 opposes many of the regulatory effects of PAM on LDCV metabolism; the factors that control Uhmk1 localization are unknown. P, Phosphorylation; SG, secretory granule.
Uhmk1 affects this signaling pathway at multiple points. PAM with phosphomimetic mutations at its Uhmk1 site (Asp949) and a neighboring casein kinase 2 site (Thr946) yields less sf-CD than wild-type PAM, and coexpression of catalytically active Uhmk1 with mycCD results in nuclear exclusion of mycCD. Inactive Uhmk1K54A does not affect mycCD nuclear localization. Membrane-tethered Uhmk1 also excludes mycCD from the nucleus. Whether phosphorylation of nuclear sf-CD by nuclear Uhmk1 triggers its return to the cytosol or otherwise modulates its activity is not yet clear. Uhmk1 phosphorylation of p27Kip1 speeds p27Kip1 exit from the nucleus and its subsequent degradation by the proteasome (19).
Taken together with the observation that phosphomimetic mutations reduce the nuclear accumulation of fluorescently tagged PAM-CD, the most parsimonious explanation of our data is that P-Ser949-sf-CD is created less efficiently (8) and is also less localized to the nucleus than unmodified sf-CD. Increased levels of PAM increase the expression of a small subset of genes; transient expression of mycCD had a similar effect on expression of one of these genes, Aqp1. Overexpression of Uhmk1 diminishes Aqp1 expression, perhaps by limiting the nuclear localization of sf-CD, and one consequence of this is impaired POMC processing. Although the broad outlines of this signaling pathway are supported by our data, the specifics of many of the individual steps require further validation.
Increased PAM levels alter gene expression
Overexpression of PAM inhibits POMC processing and regulated secretion and leads to cytoskeletal rearrangement (4,34). Although phosphorylation of intact PAM by Uhmk1 and the interactions of intact PAM with Kalirin, an activator of Rac1 and RhoG, play a role in these responses, the presence of sf-CD in the nucleus suggested a role for altered gene expression. Among the up-regulated genes identified by microarray analysis, Aqp1 and Slpi were of special interest (32). Aqp1 localization and levels were also altered by transient expression of soluble mycCD or full-length PAM-1, and both Aqp1 and Slpi transcripts were elevated in wild-type mice compared with PAM+/− mice.
Although better known as a plasma membrane water channel, Aqp1 has been identified in pituitary and adrenal medullary secretory granules (32). Reducing Aqp1 levels in AtT-20 cells resulted in a reduction in secretory granule number and in the levels of several granule proteins (32). These effects were posttranslational, with increased degradation of ACTH, but not of POMC, in cell lines with reduced levels of Aqp1. Further supporting an important role for Aqp1 in granule biogenesis, pituitary ACTH levels in Aqp1 knockout mice were reduced (32). In addition to affecting Aqp1 mRNA and protein levels, expression of PAM-1, but not mycCD, makes Aqp1 appear in the TGN region. Aqp1 localized to the TGN could be involved in the condensation of secretory proteins observed in this region of PAM-1 AtT-20 cells (39).
Expression of secretory leukocyte peptidase inhibitor, a small antimicrobial and antiinflammatory peptide (31) was also increased when PAM levels rose. This response might contribute to the decrease in POMC processing seen in pituitary cells overexpressing PAM (40). Slpi and three other whey family acidic protease inhibitors (Wfdc-1, -6a, and -12) are expressed in AtT-20 cells (Supplemental Table 1); changes in expression of the other Wfdc family members could not be confirmed by RT-PCR analysis.
Regulation of gene expression by fragments of membrane proteins that enter the nucleus, as occurs with Notch signaling (41), is a well-established phenomenon. SREBP is perhaps the best known example of an intracellular membrane protein signaling information about the physiological state of the cell to the nucleus; sterol-regulated cleavage of SREBP releases its cytosolic transcription factor domain (22). The cellular response to the appearance of improperly folded proteins in the endoplasmic reticulum involves cleavage of activating transcription factor 6, again releasing a cytosolic transcription factor domain essential to the unfolded protein response (42). Calpain-1-mediated cleavage of the cytoplasmic domain of ICA512 yields a soluble, catalytically inactive tyrosine phosphatase fragment, which enters the nucleus, binds to Tyr-phosphorylated signal transducer and activator of transcription 5, prevents its dephosphorylation, and alters gene expression (2,43). Elucidating the mechanism through which sf-CD alters gene expression will require additional studies.
