Abstract
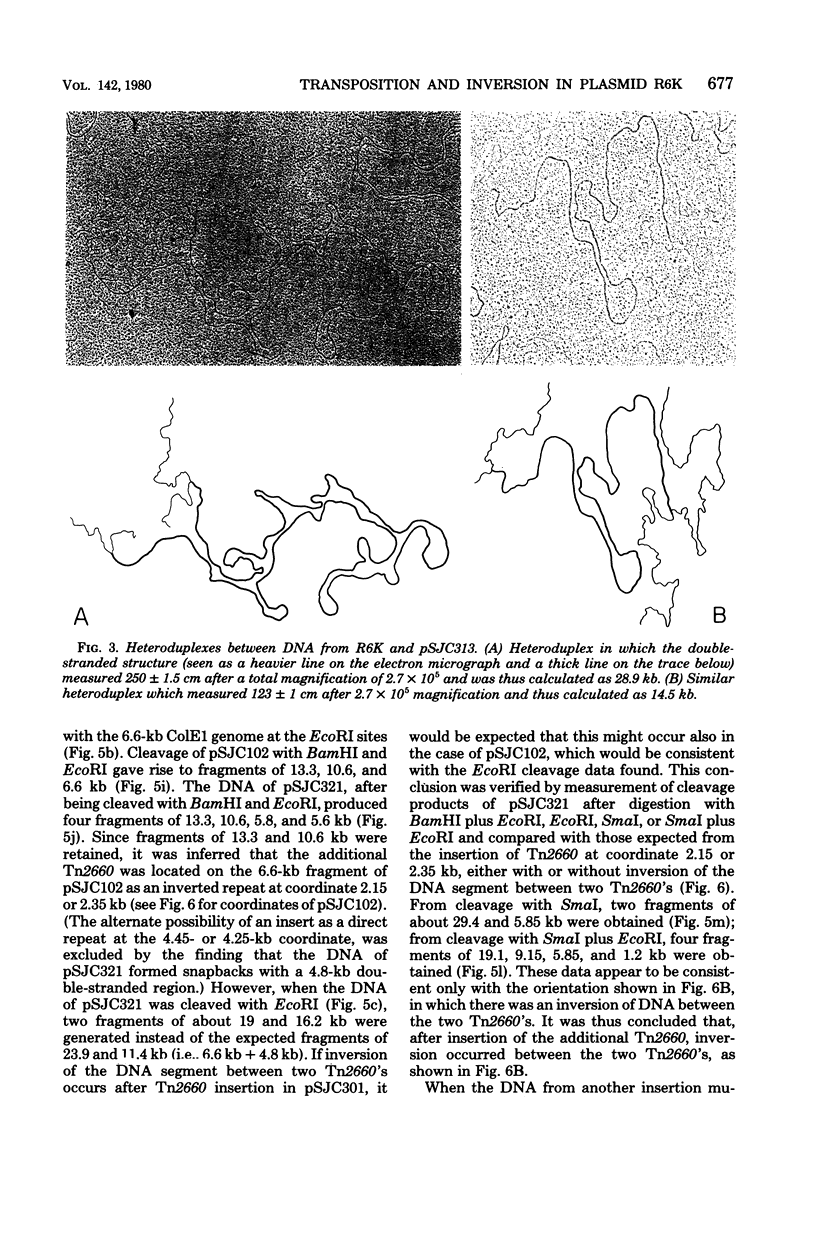
Selection was made in Escherichia coli K-12 recA hosts carrying plasmid R6K for ampicillin hyperresistance. Twenty-two selected strains were found to carry mutant plasmids, which, from electron microscopy and restriction enzyme analysis, were concluded to arise by a duplication of transposon Tn2660, which confers ampicillin resistance, in all cases the duplicate transposon being in an inverted orientation with respect to the resident Tn2660. A mutant of R6K, pSJC301, which was temperature sensitive for ampicillin resistance was produced by in vitro hydroxylamine treatment of R6K deoxyribonucleic acid. A plasmid hybrid, pSJC102, was constructed by cloning the EcoRI R6K fragment carrying the wild-type beta-lactamase gene into the EcoRI site of ColE1. pSJC301 and pSJC102 were transformed into the same recA host strain to form a stable biplasmid strain. Ampicillin-hyperresistant mutants were selected from this strain and screened for plasmids with a duplication of transposon Tn2660, which occurred with equal frequency in either pSJC301 or pSJC102; of 12 characterized, all were inverse repeats of the resident transposon. All six Tn2660 inserts into pSJC301 determined temperature-sensitive ampicillin resistance, and all six inserts into pSJC102 determined wild-type ampicillin resistance, from which it was inferred that transposition of a duplicate Tn2660 occurs predominantly as an intramolecular event, at least in the multicopy R6K plasmid. In all 28 insertion mutants of R6K, there was an inversion of the deoxyribonucleic acid between the two transposons, whereas in only one of six insertion mutants of pSJC102, inversion had occurred. These results are discussed in terms of current models of transposition.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur A., Sherratt D. Dissection of the transposition process: a transposon-encoded site-specific recombination system. Mol Gen Genet. 1979 Oct 1;175(3):267–274. doi: 10.1007/BF00397226. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calame K. L., Yamada Y., Shanblatt S. H., Nakada D. Location of promoter sites on plasmid NTP1 which contains the ampicillin resistance transposon Tn1701. J Mol Biol. 1979 Feb 5;127(4):397–409. doi: 10.1016/0022-2836(79)90229-8. [DOI] [PubMed] [Google Scholar]
- Chou J., Casadaban M. J., Lemaux P. G., Cohen S. N. Identification and characterization of a self-regulated repressor of translocation of the Tn3 element. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4020–4024. doi: 10.1073/pnas.76.8.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Casadaban M. J., Chou J., Tu C. P. Studies of the specificity and control of transposition of the Tn3 element. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1247–1255. doi: 10.1101/sqb.1979.043.01.141. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Williams P., Helinski D. R. Plasmid ColE1 DNA replication in Escherichia coli strains temperature-sensitive for DNA replication. Mol Gen Genet. 1975;136(4):273–289. doi: 10.1007/BF00341713. [DOI] [PubMed] [Google Scholar]
- Dougan G., Saul M., Twigg A., Gill R., Sherratt D. Polypeptides expressed in Escherichia coli K-12 minicells by transposition elements Tn1 and Tn3. J Bacteriol. 1979 Apr;138(1):48–54. doi: 10.1128/jb.138.1.48-54.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Preparation of covalently closed and open circular DNA molecules of phage lambda. Biochim Biophys Acta. 1976 Apr 15;432(1):113–117. doi: 10.1016/0005-2787(76)90047-2. [DOI] [PubMed] [Google Scholar]