Uhmk1 cycles between nucleus and cytosol and functions in both compartments
With substrates such as PAM and stathmin in the cytosol and p27KIP1 and SF1 in the nucleus, localization is a critical determinant of Uhmk1 function (Fig. 9) (18,19,44). In AtT-20 cells, as in other cell lines (19,20), Uhmk1 is largely localized to the nucleus, where it is excluded from nucleoli and regions of concentrated DNA. The isolated kinase domain of Uhmk1 localized to the nucleus but its RBD did not; removal of the RBD did not diminish the ability of Uhmk1cc to localize to the nucleus. Uhmk1 moved from the cytoplasm into nucleus with a half-time similar to that observed for enhanced GFP (EGFP) entry into the nucleus (27). When the cytoplasm was photobleached, Uhmk1 left the nucleus, but did so more slowly; the τ1/2 for Uhmk1 exit from the nucleus (156 sec) was 10-fold less than its τ1/2 for motion within the nucleus (16 sec).
Partitioning of Uhmk1 between nucleus and cytoplasm is governed by its interactions with immobile binding partners as well as nuclear import and export processes. The factors regulating Uhmk1 localization are not yet clear. Uhmk1 catalytic activity plays a role, because mutating a single residue in its ATP-binding site appeared to reduce its nuclear retention (Fig. 5B). FRAP studies revealed that GFP-Uhmk1 in the nucleus was 8 times less mobile than GFP, indicating that Uhmk1 was interacting with nuclear binding sites. The nuclear pore complex controls nuclear-cytoplasmic exchange of protein and mRNA (45,46,47). With a mass of 47 kDa, Uhmk1 is too large to traverse the nuclear pore complex by passive diffusion (48). Uhmk1 does not include a classical nuclear localization signal (49) (http://www.expasy.ch/prosite/) but has a putative nuclear export signal (L113LDVSV118; NetNES 1.1 software). As observed for some other proteins (50), the nuclear localization of Uhmk1 requires energy (data not shown).
Multiple signaling pathways clearly control secretory granule biogenesis (32,37). Genetic association studies link Uhmk1 to schizophrenia (13,14) and PAM to nicotine dependence (51) and to the response to antipsychotic medications (52). Further studies are required to understand how the interactions of Uhmk1 with PAM and other granule-signaling pathways participate in normal physiology and in pathological processes.
Materials and Methods
Expression vectors
PAM-2-GFP was constructed in pEGFP-N2 (CLONTECH, Palo Alto, CA) by fusing the C terminus of rat PAM (… PAPSS976) to the N terminus of EGFP (M1VSKG …) using an engineered SmaI site that placed the linker sequence –PGIHRPVAT-, derived from pEGFP-N2, between PAM and EGFP. AtT-20 cells were stably transfected with PAM-2-GFP and selected using 0.5 mg/ml G-418 (10,38). GFP fusion proteins were constructed with Uhmk1 and Uhmk1K54A cloned into pEGFP-C3 between SalI and SmaI restriction sites. The putative RBD of Uhmk1 (RBD: residues 304–419) was inserted into pEGFP-C3 between XhoI and BamHI sites. Previously generated vectors include: pRK5 encoding myc-PAM-CD [myc-Gly5-Ser-Thr-PAM(891-976) (6)]; myc-His6-Uhmk1, myc-His6-Uhmk1cc (residues 1–383), and myc-His6-Uhmk1ccK54A (9) in the pEAK10 vector (9) or the dual promoter vector pCMS-EGFP (53). PAM-CD (residues 896–976) was expressed in bacteria as a glutathione-S-transferase (GST)-fusion protein using pGEX-6P-2 (Amersham Pharmacia Biotech, Piscataway, NJ); PAM-CD-5P was generated by mutation of Ser932, Ser937, Ser945, and Ser949 to Asp, plus Thr946 to Glu, using the QuikChange Site-Directed Mutagenesis kit (Stratagene). pSIREN-Uhmk1 shRNA construct: the RNAi-Ready-pSiren-DNR-DsRed-Express vector (CLONTECH) places shRNA expression under control of the human U6 promoter and DsRed-monomer expression under control of the cytomegalovirus promoter. Sequences targeted were: Uhmk1 shRNA, AATCCTGGCAGAGGACAAG (starts at nucleotide 1257 of Uhmk1); scramble control 1, AATGCTCGCACAGCACAAG; scramble control 2, AATGCACGCTCAGCACAAG. All constructs were verified by DNA sequencing. Two vectors encoding membrane-tethered Uhmk1 were constructed by PCR amplification of Uhmk1 using primers carrying the farnesylation sequence of v-Ki-ras2, (KKKKKKSKTKCVIM) (28) or the myristoylation sequence of MARCKS (MGCCFSKT) (29); constructs were verified by DNA sequencing. Membrane-tethered GFP controls were generated by removal of Uhmk1 and in-frame insertion of GFP from pEGFP-C3.
Antibody generation
PAM (965–976) synthesized with an N-terminal Cys (Cys-Tyr-Ser-Ala-Pro-Leu-Pro-Lys-Pro-Ala-Pro-Ser-Ser) and linked to keyhole limpet hemocyanin using maleimide was used in the Covance Laboratories, Inc. (Denver, PA) 77-d protocol to generate the C-Stop antibody. Immunoglobulin was enriched by ammonium sulfate precipitation. and aliquots were affinity-purified using PAM (965–976) linked to Affi-Gel 10 (Bio-Rad Laboratories, Inc., Hercules, CA) (54). Antibody specificity was established by verifying the inability of the C-Stop antibody to detect PAM-2-GFP. CT237 antibody was raised to a mixture of two Uhmk1 synthetic peptides; L329DDDYLENEDEYEDVVEDVKE349 was linked to keyhole limpet hemocyanin using glutaraldehyde, and Cys-P405LSAYKRGYLYQTLL419-NH2 was cross-linked with maleimide. Based on blocking studies, the specificity of the resulting antiserum was directed almost entirely to the first peptide.
Cell culture, transfection, and immunocytochemistry
AtT-20 mouse corticotrope tumor cells and stably transfected cell lines expressing rat PAM-1 or iPAM were maintained as described elsewhere (4). pEAK Rapid cells were maintained and used for transient expression as described (9). Cells were transfected on poly-d-lysine-coated 15-mm glass coverslips using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as described elsewhere (9).
For fluorescence microscopy, cells were fixed and stained as described (55). Images were collected using a LSM 510 confocal laser scanning microscope (Carl Zeiss, Thornwood, NY) with a 63×, 1.4 numerical aperture oil immersion objective by recording in the 488, 568, and/or 647 nm channels when appropriate. The filters used were BP505-530, BP560-615, and LP650. The beam splitters used were HFT UV/488/543/633, NFT610, and NFT545. Uhmk1 was visualized with rabbit polyclonal antibody JH1998 (1:1000), with or without affinity purification, and rabbit antipeptide antibody CT237 or with myc monoclonal antibody 9E10 (9). The C terminus of PAM-1 was visualized with affinity-purified rabbit polyclonal C-Stop antibody (CT267; 1:200). PAM-1 was visualized with rabbit polyclonal antibodies to exon 16 (JH629; 1:1000) (38) or PAM-P-Ser949 (JH2541; 1:1000) (10). mycCD was visualized with anti-CD monoclonal antibody or rabbit polyclonal C-Stop antibody. Aqp1 was visualized with a mouse monoclonal antibody (1:250; AbD Serotec, Kidlington, UK). Total POMC was visualized using an N-ACTH antibody that detects POMC and all cleaved peptides equally (JH93), whereas cleaved ACTH was detected with antibody specific for the cleaved C terminus of ACTH (antibody Kathy) (10).
Ratiometric analyses were performed with Simple PCI, using AtT-20 and AtT-20 PAM-1 cells expressing mycUhmk1 with GFP in a dual promoter vector vs. the GFP control transfection, or Uhmk1 shRNA/DsRed vs. the average of the two control shRNA/DsRed vectors. Fixed cells stained for cleaved ACTH or total POMC were visualized with Cy5-tagged secondary antibody.
FACS
To isolate transfected cells that express GFP, Uhmk1-GFP, or Uhmk1/GFP from the mixed population observed after transfection, AtT-20 cells were dissociated 16 h after transfection using trypsin to yield a single-cell suspension. Before sorting, aggregates were removed by passing the cell suspension through a 40-μm cell strainer. FACS was carried out using a BD FACS Aria II flow cytometer. Nontransfected cells and GFP control cells were used to gate for GFP expression. About 10% of the cells expressed GFP at detectable levels, and about half of those were collected as GFP-positive single-cell events by FACS. Equal numbers of cells (30,000) were used for Western blotting.
Primary hippocampal neuron cultures
Primary hippocampal neurons were isolated as described elsewhere (56), using enzymatic digestion and trituration (57) and plated at a density of approximately 600 cells/mm2 on poly-l-lysine-coated dishes. After 3 h incubation at 37 C in 5% CO2, the medium was changed to Neurobasal containing 1× B27 supplement, 1× antibiotics, 0.5 mm l-glutamine, and 25 μm l-glutamic acid. Every 4 d, half the medium was replaced with medium lacking l-glutamic acid.
Purification and labeling of recombinant PAM-CD and exon 16
GST-PAM-CD, GST-PAM-CD-5P, and exon 16 were expressed and purified; GST was removed from PAM-CD with PreScission Protease (9,10,11,58). Recombinant proteins were labeled with Alexa Fluor 647 (Molecular Probes, Eugene, OR) using the company protocol and separated from unbound dye with a Micro-Bio-Spin P6 column; the final concentrations of the Alexa Fluor 647 labeled proteins were approximately 1 mg/ml.