- Gill R., Heffron F., Dougan G., Falkow S. Analysis of sequences transposed by complementation of two classes of transposition-deficient mutants of Tn3. J Bacteriol. 1978 Nov;136(2):742–756. doi: 10.1128/jb.136.2.742-756.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., Dreisig H., Molin S., Nordström K., Uhlin B. E. DNA replication control: studies of plasmid R1. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):419–425. doi: 10.1101/sqb.1979.043.01.048. [DOI] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P. L., Clowes R. C. Transposition of a duplicate antibiotic resistance gene and generation of deletions in plasmid R6K. J Bacteriol. 1979 Feb;137(2):977–989. doi: 10.1128/jb.137.2.977-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Smith H. R., Anderson E. S. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol Gen Genet. 1976 Apr 23;145(1):101–108. doi: 10.1007/BF00331564. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Miller H. I., Adams M. L. EcoRI restriction endonuclease cleavage site map of bacteriophage P22DNA. J Mol Biol. 1978 Jan 25;118(3):347–363. doi: 10.1016/0022-2836(78)90233-4. [DOI] [PubMed] [Google Scholar]
- Jahn G., Laufs R., Kaulfers P. M., Kolenda H. Molecular nature of two Haemophilus influenzae R factors containing resistances and the multiple integration of drug resistance transposons. J Bacteriol. 1979 May;138(2):584–597. doi: 10.1128/jb.138.2.584-597.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D. The production of phage chromosome fragments and their capacity for genetic transfer. J Mol Biol. 1962 Apr;4:275–287. doi: 10.1016/s0022-2836(62)80005-9. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli: requirement for deoxyribonucleic acid polymerase I in the replication of the plasmid ColE 1 . J Bacteriol. 1973 Jun;114(3):1116–1124. doi: 10.1128/jb.114.3.1116-1124.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Brevet J., Cohen S. N. Involvement of multiple translocating DNA segments and recombinational hotspots in the structural evolution of bacterial plasmids. J Mol Biol. 1976 Dec;108(2):333–360. doi: 10.1016/s0022-2836(76)80124-6. [DOI] [PubMed] [Google Scholar]
- Kretschmer P. J., Cohen S. N. Effect of temperature on translocation frequency of the Tn3 element. J Bacteriol. 1979 Aug;139(2):515–519. doi: 10.1128/jb.139.2.515-519.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer P. J., Cohen S. N. Selected translocation of plasmid genes: frequency and regional specificity of translocation of the Tn3 element. J Bacteriol. 1977 May;130(2):888–899. doi: 10.1128/jb.130.2.888-899.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Lovett M. A., Helinski D. R. Strand and site specificity of the relaxation event for the relaxation complex of the antibiotic resistance plasmid R6K. Biochemistry. 1974 Dec 31;13(27):5484–5490. doi: 10.1021/bi00724a005. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., HUMAN M. L. A nonhereditary, host-induced variation of bacterial viruses. J Bacteriol. 1952 Oct;64(4):557–569. doi: 10.1128/jb.64.4.557-569.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisen P. D., Kopecko D. J., Chou J., Cohen S. N. Site-specific DNA deletions occurring adjacent to the termini of a transposable ampicillin resistance element (Tn3). J Mol Biol. 1977 Dec 25;117(4):975–978. doi: 10.1016/s0022-2836(77)80008-9. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Ohmori H., Ohtsubo E. Nucleotide-sequence analysis of Tn3 (ap): implications for insertion and deletion. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1269–1277. doi: 10.1101/sqb.1979.043.01.144. [DOI] [PubMed] [Google Scholar]
- Pinney R. J., Smith J. T. Fertility inhibition of an N group R factor by a group X R factor, R6K. J Gen Microbiol. 1974 Jun;82(2):415–418. doi: 10.1099/00221287-82-2-415. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Richmond M. H. Inhibition of TnA translocation by TnA. J Bacteriol. 1977 Jan;129(1):407–414. doi: 10.1128/jb.129.1.407-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J Bacteriol. 1952 Oct;64(4):489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: tandemly repeated resistance determinants in amplified forms of pAMalpha1 DNA. J Mol Biol. 1976 Apr 15;102(3):583–600. doi: 10.1016/0022-2836(76)90336-3. [DOI] [PubMed] [Google Scholar]