Microinjection of cells
For live cell time-lapse confocal imaging, AtT-20 cells plated onto glass bottom dishes (MatTek) were injected using an Eppendorf InjectMan N12 and Eppendorf Femtojet (Eppendorf, Hamburg, Germany) with a Zeiss LSM 510 Confocor 3. Injection parameters were: injection time, 0.5 sec; injection pressure, 50–80 hPa; compensation pressure, 8–16 hPa. Cells were injected and imaged at 37 C and 5% CO2 in an XL-LSM Incubator (PeCon GmbH Erbach, Germany) mounted on the stage of a Zeiss LSM 510 Confocor 3 confocal microscope equipped with a 40× 1.2 NA objective. The volume injected was <10% of the cell volume. Processing and quantitative analysis of confocal images was performed using Metamorph (Universal Imaging Corp., Downingtown, PA). A total of 71 cells were analyzed by measuring the average intensity of three nonoverlapping sample areas in the nucleus, three in the cytoplasm, and two in empty background areas. Background was removed from each individual cell, and the nucleo-cytoplasmic ratio of the averaged areas was obtained for each cell.
Western blot analysis and nuclear isolation
Cell pellets or subcellular fractions were subjected to Western blot analysis (59). Uhmk1 was visualized with JH1998 (1:1000) (9). PAM-1 was visualized with C-Stop antibody (CT267; affinity-purified, 1:200) or PAM-CD monoclonal antibody 6E6 (60). Histones were visualized with mouse monoclonal F152.C25.WJJ at 1:500 (Chemicon, Temecula, CA). Calnexin antibody (clone 37; BD Biosciences, Missisauga, Ontario, Canada) was used at 1:1000. When quantification was performed, nonsaturated signals were acquired with a GeneGnome work station using Gene-Tools software (Syngene, Frederick, MD). Nuclei were isolated from AtT-20 cells as described elsewhere (54). An equal percentage of the input, supernatant, interface, and pellet samples (1%) was analyzed by SDS-PAGE.
FRAP
Images were collected using a Zeiss LSM 510 confocal laser scanning microscope with a 63× 1.4 numerical aperture oil immersion objective by recording in the 505–530 nm channel. Selected cell regions were photobleached with 50 iterations of 488 nm laser as previously shown (61,62) but a lower laser power at 50% and followed for up to 4 min by imaging every 1.6 sec with 7.5% laser power. This protocol produced 11% photobleaching over this time period on nonphotobleached neighboring cells. Areas (14 pixels in diameter) were quantified in the photobleached nuclear area, nonbleached nuclear area, and cytoplasm. The recovery curve was fit by a single exponential function using Sigmaplot software (SPSS, Science, Chicago, IL).
RNA isolation and microarray analysis
Three clones of AtT-20 iPAM cells were examined. Each clone was maintained in control medium or medium containing 4 μg/ml of doxycycline for 48 h (63,64), and total RNA was prepared using Trizol (Invitrogen). The six samples of RNA were labeled and used to probe a MouseWG-6v2.0 Expression BeadChip using the standard Illumina protocol in the University of Connecticut Health Center Translational Genomics Core. These chips target 45,281 transcripts representing 35,000 genes. A background of expression was chosen using 10 immunoglobulin-related genes not believed to be expressed in pituitary corticotropes, which set the cutoff at 120. By comparison, POMC (Pomc1) and Ubiquitin B (Ubb) showed expression levels over 70,000, and glyceraldehyde 3-phosphate dehydrogenase (G3PDH) was 55,000. Less abundant but biochemically detectable genes were closer to background, such as prohormone convertase 1 (PCSK1) at 150. Uhmk1 was barely above background (179) and prohormone convertase 2 (PCSK2) was at background (116), as expected. Our previous analyses showed that Uhmk1 expression was high in anterior pituitary, neurointermediate pituitary, and throughout the brain (9). The levels of expression for the most abundant 15,000 genes differed among the subclones by only ±15%. The ratio of the signal for the doxycycline-induced RNA to the basal RNA was calculated for each transcript for each subclone (120 background subtracted) and averaged to yield the data in Supplemental Table 1. Approximately 11,000 transcripts with expression levels of 150 or below (less than 25% above the 120 background) have been deleted from the table. The average sd for the doxycycline/basal ratio was ±12%.
Conventional and qPCR
Tissue samples were homogenized in Trizol (Invitrogen), RNA was prepared following the manufacturer’s instructions, and cDNA was prepared using random primers and either Superscript II Reverse Transcriptase (Invitrogen) or the iScript Select cDNA Kit (Bio-Rad Laboratories). Conventional PCR was performed for 25–30 cycles at 52–58 C annealing temperature (Table 1). For qPCR, primer pairs for Aqp1, Slpi, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were chosen to give similar product lengths and melt temperatures (Table 1). Quantitative PCR was performed with iQ SYBR Green Supermix using an Eppendorf Mastercycler ep realplex machine; the standard program was used with elongation time per cycle set to 40 sec. Maximal rates of amplification of products for every primer pair were 2.0 ± 0.1 per cycle. Relative levels of transcripts were calculated by the ΔCT method (65).
Table 1.
Primers used for PCR
| Gene | Primer name | Sequence | Tm (C) | nt |
|---|---|---|---|---|
| mGAPDH | mGAPDH-for | TTGTCAGCAATGCATCCTGCACCACC | 61 | 119 |
| NM_008084.2 | mGAPDH-rev | CTGAGTGGCAGTGATGGCATGGAC | 61 | |
| mAqp1 | mAqp1-top | CACTGTGCCCTTAACCACATTGTGAACC | 61 | 128 |
| NM_007472.1 | mAqp1-bot | GGGAACGGAGACGAGTGTGCAGC | 62 | |
| mSlpi | mSlpi-top | GGTTCCATGGCTCCCGGCTCC | 62 | 127 |
| NM_011414.2 | mSlpi-bot | CTCTCCAACAGCATTTCCCTAAAGATCGG | 62 | |
| mGAPDH | mGAPDH-Lt | TTGTCAGCAATGCATCCTGCACCAC | 59 | 412 |
| NM_008084.2 | mGAPDH-Lb | GAGACAACCTGGTCCTCAGTGTAG | 62 | |
| mAqp1 | mAqp1-Lt | GCTGCACACTCGTCTCCGTTCCCTAGCAG | 67 | 316 |
| NM_007472.1 | mAqp1-Lb | CATACACAGAGCAGACAGATACAAGGTCCTGGTTC | 63 | |
| mSlpi | mSlpi-Lt | CCCTCCCAATGTCTGCCAGAGGGAC | 64 | 309 |
| NM_011414.2 | mSlpi-Lb | TGCTCTCCAACAGCATTTCCCTAAAGATCGG | 63 |
for, Forward; rev, reverse; bot, bottom; Tm, temperature; nt, nucleotide.
Supplementary Material
Acknowledgments
We thank Darlene D'Amato for her constant technical support and Anupinder Kaur and the University of Connecticut Health Center Translational Genomics Core for their help with array analyses. We thank G. Baiges for her excellent illustration work and Jodi Eipper-Mains for repeatedly helping with the array analysis, gene identification, and figures.
Footnotes
This work was supported by National Institutes of Health Grant DK-32949 (to B.A.E. and R.E.M.), and Grants NS-015190 and RR-022232 (to J.H.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 23, 2010
Abbreviations: Aqp1, Aquaporin 1; CD, cytoplasmic domain; EGFP, enhanced GFP; FACS, fluorescence-activated cell sortig; FRAP, fluorescence recovery after photobleaching; GFP, green fluorescent protein; G3PDH, glyceraldehyde 3-phosphate dehydrogenase; GST, glutathione-S-transferase; iPAM, doxycycline-inducible rat PAM-1; LDCV, large dense core vesicle; PAL, peptidyl-α-hydroxyglycine α-amidating lyase; PAM, peptidylglycine α-amidating monooxygenase; P-CIP2, PAM-COOH terminal interactor protein 2; POMC, proopiomelanocortin; qPCR, quantitative PCR; RBD, RNA binding domain; sf-CD, soluble fragment of CD; shRNA, short hairpin RNA; Slp1, secretory leukocyte peptidase inhibitor; SREBP, sterol-regulatory element-binding protein; TGN, trans-Golgi network; TMD, transmembrane domain; Uhmk1, U2AF homology motif kinase 1.
References
- Ferraro F, Eipper BA, Mains RE 2005 Retrieval and reuse of pituitary secretory granule proteins. J Biol Chem 280:25424–25435 [DOI] [PubMed] [Google Scholar]
- Trajkovski M, Mziaut H, Altkrüger A, Ouwendijk J, Knoch KP, Müller S, Solimena M 2004 Nuclear translocation of an ICA512 cytosolic fragment couples granule exocytosis and insulin expression in β-cells. J Cell Biol 167:1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo YP, Hutton JC, Angleson JK 2004 Recycling of the dense-core vesicle membrane protein phogrin in Min6 β-cells. Biochem Biophys Res Commun 324:1004–1010 [DOI] [PubMed] [Google Scholar]
- Ciccotosto GD, Schiller MR, Eipper BA, Mains RE 1999 Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J Cell Biol 144:459–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram SL, Mains RE, Eipper BA 1996 Identification of routing determinants in the cytosolic domain of a secretory granule-associated integral membrane protein. J Biol Chem 271:17526–17535 [DOI] [PubMed] [Google Scholar]
- Bell-Parikh LC, Eipper BA, Mains RE 2001 Response of an integral granule membrane protein to changes in pH. J Biol Chem 276:29854–29863 [DOI] [PubMed] [Google Scholar]
- Bruzzaniti A, Marx R, Mains RE 1999 Activation and routing of membrane-tethered prohormone convertases 1 and 2. J Biol Chem 274:24703–24713 [DOI] [PubMed] [Google Scholar]
- Rajagopal C, Stone KL, Francone VP, Mains RE, Eipper BA 2009 Secretory granule to the nucleus: role of a multiply phosphorylated intrinsically unstructured domain. J Biol Chem 284:25723–25734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BD, Darlington DN, Penzes P, Johnson RC, Eipper BA, Mains RE 1999 The novel kinase peptidylglycine α-amidating monooxygenase cytosolic interactor protein 2 interacts with the cytosolic routing determinants of the peptide processing enzyme peptidylglycine α-amidating monooxygenase. J Biol Chem 274:34646–34656 [DOI] [PubMed] [Google Scholar]
- Steveson TC, Zhao GC, Keutmann HT, Mains RE, Eipper BA 2001 Access of a membrane protein to secretory granules is facilitated by phosphorylation. J Biol Chem 276:40326–40337 [DOI] [PubMed] [Google Scholar]
- Alam MR, Caldwell BD, Johnson RC, Darlington DN, Mains RE, Eipper BA 1996 Novel proteins that interact with the COOH-terminal cytosolic routing determinants of an integral membrane peptide-processing enzyme. J Biol Chem 271:28636–28640 [DOI] [PubMed] [Google Scholar]
- Maucuer A, Le Caer JP, Manceau V, Sobel A 2000 Specific Ser-Pro phosphorylation by the RNA-recognition motif containing kinase KIS. Eur J Biochem 267:4456–4464 [DOI] [PubMed] [Google Scholar]
- Puri V, McQuillin A, Choudhury K, Datta S, Pimm J, Thirumalai S, Krasucki R, Lawrence J, Quested D, Bass N, Moorey H, Morgan J, Punukollu B, Kandasami G, Curtis D, Gurling H 2007 Fine mapping by genetic association implicates the chromosome 1q23.3 gene UHMK1, encoding a serine/threonine protein kinase, as a novel schizophrenia susceptibility gene. Biol Psychiatry 61:873–879 [DOI] [PubMed] [Google Scholar]
- Puri V, McQuillin A, Datta S, Choudhury K, Pimm J, Thirumalai S, Krasucki R, Lawrence J, Quested D, Bass N, Crombie C, Fraser G, Walker N, Moorey H, Ray M, Sule A, Curtis D, St Clair D, Gurling H 2008 Confirmation of the genetic association between the U2AF homology motif (UHM) kinase 1 (UHMK1) gene and schizophrenia on chromosome 1q23.3. Eur J Hum Genet 16:1275–1282 [DOI] [PubMed] [Google Scholar]
- Reed SI 2003 Ratchets and clocks: the cell cycle, ubiquitylation and protein turnover. Nat Rev Mol Cell Biol 4:855–864 [DOI] [PubMed] [Google Scholar]
- Murray AW 2004 Recycling the cell cycle: cyclins revisited. Cell 116:221–234 [DOI] [PubMed] [Google Scholar]
- Bièche I, Manceau V, Curmi PA, Laurendeau I, Lachkar S, Leroy K, Vidaud D, Sobel A, Maucuer A 2003 Quantitative RT-PCR reveals a ubiquitous but preferentially neural expression of the KIS gene in rat and human. Mol Brain Res 114:55–64 [DOI] [PubMed] [Google Scholar]
- Maucuer A, Camonis JH, Sobel A 1995 Stathmin interaction with a putative kinase and coiled-coil-forming protein domains. Proc Natl Acad Sci USA 92:3100–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, Nabel EG 2002 A growth factor-dependent nuclear kinase phosphorylates p27Kip1 and regulates cell cycle progression. EMBO J 21:3390–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manceau V, Kielkopf CL, Sobel A, Maucuer A 2008 Different requirements of the kinase and UHM domains of KIS for its nuclear localization and binding to splicing factors. J Mol Biol 381:748–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengoechea-Alonso MT, Ericsson J 2007 SREBP in signal transduction: cholesterol metabolism and beyond. Curr Opin Cell Biol 19:215–222 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL 1997 The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331–340 [DOI] [PubMed] [Google Scholar]
- Carson JH, Gao Y, Tatavarty V, Levin MK, Korza G, Francone VP, Kosturko LD, Maggipinto MJ, Barbarese E 2008 Multiplexed RNA trafficking in oligodendrocytes and neurons. Biochim Biophys Acta 1779:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Takano K, Yoneda Y 2005 The role of mammalian Staufen on mRNA traffic: a view from its nucleocytoplasmic shuttling function. Cell Struct Funct 30:51–56 [DOI] [PubMed] [Google Scholar]
- Gama-Carvalho M, Carmo-Fonseca M 2001 The rules and roles of nucleocytoplasmic shuttling proteins. FEBS Lett 498:157–163 [DOI] [PubMed] [Google Scholar]
- Piñol-Roma S, Dreyfuss G 1991 Transcription-dependent and transcription-independent nuclear transport of hnRNP proteins. Science 253:312–314 [DOI] [PubMed] [Google Scholar]
- Wei X, Henke VG, Strübing C, Brown EB, Clapham DE 2003 Real-time imaging of nuclear permeation by EGFP in single intact cells. Biophys J 84:1317–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocks O, Peyker A, Bastiaens PIH 2006 Spatio-temporal segregation of Ras signals: one ship, three anchors, many harbors. Curr Opin Cell Biol 18:351–357 [DOI] [PubMed] [Google Scholar]
- Mazumdar MD, Tasic B, Miyamichi K, Li L, Luo L 2007 A global double-fluorescent Cre reporter mouse. Genesis 45:593–605 [DOI] [PubMed] [Google Scholar]
- Xin X, Ferraro F, Bäck N, Eipper BA, Mains RE 2004 Cdk5 and Trio modulate endocrine cell exocytosis. J Cell Sci 117:4739–4748 [DOI] [PubMed] [Google Scholar]
- Nukiwa T, Suzuki T, Fukuhara T, Kikuchi T 2008 Secretory leukocyte peptidase inhibitor and lung cancer. Cancer Sci 99:849–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaoutova I, Cawley NX, Patel N, Kim T, Rathod T, Loh YP 2008 Aquaporin 1 is important for maintaining secretory granule biogenesis in endocrine cells. Mol Endocrinol 22:1924–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SJ, Sattar AK, Jeong EH, Satchi M, Cho JA, Dash S, Mayes MS, Stromer MH, Jena BP 2002 Aquaporin 1 regulates GTP-induced rapid gating of water in secretory vesicles. Proc Natl Acad Sci USA 99:4720–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller MR, Mains RE, Eipper BA 1997 A novel neuroendocrine intracellular signaling pathway. Mol Endocrinol 11:1846–1857 [DOI] [PubMed] [Google Scholar]
- Czyzyk TA, Ning Y, Hsu MS, Peng B, Mains RE, Eipper BA, Pintar JE 2005 Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev Biol 287:301–313 [DOI] [PubMed] [Google Scholar]
- Wolfson JJ, May KL, Thorpe CM, Jandhyala DM, Paton JC, Paton AW 2008 Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell Microbiol 10:1775–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MR, Steveson TC, Johnson RC, Bäck N, Abraham B, Mains RE, Eipper BA 2001 Signaling mediated by the cytosolic domain of peptidylglycine α-amidating monooxygenase. Mol Biol Cell 12: 629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steveson TC, Keutmann HT, Mains RE, Eipper BA 1999 Phosphorylation of cytosolic domain Ser(937) affects both biosynthetic and endocytic trafficking of peptidylglycine α-amidating monooxygenase. J Biol Chem 274:21128–21138 [DOI] [PubMed] [Google Scholar]
- Bäck N, Litonius E, Mains RE, Eipper BA 2004 Fluoride causes reversible dispersal of Golgi cisternae and matrix in neuroendocrine cells. Eur J Cell Biol 83:389–402 [DOI] [PubMed] [Google Scholar]
- Mains RE, Bloomquist BT, Eipper BA 1991 Manipulation of neuropeptide biosynthesis through the expression of antisense RNA for peptidylglycine α-amidating monooxygenase. Mol Endocrinol 5: 187–193 [DOI] [PubMed] [Google Scholar]
- Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R 2000 Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature 405:966–970 [DOI] [PubMed] [Google Scholar]
- Tsukumo Y, Tomida A, Kitahara O, Nakamura Y, Asada S, Mori K, Tsuruo T 2007 Nucleobindin 1 controls the unfolded protein response by inhibiting ATF6 activation. J Biol Chem 282:29264–29272 [DOI] [PubMed] [Google Scholar]
- Mziaut H, Trajkovski M, Kersting S, Ehninger A, Altkrüger A, Lemaitre RP, Schmidt D, Saeger HD, Lee MS, Drechsel DN, Müller S, Solimena M 2006 Synergy of glucose and growth hormone signalling in islet cells through ICA512 and STAT5. Nat Cell Biol 8:435–445 [DOI] [PubMed] [Google Scholar]
- Manceau V, Swenson M, Le Caer JP, Sobel A, Kielkopf CL, Maucuer A 2006 Major phosphorylation of SF1 on adjacent Ser-Pro motifs enhances interaction with U2AF65. FEBS J 273:577–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keminer O, Peters R 1999 Permeability of single nuclear pores. Biophys J 77:217–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Clapham DE 1999 Conformational changes of the in situ nuclear pore complex. Biophys J 77:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente SR 2000 Gatekeepers of the nucleus. Science 288:1374–1377 [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C, Pyle J, Jaconi M, Stehno-Bittel L, Clapham DE 1996 Conformational states of the nuclear pore complex induced by depletion of nuclear Ca2+ stores. Science 273:1875–1877 [DOI] [PubMed] [Google Scholar]
- Lange A, Mills RE, Lange CJ, Stewart M, Devine SE, Corbett AH 2007 Classical nuclear localization signals: definition, function, and interaction with importin α. J Biol Chem 282:5101–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn CF, Neidig JA, Freidinger KE, Stankiewicz TA, Weaver BS, McGrew J, Allison LA 2001 Nucleocytoplasmic shuttling of the thyroid hormone receptor α. Mol Endocrinol 15:512–533 [DOI] [PubMed] [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Poling J, Krauthammer M, Farrer L, Kranzler HR 2007 Genomewide linkage scan for nicotine dependence: identification of a chromosome 5 risk locus. Biol Psychiatry 61:119–126 [DOI] [PubMed] [Google Scholar]
- Müller DJ, Kennedy JL 2006 Genetics of antipsychotic treatment emergent weight gain in schizophrenia. Pharmacogenomics 7:863–887 [DOI] [PubMed] [Google Scholar]
- Ma XM, Huang J, Wang Y, Eipper BA, Mains RE 2003 Kalirin, a multifunctional Rho GEF, is necessary for maintenance of hippocampal pyramidal neuron dendrites and dendritic spines. J Neurosci 23:10593–10603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller MR, Mains RE, Eipper BA 1995 A neuroendocrine-specific protein localized to the endoplasmic reticulum by distal degradation. J Biol Chem 270:26129–26138 [DOI] [PubMed] [Google Scholar]
- Francone VP, Maggipinto MJ, Kosturko LD, Barbarese E 2007 The microtubule-associated protein tumor overexpressed gene/cytoskeleton-associated protein 5 is necessary for myelin basic protein expression in oligodendrocytes. J Neurosci 27:7654–7662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Tatavarty V, Korza G, Levin MK, Carson JH 2008 Multiplexed dendritic targeting of α calcium calmodulin-dependent protein kinase II, neurogranin, and activity-regulated cytoskeleton- associated protein RNAs by the A2 pathway. Mol Biol Cell 19:2311–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker G 1989 Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol 108:1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HY, Johnson RC, Mains RE, Eipper BA 1993 Topological switching of the COOH-terminal domain of PAM by alternative RNA splicing. Arch Biochem Biophys 301:77–84 [DOI] [PubMed] [Google Scholar]
- De M, Ciccotosto GD, Mains RE, Eipper BA 2007 Trafficking of a secretory granule membrane protein is sensitive to copper. J Biol Chem 282:23362–23371 [DOI] [PubMed] [Google Scholar]
- Milgram SL, Kho ST, Martin GV, Mains RE, Eipper BA 1997 Localization of integral membrane peptidylglycine α-amidating monooxygenase in neuroendocrine cells. J Cell Sci 110:695–706 [DOI] [PubMed] [Google Scholar]
- Herrmann F, Lee J, Bedford MT, Fackelmayer FO 2005 Dynamics of human protein arginine methyltransferase 1 (PRMT1) in vivo. J Biol Chem 280:38005–38010 [DOI] [PubMed] [Google Scholar]
- Huang Y, Qui J, Chen G, Dong S 2008 Coiled-coil domain of PML is essential for the aberrant dynamics of PML-RAR-α, resulting in sequestration and decreased mobility of SMRT. Biochem Biophys Res Commun 365:258–265 [DOI] [PubMed] [Google Scholar]
- McPherson CE, Eipper BA, Mains RE 2002 Genomic organization and differential expression of Kalirin isoforms. Gene 284:41–51 [DOI] [PubMed] [Google Scholar]
- Francone VP, Mezquita J 2005 Diversification of CDK11 transcripts during chicken testis development and regression. Mol Reprod Dev 72:273–280 [DOI] [PubMed] [Google Scholar]
- Yuan JS, Wang D, Stewart Jr CN 2008 Statistical methods for efficiency adjusted real-time PCR quantification. Biotechnol J 3:112–123 [DOI] [PubMed] [Google Scholar]
- Bäck N, Rajagopal C, Mains RE, Eipper BA 1 April 2010 Secretory granule membrane protein recycles through multivesicular bodies. Traffic 10.1111/j.1600-0854.2010.01066.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.